Abstract
Urocortin, an endogenous peptide structurally related to corticotropin-releasing factor (CRF), has potent cardiovascular effects, suggesting that it may be of significance in cardiovascular regulation. The objective of this study was to analyse the effects of urocortin and its action mechanisms on human blood vessels.
To this, 3 mm long segments from human saphenous veins were prepared for isometric tension recording in an organ bath.
In the segments at basal resting tone, urocortin did not produce any effect, but in the segments precontracted with endothelin-1 (1 – 10 nM), urocortin (1 pM – 10 nM) produced concentration-dependent relaxation.
This relaxation was not modified by the inhibitor of nitric oxide synthase NG-nitro-L-arginine methyl ester (L-NAME, 100 μM), but it was potentiated by the cyclo-oxygenase inhibitor meclofenamate (10 μM) and it was reduced by the inhibitors of high-conductance Ca2+-dependent potassium channels tetraethylammonium (TEA, 10 mM) and charybdotoxin (100 nM).
These results indicate that human saphenous veins are very sensitive to urocortin, which produces vascular relaxation by a mechanism independent of nitric oxide and dependent of high-conductance Ca2+-dependent potassium channels, and that it may be opposed by the release of vasoconstrictor prostanoids. Therefore, urocortin may be of significance for regulation of the venous circulation in humans.
Keywords: Ion channels, peptides, prostaglandins, vasodilation, veins
Introduction
Urocortin is a recently isolated 40 amino acid peptide, which has a high degree of structural homology with corticotropin-releasing factor (CRF), and belongs to a group of structurally related peptides which includes, in addition to urocortin and CRF, fish urotensin I and amphibian sauvagine (Parkes & May, 2000).
CRF and urocortin may act as neurotransmitters in the central nervous system and may also act on peripheral tissues, particularly in the cardiovascular system. These peptides produce potent effects on the cardiovascular system when administered both intravenously or directly into the central nervous system (Parkes & May, 2000; Parkes et al., 2001). It has been observed that the effects of intravenous urocortin are more potent than those of CRF, probably because the affinity of urocortin is higher than that of CRF for the subtype of CRF – R2β receptors (Vaughan et al., 1995), which are expressed in peripheral tissues (Perrin et al., 1995). Moreover, urocortin mRNA is expressed in smooth muscle cells of blood vessels and in cardiac myocites (Parkes & May, 2000). Because of that, it has been suggested that urocortin may be the endogenous CRF-like peptide that binds to cardiovascular receptors and mediates their cardiocirculatory effects (Parkes et al., 2001). In rats, intravenous urocortin produce potent and long lasting hypotension which might be due to systemic vasodilation, and tachycardia, probably in part baroreflex mediated (Vaughan et al., 1995). Indeed, it has been shown that this peptide produces relaxation of rat basilar arteries (Schilling et al., 1998) and of rat coronary circulation (Terui et al., 2001). In mice, urocotin also produces hypotension, which is abolished in knockout mice with the CRF – R2 receptor subtype inactivated (Bale et al., 2000; Coste et al., 2000). Interestingly, these knockout mice have elevated resting blood pressure, suggesting that activation of CRF – R2 receptors may play a role in regulation of basal vascular tone (Coste et al., 2000). However, there may exist species differences in the vascular effects of urocortin, as in sheep this peptide increases systemic blood pressure, in addition to increasing cardiac contractility (Parkes et al., 1997).
All these observations have aroused the interest for the possible role of urocortin in cardiovascular regulation. In addition, urocortin is produced in human hearts, and it increases in failing hearts (Nishikimi et al., 2000), suggesting that this peptide could be involved in human cardiovascular regulation under normal and pathologic conditions. Considering that there is marked species variability in the cardiovascular effects of urocortin, these effects should be also studied in human tissues before the results obtained in animals can be extrapolated to humans. To our knowledge, there is only one study analysing the response to urocortin in human blood vessels, this study shows that this peptide produces dilatation of human perfused placenta (Leitch et al., 1998). Therefore, the objective of our study was to analyse the effects of urocortin on human blood vessels, using segments from human saphenous veins. The role of nitric oxide, prostanoids and potassium channels in the vascular effects of urocortin were also examined.
Methods
For this study, saphenous veins from 12 patients (nine males and three females), aged between 45 and 83 years, were used. The veins were obtained during coronary bypass artery surgery and placed in ice isotonic cold saline solution for transportation to the laboratory. Under a dissecting microscope, they were cleaned from surrounding tissue, cut in segments 3 mm long and prepared for isometric tension recording in a 4 ml organ bath, containing modified Krebs – Henseleit solution with the following composition (millimolar): NaCl, 115; KCl, 4.6; KH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.5; NaHCO3, 25; glucose, 11. The solution was equilibrated with 95% oxygen and 5% carbon dioxide to give a pH of 7.3 – 7.4, and maintained at 37°C. Briefly, the method consists of passing through the lumen of the vascular segment two stainless steel wires, 150 μm in diameter. One wire is fixed to the organ bath wall, while the other is connected to a strain gauge for isometric tension recording, thus permitting the application of passive tension in a plane perpendicular to the long axis of the vascular cylinder. The recording system included a Universal Transducing Cell UC3 (Statham Instruments, Inc.), a Statham Microscale Accessory UL5 (Statham Instruments, Inc.) and a Beckman Type RS Recorder (model R-411, Beckman Instruments, Inc.). To establish the resting tension for maximal force development, a series of preliminary experiments was performed on vein rings of similar length and outer diameter that were exposed repeatedly to KCl 100 mM. Basal tension was increased gradually until contractions were maximal, and the optimal resting tension was 30 mN. This tension was applied to all the vascular segments. When the veins were stretched immediately after placed in the bath, they often developed spontaneous myogenic tone. To avoid this, in the present experiments, the vascular segments were kept in the bath at zero tension for 30 min before being stretched to the passive tension of 30 mN. In these conditions, they did not develop any apparent spontaneous contraction over the resting tension, and were equilibrated for 60 – 90 min at this basal passive tension until they were stimulated.
The responses to cumulative concentrations of human urocortin (1 pM – 10 nM) were recorded in the segments of saphenous veins at basal, resting tension after applying the optimal passive tension, and also in vein segments precontracted with endothelin-1 (1 – 10 nM). To asses the functional status of the endothelium, acetylcholine 10 μM was added cumulatively to the bath in the arteries precontracted with endothelin-1, after recording the effects of the last urocortin concentration. In this case, acetylcholine produced a further relaxation which was 16±7% of the remaining tone. This is the typical response to acetylcholine of intact human saphenous arteries (Sogo et al., 2000).
The relaxation to urocortin in the precontracted segments was studied in segments pretreated with the inhibitor of nitric oxide synthesis NG-nitro-L-arginine methyl ester (L-NAME, 100 μM), with the cyclo-oxygenase inhibitor meclofenamate (10 μM), and with the inhibitors of high-conductance Ca2+-dependent potassium channels tetraethylammonium (TEA, 1 – 10 mM) or charybdotoxin (100 nM), to analyse the role of nitric oxide, prostanoids and potassium channels in this relaxation to urocortin. These blockers were added to the organ bath 20 min before beginning the concentration response curve to urocortin. As the segments pretreated with TEA developed a greater level of active tone when precontracted with endothelin-1 (1 – 10 nM), a group of control segments were precontracted with a higher concentration of endothelin-1 (10 – 30 nM) to reach a level of tone similar to that in the segments pretreated with TEA plus endothelin-1, in order to test whether this elevated tone may affect by itself the relaxation to urocortin
The relaxation to urocortin is expressed as percentage of the active tone reached, and calculated as means±s.e.mean. The results were analysed by one-way ANOVA, followed by Dunnet's test to compare each experimental condition with control. P<0.05 was considered as significant.
The substances used were: charybdotoxin; meclofenamic acid (2-[(2,6-Dichloro-3-methyl-phenyl)amino]benzoic acid) sodium salt; L-NAME; tetraetylammonium chloride; and urocortin human; all from Sigma; and endothelin-1 (human, porcine) from Peninsula Laboratories Europe, Ltd.
Results
In the vein segments at basal, resting tension, urocortin (1 pM – 10 nM) did not produce any effect (six segments from three individuals) (Figure 1). In the vein segments precontracted with endothelin-1 (1 – 10 nM, mean active tone=8.5±0.75 mN), urocortin produced concentration-dependent relaxation (Figures 1 and 2). This relaxation was not significantly modified by pretreatment with L-NAME (100 μM), but it was potentiated (P<0.01) by the cyclo-oxygenase inhibitor meclofenamate (10 μM) (Figure 2). The antagonist of high conductance, Ca2+-sensitive potassium channels charybdotoxin (100 nM) reduced (P<0.05) the relaxation to urocortin. Also, TEA, which is an antagonist of this same type of potassium channels, reduced the relaxation to urocortin at a concentration of 10 mM (P<0.05) but not at 1 mM (Figure 2).
Figure 1.
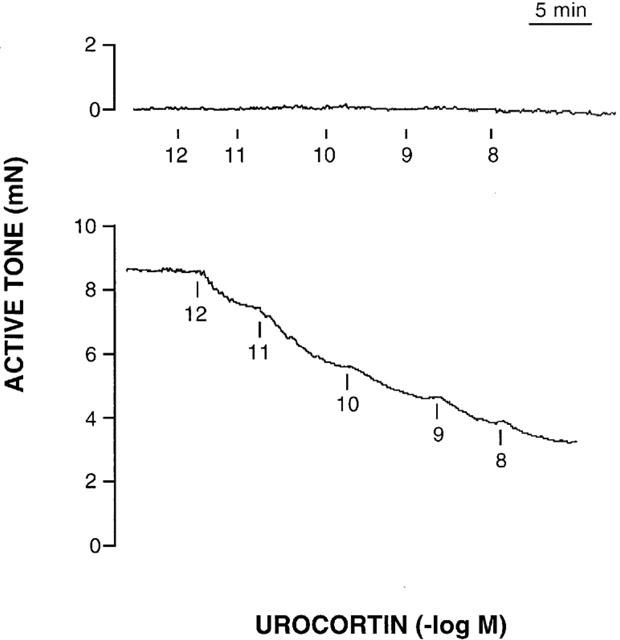
Actual recordings of the effects of urocortin (1 pM – 10 nM) on one segment of human saphenous vein at basal, resting tone (top), and on another segment of saphenous vein precontracted with endothelin-1 (5 nM) (bottom).
Figure 2.
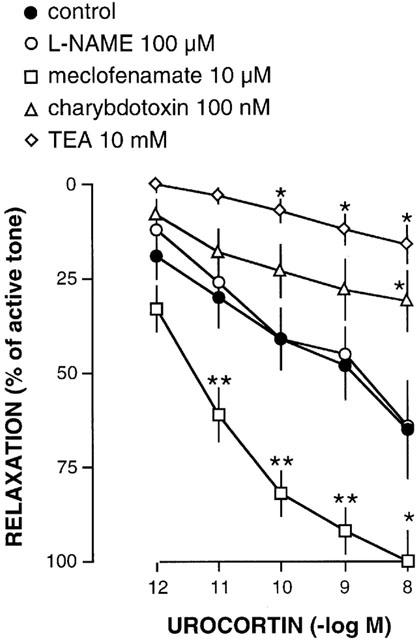
Summary of the relaxation to urocortin (1 pM – 10 nM) of human saphenous veins precontracted with endothelin-1 (1 – 10 nM) in the absence (control, 15 segments from 12 individuals) and in the presence of L-NAME (100 μM, 14 segments from 12 individuals), meclofenamate (10 μM, 14 segments from 12 individuals), charybdotoxin (100 nM, 20 segments from 12 individuals), or TEA (10 mM, seven segments from five individuals). Statistically significant compared to control (*P<0.05; **P<0.01).
The active tone developed by precontraction with endothelin-1 (1 – 10 nM) tended to be higher in the segments pretreated with L-NAME (10.9±2.2 mN), meclofenamate (15.6±3.2), charybdotoxin (16.1±2.7 mN) or TEA (1 mM, 19.9±2.5 mN; 10 mM, 25±3.7 mN) than in control segments, although this increase was statistically significant (P<0.01) only in those treated with 10 mM TEA. To test whether this elevated tone could affect the relaxation to urocortin, a group of control segments (five segments from five individuals) were precontracted with a higher concentration of endothelin-1 (10 – 30 nM) to reach a level of tone (19.3±1.2 mN) which was not significantly different from that in the segments treated with TEA plus endothelin-1. In those control segments precontracted with a higher concentration of endothelin-1 and showing a higher level of tone (19.3±1.2 mN), the relaxation to urocortin was similar to that in the control segments precontracted with a lower concentration of endothelin-1 and showing a lower level of tone (8.5±0.75 mN) (data not shown).
Discussion
The present study was performed in vitro using 3 mm long segments of human saphenous veins under isometric conditions, and the results show that urocortin produces concentration-dependent relaxations of these veins, and this relaxation was evident with very low (1 pM) concentrations of this peptide. Therefore, human saphenous veins are very sensitive to urocortin, and although our in vitro results can not be directly extrapolated to in vivo function, we may suggest that the magnitude of the observed vein relaxation might represent relatively great changes of vessel wall tension, and thus great displacements of blood volume in the in vivo venous circulation. Urocortin concentrations of 1 pM correspond to the plasma levels measured in humans (Watanabe et al., 1999), and, as urocortin can be produced by systemic tissues such as the heart (Nishikimi et al., 2000), our results suggest that urocortin may be relevant to the regulation of the venous circulation in humans. Our results in human veins compare with those obtained in rat isolated basilar artery by Schilling et al. (1998) and in human perfused placenta by Leitch et al. (1998), who found that urocortin relaxed these vascular beds at very low concentrations (1 – 10 pM). These two studies also report that the sensitivity of these vascular beds for urocortin was higher than that for CRF, suggesting that urocortin may be the physiological endogenous activator of cardiovascular CRF receptors. Urocortin also may dilate cat cerebral (Yatsushiro et al., 1997) and sheep coronary (Parkes et al., 1997) arteries in vivo.
Regarding the mechanisms of the vasodilation by urocortin, our results suggest that this effect is not due to release of nitric oxide by the vascular wall, as it was not modified by the nitric oxide synthase inhibitor L-NAME, and the concentration of this inhibitor we have used (100 μM) may be enough to markedly reduce nitric oxide synthesis (Fernández et al., 1994). This lack of nitric oxide participation agrees with that observed in the coronary circulation of rat, in which the relaxation to urocortin was not inhibited by nitric oxide inhibition (Terui et al., 2001). In the basilar artery of rat, the effect of urocortin is not modified by endothelium removal, suggesting that this peptide acts directly on the smooth muscle of the vascular wall (Schilling et al., 1998). Contrarily, the relaxation to CRF in the rat coronary circulation is partly dependent on nitric oxide (Grunt et al., 1993). Thus, there may be differences between these two peptides in their mechanisms of vascular action.
We have also observed that the relaxation to urocortin was reduced by TEA and by charybdotoxin, which are both blockers of potassium channels of the high-conductance Ca2+-sensitive subtype. Although the vessel segments pretreated with these blockers developed a higher active tone when precontracted with endothelin-1, this higher tone probably is not the cause of the reduced relaxation to urocortin, because induction of a higher tone with a higher concentration of endothelin-1 did not modify the relaxation to urocortin. In the rat isolated basilar artery also it has been observed that the relaxation to urocortin is reduced by antagonists of high-conductance Ca2+-sensitive potassium channels, such as TEA or iberiotoxin (Schilling et al., 1998), as occurred in our experiments. Therefore, it may be suggested that urocortin produces vascular relaxation by activating potassium channels in smooth muscle cells. Potassium channels seem to mediate the vascular relaxation to several substances (adenosine, prostacyclin or epoxides of arachidonic acid) which act directly on vascular smooth muscle. Opening of potassium channels produces hyperpolarization of smooth muscle membrane, and subsequent relaxation of smooth muscle cell (Jackson, 2000).
Regarding the role of prostanoids, our results suggest that the vasodilation to urocortin may be modulated by the release of cyclo-oxygenase products. This vasodilation was potentiated by the cyclo-oxygenase inhibitor meclofenamate, thus suggesting that vasoconstricting prostanoids may be released from the vascular wall of urocortin-stimulated human saphenous veins, and this release may oppose the dilating effect of urocortin. It has been reported that the relaxation to acetylcholine in human saphenous veins may also be increased by cyclo-oxygenase inhibition (Yang et al., 1991). Consequently, it may be hypothesized that vasoconstrictor prostanoids released by the venous wall may modulate the response to different vasodilator stimuli, including urocortin. Indeed, the venous endothelium may have a higher capacity to release vasoconstrictor factors than the arterial endothelium (García-Villalón et al., 1993). Our results contrast, however, with those of Terui et al. (2001), who have reported that the in vitro relaxation of rat coronary circulation to urocortin was reduced by cyclo-oxygenase inhibition, and suggested that this relaxation may be mediated by the release of vasodilating prostaglandins. The interaction of urocortin with prostanoids may be complex, as urocortin increases the release from human trophoblast tissue of the vasodilator prostaglandin E2, but also urocortin potentiates the contracting effects of prostaglandin F2α on human myometrium (Petraglia et al., 1999). It may be hypothesized that urocortin may increase the release and/or the effects of both contracting and dilating prostanoids, and that either of these may predominate depending on the type of blood vessel (arterial or venous), and perhaps of vascular bed or species.
Since the relatively recent discovery of urocortin, evidence has been found suggesting that this peptide may have significant cardiovascular functions, not only under normal conditions, but also under pathological conditions such as cardiac ischemia (Brar et al., 2000) and cardiac failure (Nishikimi et al., 2000). Our present results, showing that urocortin produces marked dilation at very low concentrations in human veins, suggest that this peptide should be considered as a factor involved in the physiology and/or pathophysiology of the venous circulation in human beings.
Acknowledgments
We are indebted to Hortensia Fernández-Lomana and María Ester Martínez by technical assistance. This study was supported by MEyC (SAF 1999-0004), FIS 899/0224) and CAM (08-4/0003/1998).
Abbreviations
- CRF
corticotropin-releasing factor
- L-NAME
NG-nitro-L-arginine methyl ester
- TEA
tetraethylammonium
References
- BALE T.L., CONTARINO A., SMITH G.W., CHAN R., GOLD L.H., SAWCHENKO P.E., KOOB G.F., VALE W.W., LEE K.F. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behavior and are hypertensive to stress. Nat. Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- BRAR B.K., JONASSEN A.K., STEPHANOU A., SANTILLI G., RAILSON J., KNIGHT R.A., TELLON D.M., LATCHMAN D.S. Urocortin protects against ischemic and reperfusion injury via a MAPK-dependent pathway. J. Biol. Chem. 2000;275:8508–8514. doi: 10.1074/jbc.275.12.8508. [DOI] [PubMed] [Google Scholar]
- COSTE S.C., KESTERSON R.A., HELDWEIN K.A., STEVENS L.S., HEARD A.D., HOLLIS J.H., MURRAY S.E., HILL J.K., PANTELY G.A., HOHIMER A.R., HATTON D.C., PHILLIPS T.J., FINN D.A., LOW M.J., RITENBERG M.B., STENZEL P., STENZEL-POORE M.P. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat. Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- FERNÁNDEZ N., MONGE L., GARCÍA-VILLALÓN A.L., GARCÍA J.L., GÓMEZ B., DIÉGUEZ G. Cooling effects on nitric oxide production by rabbit ear and femoral arteries during cholinergic stimulation. Br. J. Pharmacol. 1994;113:550–554. doi: 10.1111/j.1476-5381.1994.tb17024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCÍA-VILLALÓN A.L., FERNÁNDEZ N., GARCÍA J.L., MONGE L., GÓMEZ B., DIÉGUEZ G. Reactivity of the dog cavernous carotid artery. The role of the arterial and venous endothelium. Pflügers Arch. Eur. J. Physiol. 1993;425:256–262. doi: 10.1007/BF00374175. [DOI] [PubMed] [Google Scholar]
- GRUNT M., GLASER J., SCHMIDHUBER H., PAUSCHINGER P., BOM J. Effects of corticotropin-releasing factor on isolated rat heart activity. Am. J. Physiol. 1993;264:H1124–H1129. doi: 10.1152/ajpheart.1993.264.4.H1124. [DOI] [PubMed] [Google Scholar]
- JACKSON W.F. Ion channels and vascular tone. Hypertension. 2000;35:173–178. doi: 10.1161/01.hyp.35.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEITCH I.M., BOURA A.L.A., BOTTI C., READ M.A., WALTERS W.A.W., SMITH R. Vasodilator actions of urocortin and related peptides in the human perfused placenta in vitro. J. Clin. Endocrinol. Metab. 1998;83:4510–4513. doi: 10.1210/jcem.83.12.5356. [DOI] [PubMed] [Google Scholar]
- NISHIKIMI T., MIYATA A., HORIO T., YOSHIHARA F., NAGAYA N., TAKISHITA S., YUTANI C., MATSUO H., MATSUOKA H., KANGAWA K. Urocortin, a member of the corticotropin-releasing factor family, in normal and diseased heart. Am. J. Physiol. 2000;279:H3031–H3039. doi: 10.1152/ajpheart.2000.279.6.H3031. [DOI] [PubMed] [Google Scholar]
- PARKES D.G., MAY C.N. Urocortin: a novel player in cardiac control. News Physiol. Sci. 2000;15:264–268. doi: 10.1152/physiologyonline.2000.15.5.264. [DOI] [PubMed] [Google Scholar]
- PARKES D., VAUGHAN J., RIVIER J., VALE W., MAY C. Cardiac inotropic actions of urocortin in conscious sheep. Am. J. Physiol. 1997;272:H2115–H2122. doi: 10.1152/ajpheart.1997.272.5.H2115. [DOI] [PubMed] [Google Scholar]
- PARKES D.G., WEISINGER R.S., MAY C.N. Cardiovascular actions of CRH and urocortin: an update. Peptides. 2001;22:821–827. doi: 10.1016/s0196-9781(01)00396-5. [DOI] [PubMed] [Google Scholar]
- PERRIN M., DONALDSON C., CHEN R., BLOUNT A., BERGGREN T., BILEZIKJIAN L., SAWCHENKO P., VALE W. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc. Natl. Acad. Sci. U.S.A. 1995;92:2969–2973. doi: 10.1073/pnas.92.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETRAGLIA F., FLORIO P., BENEDETTO C., MAROZIO L., DI BLASIO A.M., TICCONI C., PICCIONE E., LUISI S., GENAZZANI A.R., VALE W. Urocortin stimulates placental adrenocorticotropin and prostaglandin release and myometrial contractility in vitro. J. Clin. Endocrinol. Metab. 1999;84:1420–1423. doi: 10.1210/jcem.84.4.5585. [DOI] [PubMed] [Google Scholar]
- SCHILLING L., KANZLER C.H., SCHMIEDEK P., EHENREICH H. Characterization of the relaxant action of urocortin, a new peptide related to corticotropin-releasing factor in the rat isolated basilar artery. Br. J. Pharmacol. 1998;125:1164–1171. doi: 10.1038/sj.bjp.0702182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOGO N., CAMPANELLA C., WEBB D.J., MEGSON I.L. S-Nitrosothiols cause prolonged, nitric-oxide mediated relaxation in human saphenous vein and internal mammary artery: therapeutic potential in bypass surgery. Br. J. Pharmacol. 2000;131:1236–1244. doi: 10.1038/sj.bjp.0703700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERUI K., HIGASHIYAMA A., HORIBA N., FURUKAWA K.-I., MOTOMURA S., SUDA T. Coronary vasodilation and positive inotropism by urocortin in the isolated rat heart. J. Endocrinol. 2001;169:177–183. doi: 10.1677/joe.0.1690177. [DOI] [PubMed] [Google Scholar]
- VAUGHAN J., DONALDSON C., BITTENCOURT J., PERRIN M.H., LEWIS K., SUTTON S., CHAN R., TURNBULL A., LOVEJOY D., RIVIER C., RIVIER J., SAWCHENKO P., VALE W. Urocortin, a mammalian neuropeptide related to fish urotensin 1 and corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- YANG Z., VON SEGESSER L., BAUER E., STULZ P., TURINA M., LÜSCHER T.F. Different activation of the endothelial L-arginine and cyclooxygenase pathway in the human internal mammary artery and saphenous vein. Circ. Res. 1991;68:52–60. doi: 10.1161/01.res.68.1.52. [DOI] [PubMed] [Google Scholar]
- YATSUSHIRO K., CHALMERS D.T., MCCULLOCH J. Corticotropin-relasing factor is a potent dilator of cerebral blood vessels in vivo. J. Cereb. Blood. Flow Metab. 1997;17 Suppl 1:S286. [Google Scholar]
- WATANABE F., OKI Y., OZAWA M., MASUZAWA M., IWABUCHI M., YOSHIMI T., NISHIGUCHI T., LINO K., SASANO H. Urocortin in human placenta and maternal plasma. Peptides. 1999;20:205–209. doi: 10.1016/s0196-9781(98)00175-2. [DOI] [PubMed] [Google Scholar]


