Abstract
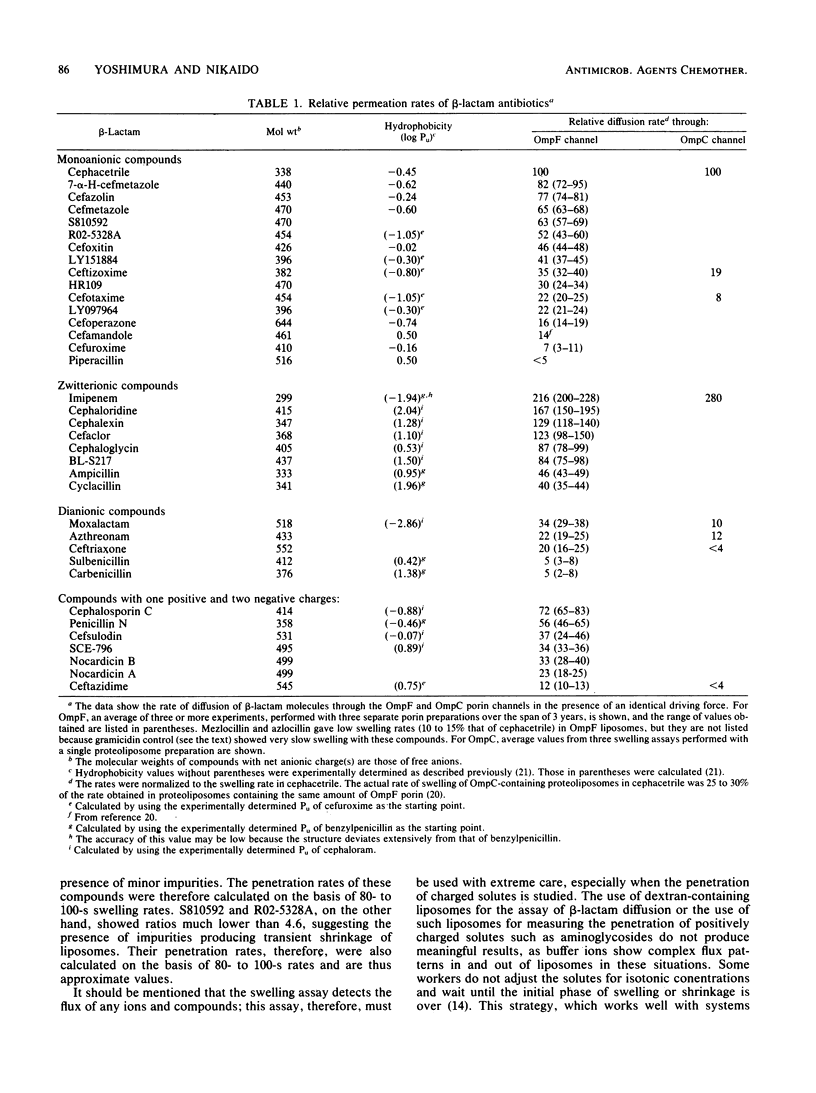
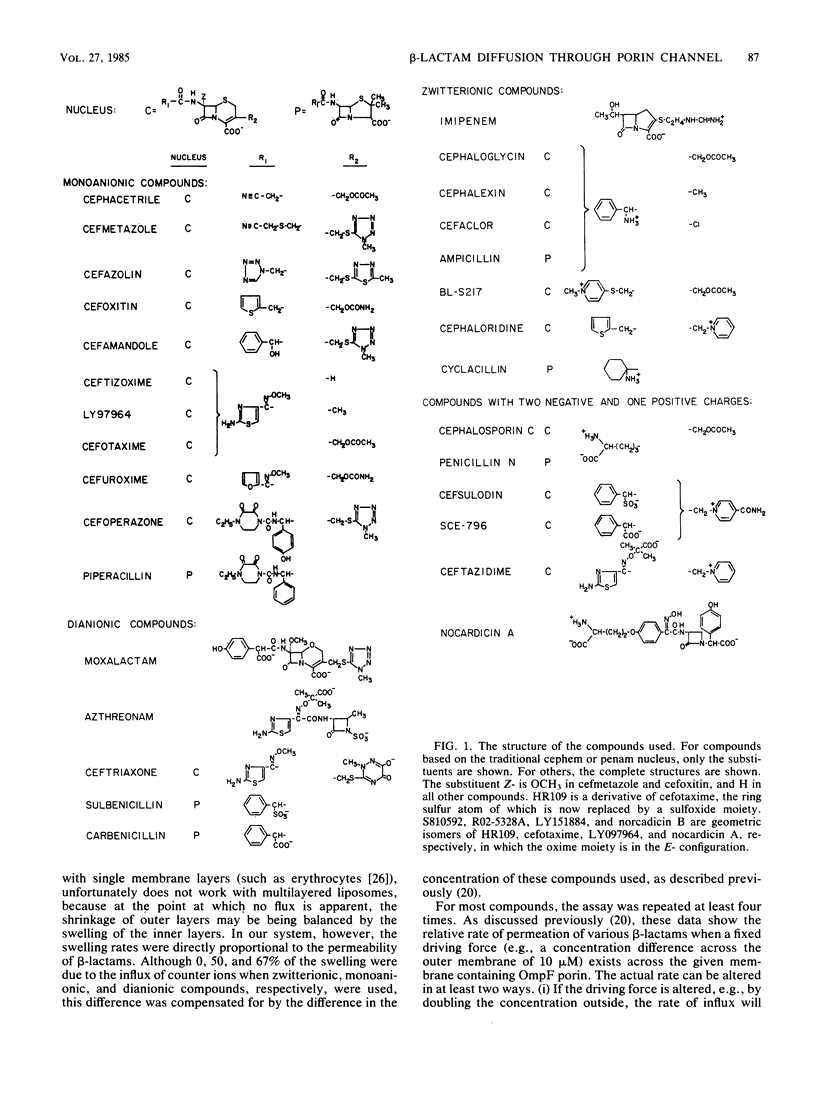
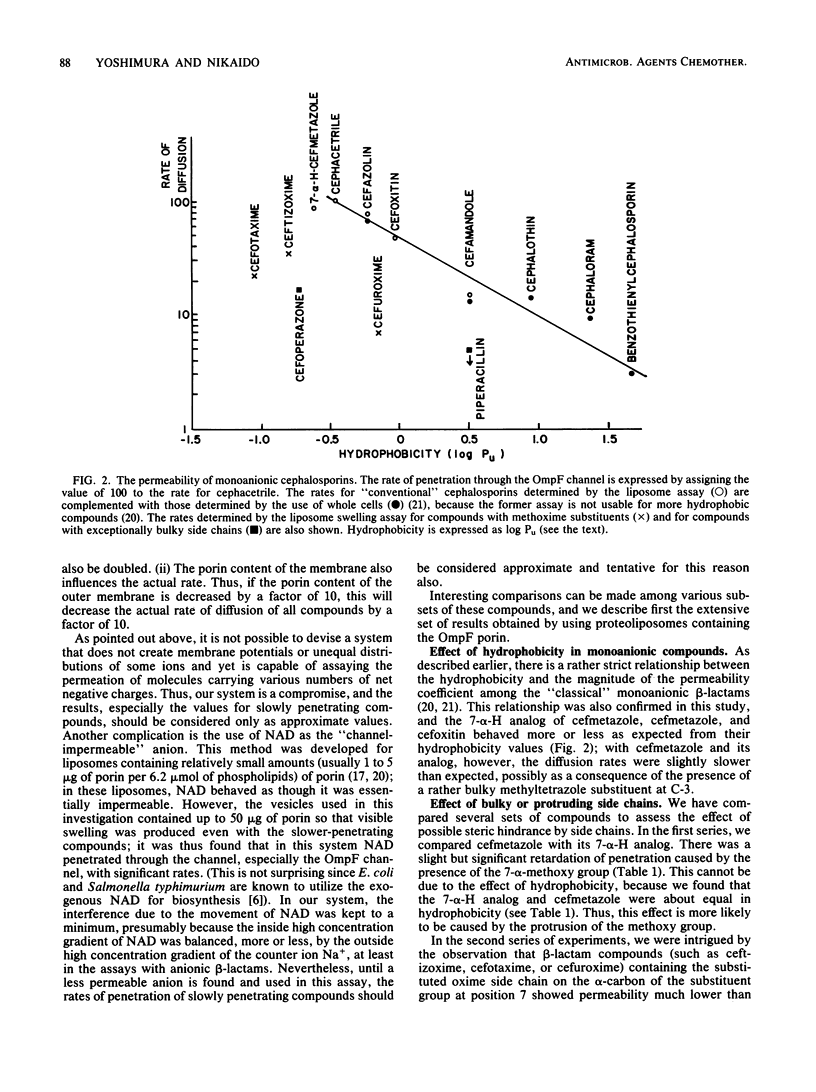
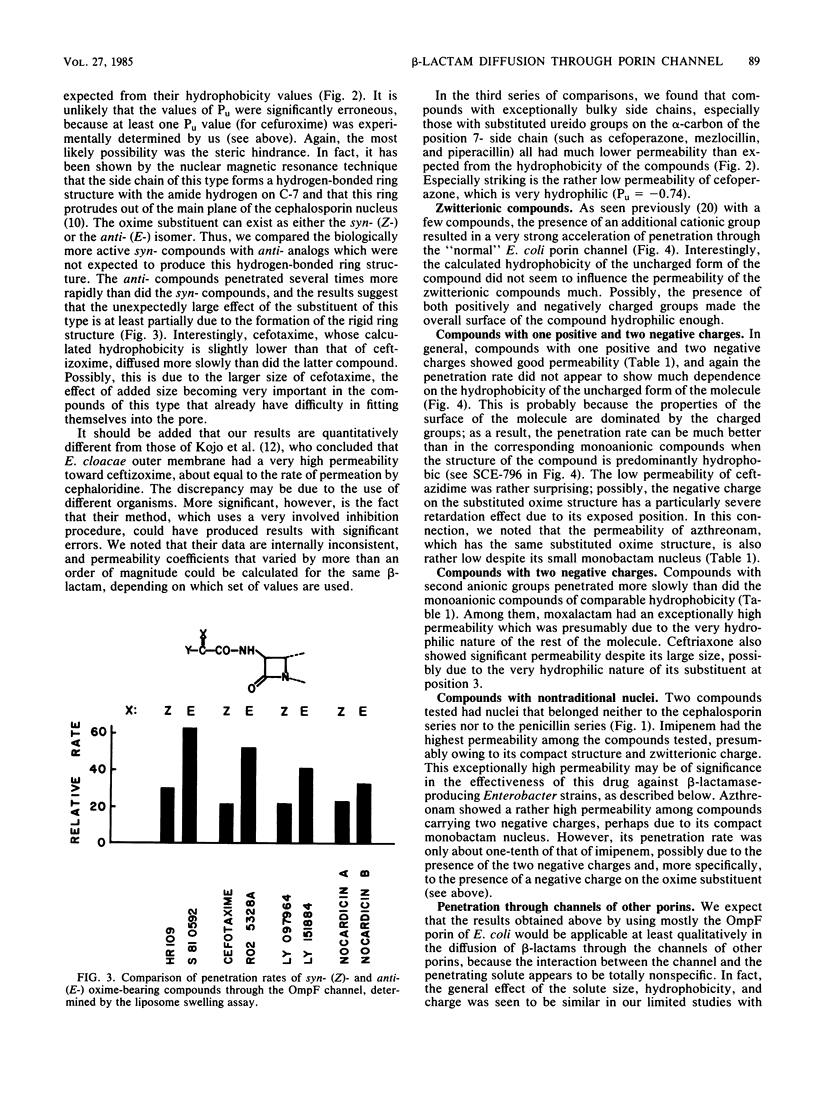
Diffusion rates of various beta-lactam antibiotics through the OmpF and OmpC porin channels of Escherichia coli K-12 were measured by the use of reconstituted proteoliposomes. The results can be interpreted on the basis of the gross physicochemical properties of the antibiotics along the following lines. (i) As noted previously (Nikaido et al., J. Bacteriol., 153:232-240, 1983), there was a monotonous dependence of the penetration rate on the hydrophobicity of the molecule among the classical monoanionic beta-lactams, and a 10-fold increase in the octanol-water partition coefficient of the uncharged molecule decreased the penetration rate by a factor of 5 to 6. (ii) Compounds with exceptionally bulky side chains, such as mezlocillin, piperacillin, and cefoperazone, showed much slower penetration rates than expected from their hydrophobicity. (iii) The substituted oxime side chain on the alpha-carbon of the substituent group at position 7 of the cephem nucleus decreased the penetration rate almost by an order of magnitude; this appears to be largely due to the steric effect. (iv) The presence of a methoxy group at position 7 of the cephalosporins also reduced the penetration rate by 20%, probably also due to the steric hindrance. (v) Zwitterionic compounds penetrated very rapidly, and the correlation between the rate and hydrophobicity appeared to be much weaker than with the monoanionic compounds. Imipenem showed the highest permeability among the compounds tested, presumably due, at least in part, to its compact molecular structure. (vi) Compounds with two negative charges penetrated more slowly than did analogs with only one negatively charged group. Among them, only moxalactam, ceftriaxone, and azthreonam showed penetration rates corresponding to, or higher than, 10% of that of imipenem.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bavoil P., Nikaido H., von Meyenburg K. Pleiotropic transport mutants of Escherichia coli lack porin, a major outer membrane protein. Mol Gen Genet. 1977 Dec 14;158(1):23–33. doi: 10.1007/BF00455116. [DOI] [PubMed] [Google Scholar]
- Dietzel I., Kolb V., Boos W. Pole cap formation in Escherichia coli following induction of the maltose-binding protein. Arch Microbiol. 1978 Aug 1;118(2):207–218. doi: 10.1007/BF00415731. [DOI] [PubMed] [Google Scholar]
- Douglas J. T., Rosenberg E. Y., Nikaido H., Verstreate D. R., Winter A. J. Porins of Brucella species. Infect Immun. 1984 Apr;44(1):16–21. doi: 10.1128/iai.44.1.16-21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Moat A. G. Nicotinamide adenine dinucleotide biosynthesis and pyridine nucleotide cycle metabolism in microbial systems. Microbiol Rev. 1980 Mar;44(1):83–105. doi: 10.1128/mr.44.1.83-105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein F. W., Gutmann L., Williamson R., Collatz E., Acar J. F. In vivo and in vitro emergence of simultaneous resistance to both beta-lactam and aminoglycoside antibiotics in a strain of Serratia marcescens. Ann Microbiol (Paris) 1983 May-Jun;134A(3):329–337. [PubMed] [Google Scholar]
- Harder K. J., Nikaido H., Matsuhashi M. Mutants of Escherichia coli that are resistant to certain beta-lactam compounds lack the ompF porin. Antimicrob Agents Chemother. 1981 Oct;20(4):549–552. doi: 10.1128/aac.20.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M., Komori T. A., Kamiya T. Letter: Nocardicin A and B, novel monocyclic beta-lactam antibiotics from a Nocardia species. J Am Chem Soc. 1976 May 12;98(10):3023–3025. doi: 10.1021/ja00426a063. [DOI] [PubMed] [Google Scholar]
- Jaffé A., Chabbert Y. A., Derlot E. Selection and characterization of beta-lactam-resistant Escherichia coli K-12 mutants. Antimicrob Agents Chemother. 1983 Apr;23(4):622–625. doi: 10.1128/aac.23.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojo H., Shigi Y., Nishida M. Enterobacter cloacae outer membrane permeability to ceftizoxime (FK 749) and five other new cephalosporin derivatives. J Antibiot (Tokyo) 1980 Mar;33(3):317–321. doi: 10.7164/antibiotics.33.317. [DOI] [PubMed] [Google Scholar]
- Komatsu Y., Murakami K., Nishikawa T. Penetration of moxalactam into its target proteins in Escherichia coli K-12: comparison of a highly moxalactam resistant mutant with its parent strain. Antimicrob Agents Chemother. 1981 Nov;20(5):613–619. doi: 10.1128/aac.20.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae R., Nakae T. Diffusion of aminoglycoside antibiotics across the outer membrane of Escherichia coli. Antimicrob Agents Chemother. 1982 Oct;22(4):554–559. doi: 10.1128/aac.22.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Proteins forming large channels from bacterial and mitochondrial outer membranes: porins and phage lambda receptor protein. Methods Enzymol. 1983;97:85–100. doi: 10.1016/0076-6879(83)97122-7. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J Bacteriol. 1983 Jan;153(1):241–252. doi: 10.1128/jb.153.1.241-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Song S. A., Shaltiel L., Nurminen M. Outer membrane of Salmonella XIV. Reduced transmembrane diffusion rates in porin-deficient mutants. Biochem Biophys Res Commun. 1976 May 23;76(2):324–330. doi: 10.1016/0006-291x(77)90728-8. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Emergence of resistance to cefamandole: possible role of cefoxitin-inducible beta-lactamases. Antimicrob Agents Chemother. 1979 Jun;15(6):792–797. doi: 10.1128/aac.15.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Hiruma R., Kawana N., Kaneko M., Taniyasu F., Inami A. Outer membrane permeation of beta-lactam antibiotics in Escherichia coli, Proteus mirabilis, and Enterobacter cloacae. Antimicrob Agents Chemother. 1982 Oct;22(4):585–592. doi: 10.1128/aac.22.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg A. H., Tolxdorff-Neutzling R. M., Wiedemann B. Chromosomal beta-lactamases of Enterobacter cloacae are responsible for resistance to third-generation cephalosporins. Antimicrob Agents Chemother. 1983 Jun;23(6):918–925. doi: 10.1128/aac.23.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha'afi R. I., Rich G. T., Mikulecky D. C., Solomon A. K. Determination of urea permeability in red cells by minimum method. A test of the phenomenological equations. J Gen Physiol. 1970 Apr;55(4):427–450. doi: 10.1085/jgp.55.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Then R. L., Angehrn P. Trapping of nonhydrolyzable cephalosporins by cephalosporinases in Enterobacter cloacae and Pseudomonas aeruginosa as a possible resistance mechanism. Antimicrob Agents Chemother. 1982 May;21(5):711–717. doi: 10.1128/aac.21.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. Outer membrane protein e of Escherichia coli K-12 is co-regulated with alkaline phosphatase. J Bacteriol. 1980 Jul;143(1):151–157. doi: 10.1128/jb.143.1.151-157.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A., Hiruma R., Sawai T. Phospholipid bilayer permeability of beta-lactam antibiotics. J Antibiot (Tokyo) 1982 Dec;35(12):1692–1699. doi: 10.7164/antibiotics.35.1692. [DOI] [PubMed] [Google Scholar]
- Young J. D., Blake M., Mauro A., Cohn Z. A. Properties of the major outer membrane protein from Neisseria gonorrhoeae incorporated into model lipid membranes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3831–3835. doi: 10.1073/pnas.80.12.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]


