Abstract
Urotensin-II (U-II) and its receptor (UT) represent novel therapeutic targets for management of a variety of cardiovascular diseases. To test such hypothesis, it will be necessary to develop experimental animal models for the manipulation of U-II/UT receptor system. The goal of this study was to clone mouse and primate preproU-II and UT for pharmacological profiling.
Monkey and mouse preproU-II genes were identified to encode 123 and 125 amino acids. Monkey and mouse UT receptors were 389, and 386 amino acids, respectively. Genomic organization of mouse genes showed that the preproU-II has four exons, while the UT receptor has one exon.
Although initially viewed by many exclusively as cardiovascular targets, the present study demonstrates expression of mouse and monkey U-II/UT receptor mRNA in extra-vascular tissue including lung, pancreas, skeletal muscle, kidney and liver.
Ligand binding studies showed that [125I]h U-II bound to a single sites to the cloned receptors in a saturable/high affinity manner (Kd 654±154 and 214±65 pM and Bmax of 1011±125 and 497±68 fmol mg−1 for mouse and monkey UT receptors, respectively). Competition binding analysis demonstrated equipotent, high affinity binding of numerous mammalian, amphibian and piscine U-II isopeptides to these receptors (Ki=0.8 – 3 nM). Fluorescein isothiocyanate (FITC) labelled U-II, bound specifically to HEK-293 cells expressing mouse or monkey UT receptor, confirming cell surface expression of recombinant UT receptor.
Exposure of these cells to human U-II resulted in an increase in intracellular [Ca2+] concentrations (EC50 3.2±0.8 and 1.1±0.3 nM for mouse and monkey UT receptors, respectively) and inositol phosphate (Ip) formation (EC50 7.2±1.8 and 0.9±0.2 nM for mouse and monkey UT receptors, respectively) consistent with the primary signalling pathway for UT receptor involving phospholipase C activation.
Keywords: GPR14, G protein-coupled receptor, U-II, Urotensin-II, UT
Introduction
U-II peptide was originally isolated from the urophysis, a gland located at the caudal end of the spinal cord of teleost fish (Bern et al., 1969; Pearson et al., 1980) and subsequently from the brain of an anuran amphibian (Conlon et al., 1992). This peptide exhibits a wide range of pharmacological activities not only in fish but also in amphibian and mammalian species (Bern et al., 1985; Conlon et al., 1996; 1997; Douglas & Ohlstein, 2000; Winter et al., 1999; Yano et al., 1994; 1995). U-II has potent endocrine and osmoregulatory actions (Bern et al., 1985; Conlon et al., 1996; 1997; Douglas & Ohlstein, 2000; Winter et al., 1999), affects habituated locomotor activity following CNS administration (Gartlon et al., 2001) and exerts potent bronchospactic actions in vitro (Hay et al., 2000). However, U-II is probably best characterized as a peptide, which influences cardiovascular function (Ames et al., 1999; Douglas & Ohlstein, 2000). Indeed, U-II is the most potent mammalian spasmogen identified to date (Ames et al., 1999). In addition to its vasoconstrictor action, U-II also exerts profound inotropic/arrhythmogenic actions in isolated cardiac tissue (Russell et al., 2001), has potent vasodilator effects (Bottrill et al., 2000; Gray et al., 2001; Strirrat et al., 2001) and has even been shown to have an effect on human microcirculatory function in vivo (Leslie, 2000).
U-II orthologues have been identified (See Paul's pullquote example: ) in lower vertebrates such as fish (Bern et al., 1969; Pearson et al., 1980), frogs (Coulouarn et al., 1998) and in mammals including man (Ames et al., 1999; Coulouarn et al., 1998; Mori et al., 1999). Human U-II splice variants are synthesized as 124 and 138 amino acids preproproteins (Ames et al., 1999; Coulouarn et al., 1998). As is the case with many neurohumoral vasoactive factors (e.g. angiotensin-II, and endothelin-1 ‘ET-1'), the presence of proteolytic cleavage sites coupled with the identification of truncated mature proteins by HPLC from mammalian hypothalamus and spinal cord suggest that the molecule is processed from a prepro-/pro-form by proteolytic cleavage. As a result of this proteolytic cleavage, the mature protein of 11, 12, 14 amino acids for human, pig, and rat, respectively, is formed (Ames et al., 1999; Coulouarn et al., 1998; Mori et al., 1999). One of the most intriguing aspects of the preproU-II isopeptides is the divergent primary structure seen across different animal species. For example, human preproU-II shares only 25% amino acid identity with the putative 125 amino acids carp preproU-II (Ames et al., 1999; Coulouarn et al., 1998; Pearson et al., 1980). Interestingly, however, the cyclic octapeptide C-terminus present in the mature protein is highly conserved amongst all animal isopeptides (Douglas & Ohlstein, 2000). This is also the minimally active fragment of the U-II isopeptides (Douglas & Ohlstein, 2001; Itoh et al., 1988).
Human U-II exerts its biological effects via an interaction with a G-protein coupled receptor (GPCR) superfamily member, originally termed GPR14 (Ames et al., 1999; Liu et al., 1999; Mori et al., 1999; Nothacker et al., 1999) and recently renamed the UT receptor (Douglas & Ohlstein, 2001). Radioligand binding and functional studies demonstrate that the human UT receptor binds goby and human U-II isoforms with identical affinity (Ames et al., 1999; Russell et al., 2001). U-II binding to the UT receptor initiates a signal transduction pathway, principally a GTP-binding protein (G-protein)-mediated activation of phospholipase C and a subsequent inositol trisphosphate-mediated increase in intracellular Ca2+ levels (Ames et al., 1999; Opgaard et al., 2000).
U-II mediates important physiological and pathophysiological effects. Recently, it was demonstrated that U-II was the most potent mammalian vasoconstrictor identified to date (Ames et al., 1999; Douglas & Ohlstein, 2000). In vitro, U-II induces potent vasoconstriction in isolated vessels from the rat, rabbit, dog, pig, marmoset (Douglas et al., 2000b), and man (Russell et al., 2001; Strirrat et al., 2001), which differ greatly between regional vascular beds and between species. Further, U-II exerts potent inotropic and pro-arryhtmogenic actions in human isolated trabeculae (Russell et al., 2001), and vasoconstrictor effects in man upon in vivo administration (Gray et al., 2001). Further, U-II was shown to induce a profound vasoconstriction and lethal myocardial dysfunction upon systemic administration in the intact primate (Ames et al., 1999). These intriguing pharmacological properties ofU-II indicate that U-II and the UT receptor may play an important role in cardiovascular disorders characterized by abnormal vasoconstriction and/or cardiac dysfunction such as congestive heart failure, pulmonary hypertension/chronic obstructive pulmonary disease (COPD) and coronary artery disease/atherosclerosis.
The perceived (patho) physiological significance of the pharmacological effects of U-II mediated by its receptor(s) underscores the importance of utilizing molecular biological approaches to dissect the components of U-II physiology. Indeed, to this end it has become apparent that consideration should be made to experimental animal species being studied in order to understand the pathophysiological effects of U-II on peripheral haemodynamics. For example, systemic i.v. administration of U-II elevates total peripheral resistance in the primate (Ames et al., 1999), yet, in contrast, decreases regional vascular resistance in the rat (Gardiner et al., 2001; indeed, no contractile activity in the mouse). In view of these regional differences in vascular reactivity to U-II seen between species (Ames et al., 1999; Douglas et al., 2000b; Gardiner et al., 2001), it is therefore important to dissect the molecular components of U-II and UT receptors in animal species in order to define the basic pharmacology of these receptors in an in vitro system. For example, it is not possible to test UT receptor antagonists in the rat model of hypertension since U-II is a systemic vasodilator in this species. Rather, one would have to study such a moiety in a primate model (where U-II elevates vascular resistance). Accordingly, one needs to clone the primate receptor to determine that such an antagonist exhibits affinity for such a receptor. The present study describes the identification, cloning, tissue localization, genomic structure and expression of the genes from mouse and non-human primate (cynomolgus monkey) in human embryonic kidney cells (HEK-293) cells. In addition, the present study also shows that U-II/UT receptor are observed in extra-vascular and extra-neuronal tissue (liver, skeletal muscle etc) providing evidence that additional physiological actions warrant future investigation. The characterization of the preproU-II and UT receptor orthologues should provide important tools for isolation and characterization of small molecular antagonists for U-II action and for future studies delineating specific mechanisms underlining U-II action in the heart and vascular system.
Methods
Materials
Human and porcine U-II (A and B isoforms) peptides were synthesized at GlaxoSmithKline, King of Prussia, PA, U.S.A. (J. Martin, Protein Biochemistry). Rat and mouse U-II isoforms and FITC-human U-II were purchased from Phoenix Pharmaceuticals Inc. (Mountain View, CA, U.S.A.). Monoiodinated human U-II ([125I]Tyr9, was custom synthesized through Amersham (Arlington Heights, IL, U.S.A.). The bicinchoninic acid (BCA) protein assay kit was obtained from Pierce Chemicals Co. (Rockford, IL, U.S.A). All other reagents were from Sigma Chemical Co. (St. Louis, MO, U.S.A.). All experiments and surgical procedures were performed as described in the Guide for the Care and Use of Laboratory Animals (DHSS publication NIH 85-23) and according to the requirements of the United Kingdom Animals (Scientific Procedures) Act (1986) and strictly conformed to the ethical standards of GlaxoSmithKline and adhered to the AALAC guidelines.
Cloning of mouse and monkey preproU-II
Mouse preproU-II was cloned as described previously (Coulouarn et al., 1999). Primers were synthesized corresponding to the 5′ and 3′ end of the genes as followed: Sense (outer 5′-CCAGAGCAGACGCCCAGACGGAC-3′ inner 5′-GACTTCTCGCCGCATCATGGAC-3′), Anti-sense primer (outer 5′-CTGAATGTAATGGTTCTGAGAGAC, inner 5′-CAACGGG CGCTTGTGTCTCCTC). These primers were used to obtain the full-length preproU-II complementary DNA (cDNA) clone from mouse brain cDNA template (Clonetech) by polymerase chain reaction (PCR).
For monkey preproU-II, primers were synthesized corresponding to the amino terminal and carboxyl terminal of the human preproU-II: Sense (outer 5′-ATGAAAGATTAAA AAATGG, inner 5′-AAATGGAAACCAACGTATTTCATC), anti-sense (outer 5′-CTGAGCT GACTAACAGATG, inner 5′-CTTATTTCACTTCAGACACAG). These primers were used to obtain the monkey full-length preproU-II cDNA clone from monkey brain cDNA as a template (Clonetech) by PCR.
Cloning of mouse and monkey UT receptor
Mouse genomic library was screened by hybridization to nitrocellulose replicates using 32P-labelled human UT receptor cDNA coding sequence as a probe in 20% formamide, 5×150 mM NaCl, 15 mM sodium citrate (SSC), 5× Denhardt's solution, 0.1% sodium dodecyl sulphate (SDS), and 0.2 mg ml−1 of E. coli tRNA at 42°C (Elshourbagy et al., 1993). Filters were washed with 2×SSC, 0.1% SDS at 42°C. Ten positive clones were isolated and characterized. Preliminary sequence analysis of these clones showed seven of them encode putative mouse UT receptor clones; the other three clones were unrelated genes. For monkey UT receptor, primers were synthesized corresponding to the 5′ and the 3′ untranslated region of the human UT receptor: Sense (outer 5′-CCACAGGCTGA GCTGGTTGCCCACAGG-3′, inner 5′-CCATCTCAGGGAGTGTCCACCCAGC-3′), anti-sense (outer 5′-GCAGGGCCT GTGATTTGGGAGTTGG-3′, inner 5′-TGGACTCCAGC CGCCCCTCCGCGTG-3′). These primers were used to obtain the monkey full-length sequence from human genomic DNA using PCR reaction. The fragments containing the entire mouse and monkey cDNA coding sequences was subcloned into the mammalian expression vector pCDN (Aiyar et al., 1994) and were then sequenced from both strands using the ABI automated sequencer (Applied Biosystems, Foster City, CA, U.S.A.).
PreproU-II and UT receptor genomic cloning
The genomic clones for mouse preproU-II and UT were isolated from the mouse RPCI-22 mouse BAC library (Research Genetics, Huntsville, AL, U.S.A.). Both PCR reactions were performed as 35 cycles of 94°C (30 s), 60°C (30 s), and 72°C (60 s). For the UT receptor the pooled library was screened by PCR and the positive clone confirmed by PCR of the appropriate plate using the following 5′ primer: GCGTGGTGGGCAATGTGT and 3′ primer: GGCTACGATGAAGGGAAT.
For preproU-II the high density BAC library membranes were screened with a 32P-labelled preproU-II specific probe. The probe fragment was derived by PCR of total mouse genomic DNA using primers specific to the 3′ end of the mouse preproU-II open reading frame 5′ primer: GCAAAATTCTAACACTGTACTGAGTCG; 3′ primer: CATAATGAAGAACA AGGCGTCGTTAGC). BAC DNA was prepared using the BAC mini-prep protocol according to the manufacturer instructions.
A number of restriction fragments containing preproU-II or UT receptor genomic sequence were isolated and subcloned into pBluescript II (Stratagene Inc., La Jolla, CA, U.S.A.), and used to generate restriction maps of the loci. The sequences of the loci were determined from these subclones using the Big Dye Terminator cycle sequencing chemistry and sequencing reactions were run on an ABI 377XL automated sequencer and analysed using Sequence Analysis software version 3.3.
Reverse-transcription polymerase chain reaction (RT – PCR)
Tissues isolated from adult C57 Bl6 mice and cynomolgus monkeys and immediately flash frozen in liquid N2 (prior to processing, all tissue was stored at −80°C). Tissue was homogenized in liquid N2 using a mortar and pestle and total RNA extracted using RNAzol (GIBCO BRL, Gaithersburg, MD, U.S.A.).
RT – PCR was performed using DNase-I-treated monkey (derived from an individual primate) and mouse (samples pooled from 5 – 20 mice, depending on the size of the particular organ in question) total RNA. cDNA was generated using oligo (dT16)-primed MultiScribe reverse transcriptase (TaqMan, Pekin Elmer, Branchburg, NJ, U.S.A.). The resultant samples were subjected to PCR amplification (94°C, 30 s; 60°C, 30 s; 72°C, 30 s: 33 cycles for mouse and monkey UT receptor, 42 cycles for mouse preproU-II and 25 cycles for monkey preproU-II followed by an additional 25 cycles using 58°C, 30 s annealing temperature; Perkin Elmer Model 9600 GeneAmp PCR System) in standard buffer containing 0.2-μM universal UT receptor primers designed against nucleotide sequences conserved between human, monkey, rat and mouse (480 basepair ‘bp' amplicon, 5′-GCC AGC ATC TTC ACG CTG ACC-3′ and 5′-GTA CTG GGC GAG CAG CTG CCA-3′). Species-specific primer sequences were used for amplification of mouse (306 bp amplicon, 5′-GAC TCT TCA GCT TCC AGT GC-3′ and 5′-CAA CGG GCG CTT GTG TCT CC-3′) and monkey (296 bp amplicon, 5′-GAA GTA TCC CTT CAA CTC TC-3′ and 5′-TCA GAC ACA GTA TTT CCA GAA G-3′) preproU-II.
Amplification fidelity was confirmed by (a) omission of Taq polymerase or reverse transcriptase from the initial RT step, (b) Southern blot analysis (see below) using full-length UT receptor or preproU-II probe and by (c) subcloning/sequencing of the PCR product. All cDNA samples produced the predicted 620 bp amplicon using universal GAPDH primers. As a control reaction, amplification was performed using 100 ng of template DNA (genomic or plasmid DNA for UT receptor and preproU-II, respectively).
Southern blot analysis
Upon completion of the cDNA electrophoresis above, agarose gels were denatured in 0.5 M NaOH/1.5 M NaCl for 60 min. Following neutralization (0.5 mM Tris HCl/1.5 M NaCl (pH 7.5)), cDNA(s) were transferred to GeneScreen Hybridization membranes (NEN Life Science, Boston, MA, U.S.A.) using 10× SSC buffer to which they were u.v. cross-linked. Membranes were prehybridized for 2 h at 42°C in the presence of denatured ssDNA following which they were incubated with denatured full-length UT receptor or preproU-II cDNA probe (overnight at 42°C in the presence of 1×106 c.p.m. ml−1 radiolabel in 50% deionized formamide, 1.5 M NaCl, 1% SDS and 10% dextran sulphate buffer). Probe was labelled with [32P]dCTP using a standard T7 DNA polymerase-random primer protocol (QuickPrime, Pharmacia Biotech, Piscataway, NJ, U.S.A.).
Finally, membranes were washed under conditions of low stringency (three 15 min washes in 1×SSC, 0.1% SDS at 25°C) followed by a 30 min high stringency wash (0.2× SSC, 0.1% SDS) at 55°C. Hybridization signals were detected using conventional X-ray autoradiography (Hyperfilm, Amersham Life Science, U.K.) or phosphorimaging (STORM 860, Molecular Dynamics, Sunnyvale, CA, U.S.A.).
Expression of mouse and monkey UT receptors in HEK-293 cells
HEK 293 cells were cultured in DMEM/Earle's salts, 2.2 g L−1 sodium bicarbonate, L-glutamine, and non-essential amino acids with 10% foetal bovine serum (JRH Bioscience, Lenexa, KS, U.S.A.). For transient transfection, HEK-293 cells were seeded in a 6-well dish at a concentration of 3×105 cells per well in complete media. pCDN monkey UT receptor and pCDN mouse UT receptor plasmids were transfected into HEK-293 cells using Lipofectamine reagent (Gibco/Life Technologies, Gaithersburg, MD, U.S.A.) at a ratio of 1 μg plasmid DNA to 5 μl Lipofectamine reagent per well according to manufacture instructions. After 48 h, cells were trypsinized and resuspended in complete media containing 0.4 mg ml−1 G-418. G-418 resistant clones were selected and tested for binding to [125I]h U-II. Fifteen 293 per monkey UT receptor and 10 293 per mouse UT receptor clonal cell lines were assessed under saturation conditions to determine radioligand binding density (Bmax) and affinity (Kd).
Identification of FITC-labelled human U-II binding sites in COS-1 cells transiently expressing recombinant mouse and monkey UT receptors
African green monkey kidney cells (COS-1) in 10% FBS Dulbecco's modified Eagles medium (DMEM) were cultured under standard conditions on RS-treated Lab-Tek II chamber slides (Nalge Nunc Int., Naperville, IL, U.S.A.) in humidified incubators at 37°C (5×105 cells per slide). Once 80 – 90% confluence had been reached, COS-1 were transiently transfected with either monkey or mouse UT receptors (in pCDN mammalian expression vector). Briefly, 1 μg plasmid DNA was preincubated in 50 μl serum-free Opti-MEM I culture medium containing 2.5 μl LipofactAMINE 2000 (Life Technologies, Gaithersburg, MD, U.S.A.). Cells were exposed to the transfection medium for 2 h at 37°C at which point cells were re-exposed to serum-containing media. Labelling studies were performed 48 h following transfection.
Cells were washed two times with cold phosphate buffered saline (PBS) containing 10 mM MgCl2, 0.1% (w v−1) D-glucose and 0.2% (w v−1) bovine serum albumin (BSA). Subsequently, cells were exposed to 10 nM fluorescein isothiocyanate (FITC)-labelled human U-II (Ki determined as ∼2 nM at recombinant UT receptor in a radioligand binding assay; N. Aiyar, unpublished observation) either alone or in the presence of excess cold, unlabelled U-II (10 μM). Cells were incubated with U-II isopeptides for 30 min at 25°C at which point cells were washed on the chamber slides with 3 ml cold buffer a total of four times and examined using confocal microscopy (‘control' cells transfected with empty vector were devoid of any FITC-human U-II binding sites). Images were collected using a Zeiss LSM-510 confocal laser scanning microscope (Carl Zeiss Inc., Thornwood, NY, U.S.A.) equipped with a 250 mW argon/krypton laser (Omnichrome Inc., Chino, CA, U.S.A.). FITC images were collected using appropriate bandpass filters at an excitation wavelength of 488 nm and an emission wavelength of 520 nm. Differential interference contrast (DIC) images were collected simultaneously and the images were merged using the LSM-510 software.
Membrane preparation
For radioligand binding studies, the HEK-293 cells were washed with Dulbecco's phosphate-buffered saline containing a protease inhibitor mixture (5 mM EDTA, 0.5 mM phenylmethylsulphonyl fluoride (PMSF), 5 μg ml−1 leupeptin, and 0.1 μ ml−1 aprotinin) and scraped in the same buffer. The membranes were prepared as described previously (Elshourbagy et al., 1998). Protein content was determined by the bicinchoninic acid method using bovine serum albumin as the standard.
Radioligand binding studies
[125I]h U-II binding assays were performed using the membranes prepared from HEK-293 cells stably transfected with mouse or monkey UT receptor using a scintillation proximity assay (SPA). Conditions were optimized for membrane protein concentration and amount of wheat germ agglutinin (WAG) coated SPA beads (Amersham, Arlington Heights, IL, U.S.A.). Binding assay consisted of [125I]h U-II (20 – 600 pM for saturation binding and 300 pM for competition binding) in the presence or absence of unlabelled ligand in assay buffer (20 mM Tris-HCl (pH 7.4), 5 mM MgCl2 and 0.05% BSA) and cell membranes precoupled wheatgerm agglutinin-scintillation proximity assay (WGA-SPA) beads at a concentration of 5 – 10 μg membrane protein and 0.5 mg of WGA-SPA beads. The total assay volume was 150 μl. Ninety-six well plates (Packard Optiplate) was used for assays. Competition binding was done with 10 different concentrations (1 pM – 1 μM) of unlabelled U-II (human, goby, rat, mouse and both forms of porcine isopeptides). The assay plate was sealed, shaken gently for 45 min at room temperature. The plates were then centrifuged at 2000×g for 10 min and counted in a Top Count Scintillation Counter (Packard). The binding data were analysed by nonlinear regression using the program Graph PAD Prism (Graph PAD Software, San Diego, CA, U.S.A.) to calculate Bmax, Kd, Ki and Hill coefficient (nM).
Phosphoinositide (PI) turnover
Stable HEK-293 cells expressing recombinant UT receptors (mouse and monkey) cells were exposed to inositol-free DMEM containing 1 uCi ml−1 D-myo-[2-3H]-inositol (Amersham, 94 Ci mmol−1) for 24 h. On the day of the experiment, the medium was removed, the cells were washed twice with Dulbecco's phosphate-buffered saline (DPBS) and then suspended in 5 ml of DPBS containing 10 mM LiCl. The cells were then aliquoted (500 ul) into 12×75 mm tubes and then incubated for 10 min at 25°C. The experiment was initiated by the addition of indicated concentrations of human U-II, and the incubation continued for an additional 5 min at 37°C after which the reaction was terminated by the addition of 1% (final concentration) trichloroacetic acid and centrifugation. The supernatants were extracted with ether saturated with water and the inositol phosphates (Ips) were separated using Dowex columns AG-1×8 anion exchange column. The [3H] inositol and inositol 1-, 2- and 3-phosphates were fractionated as described previously (Aiyar et al., 1986) and the aliquots were counted in Beckman scintillation counter using Beckman Ready Safe scintillant.
Ca2+-mobilization
For measurements of [Ca2+]i, stable HEK-293 cells expressing recombinant UT receptor (mouse and monkey) cells were loaded with 2 μM fura-2/AM (Calbiochem, La Jolla, CA, U.S.A.) in growth medium for 45 min after which fresh growth medium was added for 15 min to allow residual ester hydrolysis. The cells were then rinsed in Dulbecco's PBS, trypsinized, resuspended in growth medium, centrifuged, and resuspended into modified Kreb's Ringer's Henseleit (KRH) buffer, pH 7.4, containing 0.1% gelatin. The KRH is modified by replacing sodium bicarbonate with 25 mM HEPES. The cells were stored on ice at a concentration of 2×106 cells ml−1 and diluted to 1×106 cells ml−1 with fresh KRH buffer at 37°C just prior to use. Fluorescence of fura-2 in cells suspended in 2 ml of buffer was measured with a dual channel fluorometer (University of Pennsylvania Biomedical Instruments Group) (Lysko et al., 1994). Human U-II was added from concentrated stock solutions in water at a volume of <1%.
Results
Cloning of mouse and monkey U-II and UT receptor
The nucleotide sequence of the mouse and the monkey PCR products revealed that the full-length mouse preproU-II is 371 nucleotides in length (Coulouarn et al., 1999) and the full-length monkey cDNA is 378 bases in length (encoding proteins of 123 and 125, respectively; Figure 1). Analysis of the structure of the mouse preproU-II sequence revealed that the prepropeptides contain two potential dibasic proteolytic cleavage sites for the preproU-II (position 85 – 86 and 105 – 106). Cleavage at these sites would generate a putative 17-residue peptide mature protein, containing the conserved cyclic hexapeptide sequence (CFWKYC) motif (Coulouarn et al., 1999). In monkey the preproU-II contains three putative polybasic proteolytic cleavage sites at position 70 – 71, 86 – 87, and 112 – 113. Cleavage at the latter dibasic site would yield an 11 amino acid mature U-II, which is identical to the human U-II (Figure 1).
Figure 1.

Amino acid sequence alignments of the human, monkey, mouse, rat, frog, and fish preproU-II. The potential cleavage sites of the proU-II is underlined. The mature U-II peptide is highlighted.
Cloning the mouse UT receptor cDNA was conducted using genomic library screening. The human UT receptor cDNA (Ames et al., 1999) was used to probe mouse genomic λ phage library. Ten positive clones were identified. Nucleotide sequence analysis revealed that seven positive clones encoded for putative mouse UT receptor, while the other three clones were unrelated sequences. The full-length monkey UT receptor cDNA was cloned by PCR using oligonucleotide primers corresponding to the 5′ non-coding region of human UT receptor cDNA and the 3′ noncoding region of the human UT receptor using the human monkey genomic DNA as a template. Sequence analysis of the mouse and monkey genes revealed open reading frames (ORF) of 1158 and 1167 nucleotides, respectively. The deduced polypeptides of the mouse and the monkey receptors consisted of 386, 389 amino acid residues, respectively, with predicted molecular weights of approximately 42 kDa (Figure 2).
Figure 2.

Amino acid sequences alignments of the human, monkey, mouse and rat UT. Deduced amino acid residues are indicated beginning with the initiation methionine. The regions identifying the positive transmembrane as domains 1 – 7 are underlined and numbered sequentially. The potential N-glycosolation site (O), the conserved cysteins (*), and the potential palmytalation site (boxed), are indicated. The optimal alignment of the deduced amino acid sequences of the mouse and monkey UT receptor were compared to the rat and human UT receptor using the Wisconsin program obtained from Devereux et al. (1984).
Genomic organization of the mouse preproU-II and UT
The structures of the mouse preproU-II and UT receptor genes is shown in Figure 3a,b. The preproU-II gene has four coding exons corresponding to the full-length cDNA sequence described above and encodes a putative protein of 123 amino-acids (Figure 3a). The sizes of the exons and introns and the sequences of the exon/intron boundaries are indicated in Table 1. The boundaries of each of the three introns conform to the consensus sequences for mammalian gene (Padgett et al., 1986). The mouse UT receptor gene has a single coding exon, and encodes a putative protein of 385 amino acids (Figure 3b). The mouse UT receptor maps to chromosomal 11. This is near the locus encoding mouse thymidine kinase and syntenic with human 17q23 – 25 (Protopopov et al., 2000). Thus the mouse gene maps syntenically with the human gene that is the receptor for U-II.
Figure 3.
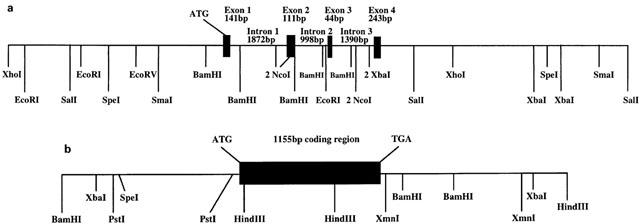
Genomic structure of the mouse U-II and its receptor. Mouse U-II and UT receptor BAC clones were isolated as described in Methods. Restriction fragments containing U-II or UT receptor genomic sequence were isolated and subcloned into pBluescript II, and used to generate restriction maps of the loci which were determined by sequence analysis. (a) Restriction map of the genomic structure. Note that for NcoI, BamHI, and SpeI only the sites, which have been confirmed by sequence analysis, are indicated. The restriction map upstream of exon I and downstream of exon 4 shows approximately site locations as determined by restriction digest of genomic subclones. (b) Restriction map of the genomic structure of UT receptor indicating one coding exon.
Table 1.
Intron and exon sizes, and the sequence of intron-exon junctions in the mouse preprourotensin-II genes
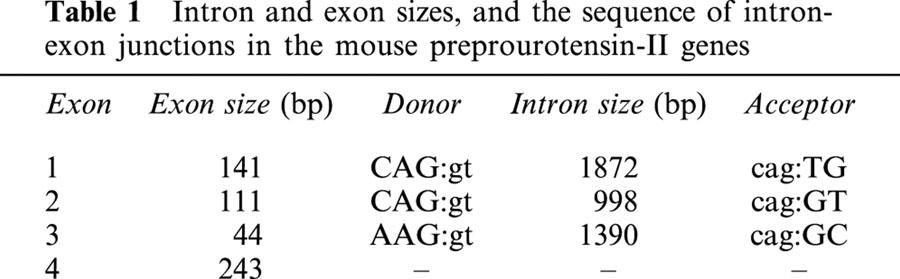
Tissue distribution of mouse and monkey UT receptor
As originally observed with human UT receptor (using both conventional RT – PCR and Northern blot approaches), RT – PCR analysis of mouse UT receptor mRNA distribution revealed that the expression of monkey and mouse UT receptor mRNA was cardiovascular in origin, namely the heart and the thoracic aorta (Figure 4a). Interestingly, the mouse UT mRNA expression was not detected in the abdominal portions of this vessel. Furthermore, mouse UT receptor mRNA expression was also observed in the bladder, pancreas, skeletal muscle, oesophagus, lung and adipose tissue. Using the amplification paradigm employed in the current study, the expression of UT receptor mRNA was also detected in the mouse brain, testis, stomach, intestine (small and lower (colon)), adrenal gland, kidney or spleen. In contrast, amplification of GAPDH resulted in the generation of the predicted 620 bp transcript in all tissue samples studied. Amplification fidelity was confirmed by the observations that PCR generated transcripts of the predicted (480 bp) size from both (a) complementary and (b) genomic DNA, (c) the lack of a PCR amplicon when Taq polymerase is omitted from the reaction mix, (d) the lack of amplicon generation using RNA samples not subjected to RT and (e) hybridization of the amplified cDNA with full-length human UT receptor (Southern blot analysis).
Figure 4.
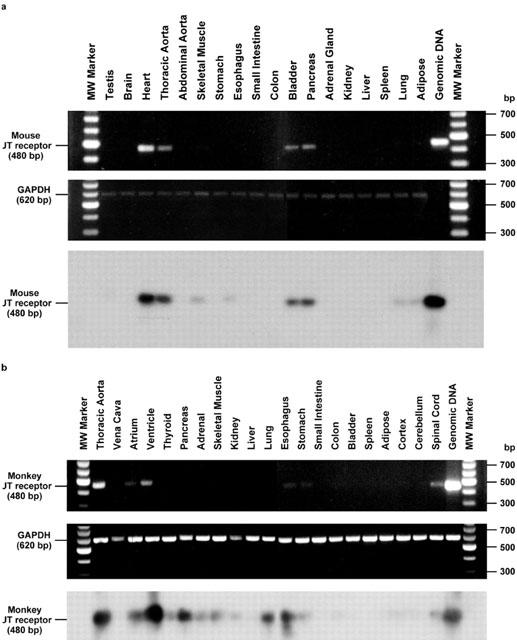
Tissue distribution of the mouse and monkey UT receptor: (a) Tissue distribution of mouse UT receptor cDNA transcripts by RT – PCR revealed expression within cardiac and vascular (thoracic but not abdominal aorta) tissue in addition to bladder and pancreas. Trace levels of expression are also observed in skeletal muscle, oesophagus, lung and adipose tissue. (Middle panel) Amplification of GAPDH cDNA did not differ significantly between tissues. The specificity of the RT – PCR amplification of UT receptor transcripts was confirmed (Lower panel) by Southern analysis using full-length UT receptor cDNA probe. (b) Tissue distributions of monkey UT receptor cDNA transcripts by RT – PCR revealed expression within heart (ventricle>atrium) and arterial blood vessels (aorta not vena cava), pancreas. Detectable levels of expression were also observed in the skeletal muscle, lung, thyroid and adrenal glands, kidney, upper portions of the gastrointestinal tract (oesophagus, stomach and small intestine but not colonic tissue) and spinal cord (but not in the cortical or cerebellar samples isolated). No detectable transcripts were derived from hepatic, bladder, adipose tissue or splenic tissue. (Middle panel) Amplification of GAPDH cDNA did not differ significantly between tissues. The specificity of the RT – PCR amplification of UT receptor transcripts was confirmed (lower panel) by Southern analysis using full-length UT receptor cDNA probe.
In accord with the observations described above for mouse UT receptor, RT – PCR analysis of monkey tissue also revealed that the mRNA expression of monkey UT receptor was cardiovascular in origin, namely the heart (ventricle>atrium) and aorta (Figure 4b). As was observed previously in human tissue (Ames et al., 1999), no transcript was detected in venous tissue (vena cava), monkey blood vessels known to be refractory in vitro to the vasoconstrictor actions of human U-II. As is seen in humans and mice (see above), UT receptor mRNA is also expressed in monkey pancreas. In contrast to the observations reported above using murine tissue, however, no monkey UT receptor mRNA expression was detected in the bladder. As with the mouse, detectable levels of expression were observed in the monkey skeletal muscle, oesophagus and lung, (but not adipose tissue). Detectable expression was also evident in endocrine organs (thyroid and adrenal glands), kidney and upper portions of the gastrointestinal tract (in addition to the oesophagus, expression was evident in both stomach and small intestine was not detected at the mRNA level in colonic tissue under the present amplification conditions). In addition, detectable expression was evident in spinal cord tissue (not in the cortical or cerebellar samples isolated). No detectable transcripts were derived from hepatic or splenic tissue. As anticipated, amplification of GAPDH resulted in the generation of the predicted 620 bp transcript in all tissue samples studied. Further, amplification fidelity was confirmed using the approaches described above for studies performed using material derived from mice.
Tissue distribution of mouse and monkey preproU-II
As with the UT receptor, RT – PCR analysis of mouse preproU-II distribution revealed that the mRNA expression of the ligand was cardiovascular in origin, namely the heart and the thoracic aorta (Coulouarn et al., 1999; Figure 5a). Interestingly, the preproU-II mRNA expression was also observed in testes and brain of the mouse. The mRNA expression of mouse preproU-II was also recorded in skeletal muscle, liver, kidney and spleen, which clearly indicates that the U-II should not be merely viewed as a simple neuropeptide. Further to this, using the amplification paradigm employed in the current study, detectable expression of preproU-II mRNA was observed in the mouse gastrointestinal tract (stomach, oesophagus, small intestine and colon), bladder, pancreas, adrenal, lung and adipose tissue. In contrast, amplification of GAPDH resulted in the generation of the predicted 620 bp transcript in all tissue samples studied.
Figure 5.
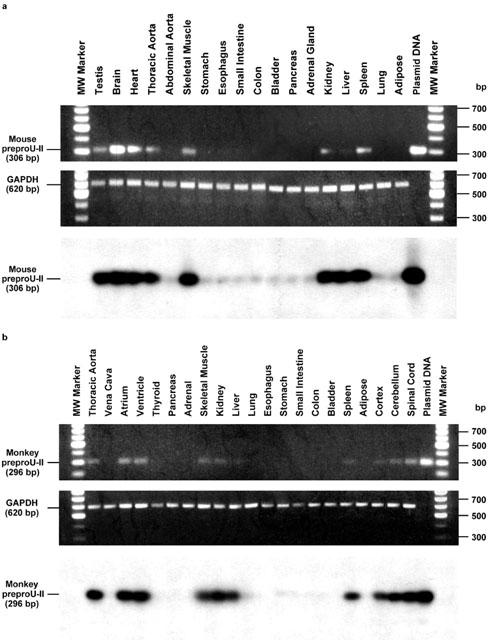
Tissue distribution of the mouse and monkey U-II. (a): Tissue distribution of mouse preproU-II cDNA transcripts by RT – PCR revealed expression within heart, thoracic aorta, testes, brain, skeletal muscle, liver, kidney and spleen (upper panel). Negligible expression of preproU-II was observed in the mouse gastrointestinal tract (stomach, oesophagus, small intestine and colon), bladder, pancreas, adrenal, lung and adipose tissue. Amplification of GAPDH cDNA did not differ significantly between tissues (middle panel). The specificity of the RT – PCR amplification of preproU-II transcripts was confirmed by Southern analysis using full-length preproU-II cDNA probe (lower panel). (b) Tissue distribution of monkey preproU-II cDNA transcripts by RT – PCR revealed expression within heart (ventricle and atrium), thoracic aorta, CNS (spinal cord, cerebellum and cortex), skeletal muscle, kidney, liver and spleen (upper panel). No detectable transcripts were derived from vena cava, endocrine tissues including thyroid, pancreas and adrenal glands, lung, gastrointestinal tissue (oesophagus, stomach, small intestine, colon), bladder or adipose tissue. Amplification of GAPDH cDNA did not differ significantly between tissues (middle panel). The specificity of the RT – PCR amplification of preproU-II transcripts was confirmed by Southern analysis using full-length preproU-II cDNA probe (lower panel).
Amplification fidelity was confirmed by the observations that PCR generated transcripts of the predicted (306 bp) size from both (a) complementary and (b) plasmid DNAs, (c) the lack of a PCR amplicon when Taq polymerase is omitted from the reaction mix, (d) the lack of amplicon generation using RNA samples not subjected to RT and (e) hybridization of the amplified cDNA with full-length human preproU-II (Southern blot analysis). Further to this, amplification fidelity was confirmed by subcloning and sequencing the PCR amplicon.
In accord with the observations described above for mouse preproU-II and for mouse/monkey UT, RT – PCR analysis of monkey tissue also revealed that the preproU-II mRNA expression was cardiovascular in origin, namely the heart (ventricle and atrium) and thoracic aorta (Figure 5b). As was observed previously in human and monkey tissue for UT receptor, no preproU-II transcript was detected in venous tissue (vena cava), blood vessels known to be refractory in vitro to the vasoconstrictor actions of U-II. In addition, expression was detected within the CNS of the monkey (from caudal regions such as the spinal cord and cerebellum to rostral regions of the brain, namely the cortex). As with mouse preproU-II, monkey preproU-II expression was also evident in skeletal muscle, kidney, liver and spleen. Using this technique, no monkey preproU-II mRNA expression was evident in the major endocrine tissues examined (thyroid, pancreas and adrenal glands), lung, gastrointestinal tissue (oesophagus, stomach, small intestine, colon), bladder or adipose tissue. As anticipated, amplification of GAPDH resulted in the generation of the predicted 620 bp transcript in all tissue samples studied. Further, amplification fidelity was confirmed using the approaches described above for studies performed using material derived from mice.
Identification of FITC-labelled U-II binding sites to mouse and monkey UT receptor
In order to demonstrate that U-II binds to membrane associated mouse and monkey UT receptor, we examined the binding of FITC-labelled human U-II in cells transiently expressing the cloned putative mouse and monkey UT receptor. Using confocal microscopy, no FITC-human U-II binding was evident in mock transfected COS-1 cells (Figure 6a – c). However, this contrasted those cells transiently expressing either monkey (not shown) or mouse (Figure 6d – i) UT receptor, which clearly bound the fluorescein-conjugated ligand (10 nM) to the plasma membrane of COS-1 cells (Figure 6d – f and g – l). Binding of the fluorescein conjugate was competitive in as much as it was abolished in the presence of 1000 fold excess cold human U-II (10 μM; Figure 6j – l).
Figure 6.
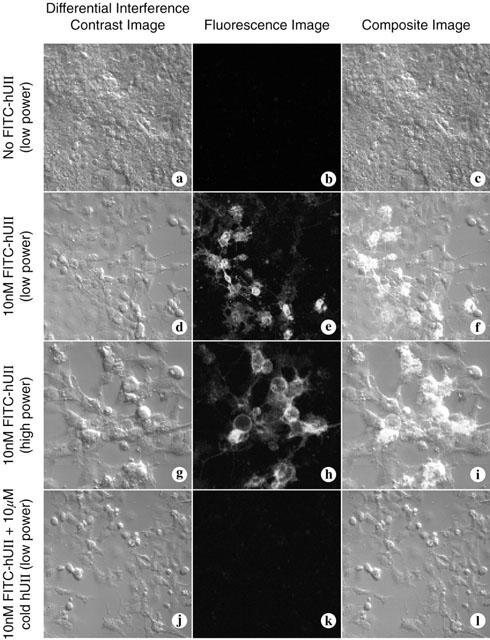
Binding of FITC-labelled human U-II to COS-cells transfected with mouse and monkey UT receptor. FITC-labelled human U-II (10 nM) does not bind to control COS-1 cells ‘mock' transfected with empty vector (panels a – c). Binding is evident at the plasma membrane of cells transfected with mouse UT receptor (two different fields of view either at original magnification of 20× or 40×, panels d – f and g – i, respectively). FITC- human U-II binding to the cell surface of COS-1 cells expressing mouse UT receptor is inhibited by the presence of 1000 fold excess ligand (10 μM cold human U-II; panels j – l). In each set of three images, differential interference contrast (DIC) images appear on the left, fluorescence images are in the centre, and computer-generated composites of the DIC and fluorescence image are on the right.
Ligand binding properties
The preliminary FITC-h U-II studies described above using intact COS-1 cells were extended using [125I]h U-II in order to determine the kinetic characteristics of this interaction. Initial characterization of [125I]h U-II (125 pM) binding to HEK-293 cell membranes containing either mouse or monkey UT receptor revealed that specific binding at this concentration was >90% of total binding as defined in the presence of unlabelled 1 μM U-II. [125I]h U-II bound to membranes from HEK-293 cells containing either mouse or monkey UT receptor in a specific and saturable manner. No such binding was detected on membranes prepared from vector DNA transfected HEK-293. Scatchard plot analysis of [125I]h U-II binding (Figure 7a and c inset) showed a single set of sites with Kd of 654±154 and 214±65 pM and for mouse and monkey UT receptor and the Bmax were 1011±125 and 497±68 fmol mg−1 protein for mouse and monkey UT receptor, respectively.
Figure 7.
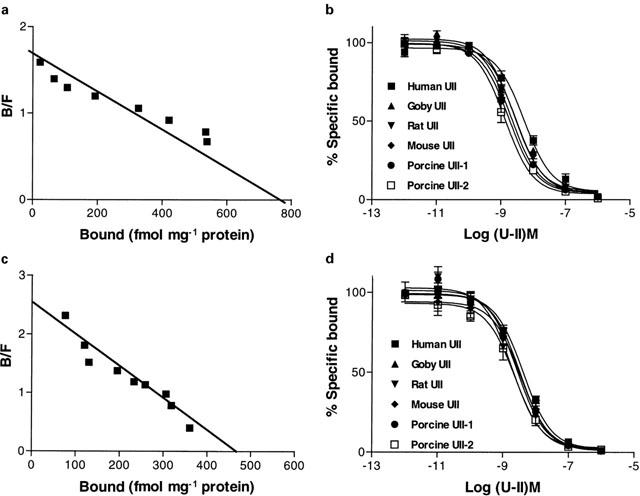
[125I]-U-II binding to HEK-293 cells transfected with mouse and monkey UT receptor cDNAs: Scatchard plots of [125I] h- U-II saturation binding to cell membranes from HEK-293 cells stably transfected with mouse (a) or monkey (c) UT receptor. Membranes (10 – 15 μg) were incubated with increasing concentrations of [125I] human U – II (0.02 – 0.6 nM) in a total volume of 150 μl at 25°C for 45 min and assayed as described in Methods. Competition binding curves for iso U-II peptides (human, goby, rat, mouse, porcine-1 and porcine-2) of mouse (b) or monkey (d) UT receptor (respectively). Membranes (10 – 15 μg) were incubated with 0.18 nM [125I] human U-II for 45 min at 25°C in the presence of increasing concentrations of U-II isopeptides. Data are the means of duplicate determinations and are representative results from one of three independent experiments. Ki values for pooled data are shown in Table 2.
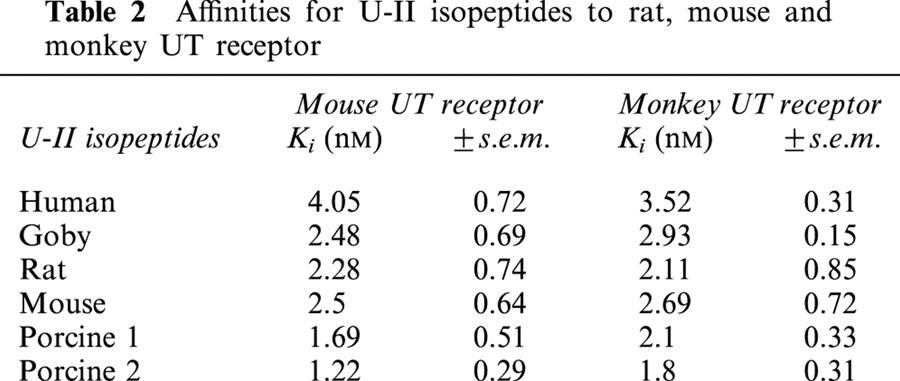
The specificity of U-II binding to the recombinant mouse and monkey UT receptor was assessed by examining the ability of human U-II and related peptides from goby, rat, mouse and porcine A and B isoform to compete [125I]h U-II binding sites (Figure 7b,d). Competition binding revealed that all six isoforms of U-II displaced the radioligand with comparable Ki (Figure 7b,d) (Table 2). In all cases, the peptides displayed monophasic competition curves with Hill coefficients, which were not significantly different from unity (indicative of interaction with single class of binding sites).
Table 2.
Affinities for U-II isopeptides to rat, mouse and monkey UT receptor
The activation of signal transduction pathways through U-II ligand-receptor coupling was investigated using HEK-293 cells expressing recombinant mouse and monkey UT receptor. Human and goby U-II stimulated PI metabolism in a concentration-dependent manner Figure 8a,b). No U-II-induced response of PI hydrolysis was observed in cells transfected with the vector DNA alone (data not shown). PI hydrolysis was measured by incubating cells labelled with [3H] myo-inositol with human U-II for 20 min and monitoring the maximal formation of a mixture of inositol phosphates while blocking further dephosphorylation by LiCl. The EC50 for the activation of IPs accumulation in mouse and monkey UT receptor were 7.2±1.8 and 0.88±0.2 nM respectively for human U-II and 2.6±0.8 and 0.7±0.2 nM respectively for goby U-II (Figure 8a,b). There was a good agreement between the peptide binding specificity of each receptor and its ability to increase inositol phosphate formation. To determine whether the stimulation of PI hydrolysis results in enhancement of intracellular [Ca2+]i mobilization, human U-II-mediated changes in the levels of intracellular Ca2+ were also examined. As shown in Figure 9a,b human U-II increased intracellular Ca2+ in a concentration-dependent manner with EC50 of 3.2±0.8 and 1.1±0.3 nM respectively for mouse and monkey UT receptor. U-II failed to modulate the forskolin-activated adenylate cyclase (data not shown).
Figure 8.
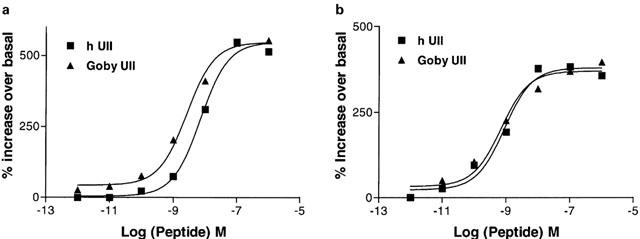
U-II-mediated inositol phosphates accumulation: Inositol phosphates accumulation in response to human and goby U-II (1 pM – 1 μM) in HEK-293 cells stably transfected with mouse (a) or monkey (b) UT receptor. The cells were treated with myo-[3H]inositol (1.0 μCi ml−1) overnight and then washed to remove excess myo-[3H]inositol. The cells were challenged with different concentrations of human or goby U-II for 5 min, and the inositol phosphates were separated from free inositol using ion-exchange chromatography. Data are the mean±s.e.mean values from three different experiments for the accumulation of inositol trisphosphate. Similar observation was made for inositol mono and diphosphates.
Figure 9.
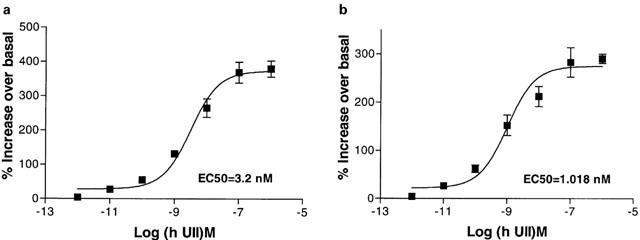
U-II-mediated [Ca2+]i release. Concentration-response curve for human U-II-mediated [Ca2+]i increase in mouse (a) or monkey (b) UT receptor stably transfected in HEK-293 cells. Each point represents the mean±s.e.mean from three separate experiments.
Discussion
The discovery of U-II as the cognate ligand for UT receptor (Ames et al., 1999; Liu et al., 1999; Mori et al., 1999; Nothacker et al., 1999) has attracted the interest of many investigations in biochemical, physiological and pharmacological research. U-II is the most potent mammalian vasoconstrictor identified to date. Its action is sustained, and resistant to washout (Ames et al., 1999). In human tissue, the functional responses of U-II include positive inotropic and arrythmogenic effects in isolated cardiac trabeculae (Russell et al., 2001), vasoconstriction (Douglas et al., 2000b; Maguire et al., 2000) and vasodilation in human resistance and microvasculature preparations in vitro and in vivo (Leslie, 2000; Maclean et al., 2000; Strirrat et al., 2001), rat cardiac myocyte and fibroblast fibrosis and hypertrophy (Tzanidis et al., 2000) and rat and rabbit smooth muscle cell hyperplasia (Sauzeau et al., 2001; Watanabe et al., 2001). These responses suggest that U-II may be involved in the pathophysiology of a number of vascular diseases characterized by aberrant and peripheral vascular and cardiac tone/remodelling such as hypertension, angina pectoris, congestive heart failure, cardiac arrythmias and other related pathological conditions (Douglas & Ohlstein, 2000). As part of the efforts to validate the UT receptor and U-II as targets for therapeutic intervention, it was necessary to characterize the mouse and monkey receptors. The characterization of the mouse gene would allow the development of knockout and/or transgenic mice for modelling many of the vascular actions of U-II. Furthermore, the characterization of primate genes for modelling is essential since primate and rat models have very different haemodynamic profiles in response to U-II administration. Thus, the rat model is not the most appropriate model for testing antagonists for antihypertensive action (since, unlike the monkey where U-II is a systemic vasoconstrictor, U-II actually increases regional vascular conductances in the intact rat; Ames et al., 1999; Gardiner et al., 2001). The outcome of the present study would provide important information not only for understanding the biology of the interaction of the receptor/ligand but also for the utilization of these animals as models for drug testing.
The present study describes the cloning of cDNAs encoding the mouse and monkey preproU-II, their tissue distribution, genomic structure as well as their pharmacological properties. Although the primary structure of the preproU-II is divergent between lower and higher vertebrates (Ames et al., 1999; Coulouarn et al., 1998; 1999; Pearson et al., 1980), there are important similarities among the overall organization in their structure. The most common feature is that U-II is synthesized as a preproprotein with an amino terminus signal peptide. The preproU-II is then cleaved by putative polybasic endopeptidase to form the mature protein (Ames et al., 1999; Coulouarn et al., 1998; Mori et al., 1999). This latter polybasic cleavage site is conserved in human, monkey, rat, frog, and carp, and as a result the mature U-II for the human, monkey, rat, frog, and carp would generate 11, 11, 14, 13, and 12 amino acids, respectively (Figures 1 and 3, Ames et al., 1999; Coulouarn et al., 1998; 1999; Pearson et al., 1980). In mouse, however, there is one putative cleavage site at position 105 – 106 (Figure 1). Cleavage at these sites would generate 17-residue peptide containing the U-II signature motif at its C-terminus.
Amino acid comparisons of the primate (human, monkey), rodent, amphibian and carp preproU-II sequences revealed an overall low identity between higher vertebrates and lower vertebrates (93% human and monkey; 48% between human and mouse; 25% human and carp). The only domain that exhibited significant amino acid sequence identity among the different species is the extreme C-terminus of the precursors which encodes the mature U-II peptide sequence. The most intriguing aspect is that this region is the cyclic hexapeptide flanked by acidic D/E N-terminus residue and an extreme I/V carboxyl residue (Figure 1), which is identical in all species examined. This octapeptide is the minimal sequence required for retaining full biological activity (Itoh et al., 1988). This was based on the observation that truncation of the N-terminus is tolerated up until the last eight residues (Itoh et al., 1988), and the fact that all mammalian, amphibian, piscine U-II isopeptides are equipotent agonists at recombinant UT receptors (Gardiner et al., 2001; Russell et al., 2001).
The structural signature of the putative mouse and monkey UT receptor revealed that it belongs to the GPCR superfamily. The most characteristic feature of this family is the presence of seven hydrophobic regions, putative transmembrane (TM) domains (Probst et al., 1992). There are also two potential N-linked glycosylation sites in the N-terminus extracellular domain, palmitoylation site (NPxxLY) in the TM-7 motif, as well as the presence of several conserved cysteine residues in the first and second extracellular loop and proline residues in TM3 and TM6, in addition to sequence similarity in putative TM domains (Probst et al., 1992). These domains were present in human, monkey, rat, and mouse indicating that the UT receptor is a member of the G protein-coupled receptor super-family (Probst et al., 1992). The structure of UT receptor peptide sequence is distantly related to the somatostaitin-4 (Rohrer et al., 1993), galanin (Habert-Ortoli et al., 1994) and kappa opioid (Mansson et al., 1994) receptors with 25 – 26% identity. The deduced amino acid sequence identity of the monkey UT receptor is 97 and 77% identical to the human and the rat sequence, while the mouse UT receptor is 76 and 93% identical to human and rat respectively. It is apparent that the recombinant UT receptors from the mouse and monkey are the likely orthologues of the human gene. This is supported by the high degree of homology of mouse, rat, monkey, and human UT receptors. For more supportive evidence of the cloned mouse gene as the orthologue of the human UT receptor, Southern analysis of the mouse genomic DNA digested with EcoRI, and screened under low homology (condition used to identify 60% paralogues i.e. ETA and ETB receptors; Elshourbagy et al., 1993) with human UT receptor cDNA identified only one 5 kb-band. Sequence analysis of the entire 5 kb-band, confirmed the existence of the mouse orthologue of UT receptor.
The expression patterns of the mouse and monkey UT receptor and preproU-II mRNAs were cardiovascular in nature. As was observed previously in human tissue (Ames et al., 1999), the expression of monkey UT receptor mRNA were detectable in arterial tissue as compared to the venous vasculature supporting the observation that U-II constriction is limited to arterial tissue in non-human primates. Similar data showed that rat UT receptor is present in thoracic aorta, and that U-II is capable of causing contractions of rat vessels where expression of UT receptor mRNA as well as the radioligand U-II binding sites exists (Douglas et al., 2000b; Itoh et al., 1988). Yet, Maguire et al. (2000) have reported contraction of human isolated saphenous vein, which suggest that some venous tissue may express the UT receptor that may account for the vasoconstrictor properties of U-II in the venous tissues. In mouse, however, it has been reported that UT receptor expression is greater in the thoracic aorta versus the abdominal aorta, yet neither of these vessels respond to U-II with contractile response (Douglas et al., 2000b; Itoh et al., 1988). Several explanations could account for the lack of contractile response of mouse aorta in response to U-II: the low level of expression of the receptor in these tissues, or lack of coupling mechanism, or the receptor could be coupled to a different signal transduction mechanism. Further experimentation is required to delineate this process.
The mRNA expression data demonstrate that, in addition to the vasculature, the preproU-II and the UT receptor mRNAs are expressed in several peripheral tissues; namely the pancreas and lung for the receptor and skeletal muscle, liver and kidney for ligand. These data suggest that U-II should not only be considered as a neuropeptide or a modulator of cardiovascular function, but also as an agent with a wider range of multi-physiological function. In support of this hypothesis, U-II has been shown to modulate insulin release from the rat pancreas in vitro (Silvestre et al., 2001). Furthermore, putative endocrine actions have been postulated for U-II previously (Douglas & Ohlstein, 2000), actions which include the regulation of cortisol and norepinephrine release in fish (Kelsall & Balment, 1998) and, most recently, secretion of circulating prolactin and thyroid-stimulating hormone in the conscious rat (following intracerebroventricular administration; Gartlon et al., 2001). As such, U-II may play a role in the regulation of mammalian endocrine function. In the skeletal muscle, central administration of U-II also influenced motor function in the intact rat. Such an observation is of interest since U-II is reported to be expressed in ventral horn motorneurones and receptor is also located in locomotor nuclei within the brain stem (Ames et al., 1999; Chartrel et al., 1996; Coulouarn et al., 1998; 2001; Dun et al., 2001; Liu et al., 1999). Further, Maguire et al. (2000) recently identified human skeletal muscle as a tissue which exhibited an appreciable number of [125I]hU-II radioligand-binding sites in a quantitative receptor autoradiography study. This observation is not surprising since the UT receptor was originally identified as one expressed by cardiac myocytes, striated cells derived from a common embryonic origin as skeletal muscle (Ames et al., 1999). U-II expression was also observed in the mammalian kidney (including the human; Nothacker et al., 1999), an observation of note since U-II has been postulated to be an osmoregulatory factor in lower vertebrates and has been shown to regulate ion transport across epithelial membranes (Douglas & Ohlstein, 2000). The finding that preproU-II was detectable in liver was of interest since it has been proposed that U-II regulates glucose mobilization as indicated by decreased liver glycogen content and increased liver glucose-6-phosphate activity in fish (Sheridan et al., 1987). Detection of UT receptor in the lung is consistent with reports demonstrating that U-II is a potent bronchoconstrictor (Hay et al., 2000). In the GI tract, U-II showed to have a spasmogen of the GI smooth muscle tissue from fish and amphibians (Douglas & Ohlstein, 2000) consistent with the present study where expression of preproU-II mRNA was observed in the mouse and monkey gastrointestinal tract. The wide range of physiological effects of U-II coupled the tissue distribution of U-II and UT receptor in the peripheral tissues suggest that U-II could be important therapeutic target for many diseases.
Genomic localization has been used to determine the possible link with certain disease. The genomic localization of the human preproU-II showed that it is localized on chromosomal 1p36. At this locus, it seems to be the hot spot for vasoactive/natriuretic peptide genes such as U-II, cortistatin (de lecea et al., 1997), and atrial natriuretic factor (ANF) (Oikawa et al., 1984). Interestingly, this region is syntenic with rat chromosomal 5, which was recently identified as stroke hotspot (Read et al., 2001). However, attempts to map mouse U-II were not successful, and it is not yet determined if it is syntenic with the human preproU-II. The mouse UT receptor maps to chromosomal 11. This is near mouse thymidine kinase and syntenic with human 17q23 – 25 (Protopopov et al., 2000). Thus the mouse UT receptor gene maps syntenically with the human UT receptor gene.
The pharmacological profile of UT receptor from mouse and monkey showed that U-II binds to UT receptor as demonstrated by the selective binding of FITC-labelled U-II to the recombinantly expressed mouse and monkey UT receptor with a Ki of 2 nM. The apparent affinity of U-II to the recombinant mouse and the monkey UT receptor is similar to that previously observed for the human and rat receptors (Ames et al., 1999). Functional and competition binding data (Figure 7) indicated that regardless of the species of U-II used, whether from human, porcine, mouse, rat, or goby, they all are equipotent in competing with [125I]hU-II binding in cells transfected with either mouse or monkey UT recombinant receptors. This is in accord with the functional studies, which showed that different isoforms of U-II are equipotent inotropic agents in human isolated trabeculae (Russell et al., 2001) and as vasodilators in conscious rats (Gardiner et al., 2001). The U-II binding to UT receptor initiates an increases in PI turnover and Ca2+ mobilization which are all consistent with the primary signalling pathway for UT receptor involving activation of phospholipase C (Douglas et al., 2000a; Opgaard et al., 2000; Saetrum et al., 2000): U-II-induced vasoconstriction is attenuated by inhibition of phospholipase C-mediated [Ca2+]i-mobilization from the sarcoplasmic reticulum (leading to a secondary membrane depolarization and [Ca2+]i-influx) and by L-type [Ca2+]-channel blockers, calmodulin antagonism and by chelation of extracellular [Ca2+]e. Thus, and as with other G-protein coupled receptors, activation of phospholipase C is one mechanism by which the signal transduction pathway of UT receptor orthologs takes place.
In summary, we have identified the mouse and monkey UT receptors and established that they are activated by a number of U-II ligands from lower (fish, amphibian) and higher vertebrate (mammals) species. The tissue distribution studies demonstrated that the U-II and UT receptors are expressed in the heart and peripheral tissues which suggests that the physiological effects of U-II are not limited to cardiovascular but are also likely to encompass much wider non-cardiovascular organs e.g. GI tract effect, metabolic effects, in addition to CNS effect. The characterization of mouse and monkey U-II and UT receptor will allow the establishment of the appropriate model needed for understanding many of the physiological and pathological effects of U-II. As with other hormones, definition of the physiological and pathological roles of the U-II/UT receptor system clearly requires potent and specific UT receptor antagonists. Such compounds may have important therapeutic potential.
Acknowledgments
The authors are grateful to Dr Ganesh Sathe and Joyce Mao for oligonucleotide synthesis, Wendy Halsey and Diana Hoult for sequence analysis, Kathryn Ellington for PCR identification of the UT receptor BAC, Drs R.W. Coatney (Dept. Laboratory Animal Sciences, SmithKline Beecham), Heather Fenderson, Jennifer Houp for technical assistance Dr R.N. Willette, Mr C.F. Sauermelch (Department of Cardiovascular Pharmacology), and Dr Derk Bergsma for their skillful assistance and comments during the preparation of this manuscript.
Abbreviations
- bp
basepair
- BSA
bovine serum albumin
- cDNA
complementary DNA
- COPD
chronic obstructive pulmonary disease
- COS
African green monkey kidney cells
- DIC
differential interence contrast
- DMEM
Dulbecco's modified Eagles medium
- EC50
effective concentration of agonist required to give a half maximal response
- ET-1
endothelin-1
- FITC
fluorescein isothiocyanate
- GPCR
G protein-coupled receptor
- G protein
GTP-binding protein
- GPR14
renamed as UT receptor by the International Union of Pharmacology (IUPHAR) Committee on Receptor Nomenclature and Drug Classification (Douglas & Ohlstein, 2001)
- HEK-293 cells
human embryonic kidney cells
- Ips
inositol phosphates
- ORF
open reading frame
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- PI
phosphoinositide
- PMSF
phenylmethylsulphonyl fluoride
- RT
reverse transcription
- SDS
sodium dodecyl sulphate
- SPA
scintillation Proximity Assay
- SSC
sodium chloride, sodium citrate
- TM
transmembrane
- U-II
Urotensin-II
- UT
Urotensin-II Receptor
- WGA
wheat germ agglutin
References
- AIYAR N., BAKER E., WU H.L., NAMBI P., EDWARDS R.M., TRILL J.J., ELLIS C., BERGSMA D.J. Human AT1 receptor is a single copy gene: characterization in a stable cell line. Mol. Cell Biochem. 1994;131:75–86. doi: 10.1007/BF01075727. [DOI] [PubMed] [Google Scholar]
- AIYAR N.V., NAMBI P., STASSEN F.L., CROOKE S.T. Vascular vasopressin receptors mediate phosphatidylinositol turnover and calcium efflux in an established smooth muscle cell line. Life Sciences. 1986;39:37–45. doi: 10.1016/0024-3205(86)90435-2. [DOI] [PubMed] [Google Scholar]
- AMES R.S., SARAU H.M., CHAMBERS J.K., WILLETTE R.N., AIYAR N.V., ROMANIC A.M., LOUDEN C.S., FOLEY J.J., SAUERMELCH C.F., COATNEY R.W., AO Z., DISA J., HOLMES S.D., STADEL J.M., MARTIN J.D., LIU W.S., GLOVER G.I., WILSON S., MCNULTY D.E., ELLIS C.E., ELSHOURBAGY N.A., SHABON U., TRILL J.J., HAY D.W., OHLSTEIN E.H., BERGSMA D.J., DOUGLAS S.A. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- BERN H.A., LEDERIS K. A reference preparation for the study of active substances in the caudal neurosecretory system of teleosts. J. Endocrinol. 1969;45:xi–xii. [PubMed] [Google Scholar]
- BERN H.A., PEARSON D., LARSON B.A., NISHIOKA R.S. Neurohormones from fish tails: the caudal neurosecretory system. I. ‘Urophysiology' and the caudal neurosecretory system of fishes. Rec. Prog. Horm. Res. 1985;41:533–552. doi: 10.1016/b978-0-12-571141-8.50016-0. [DOI] [PubMed] [Google Scholar]
- BOTTRILL F.E., DOUGLAS S.A., HILEY C.R., WHITE R. Human urotensin-II is an endothelium-dependent vasodilator in rat small arteries. Br. J. Pharmacol. 2000;130:1865–1870. doi: 10.1038/sj.bjp.0703513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHARTREL N., CONLON J.M., COLLIN F., BRAUN B., WAUGH D., VALLARINO M., LAHRICHI S.L., RIVIER J.E., VAUDRY H. Urotensin II in the central nervous system of the frog Rana ridibunda: immunohistochemical localization and biochemical characterization. J. Comp. Neurol. 1996;364:324–339. doi: 10.1002/(SICI)1096-9861(19960108)364:2<324::AID-CNE10>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- CONLON J.M., O'HARTE F., SMITH D.D., TONON M.C., VAUDRY H. Isolation and primary structure of urotensin II from the brain of tetrapod, the frog Rana ridibunda. Biochem. Biophys. Res. Commun. 1992;188:578–583. doi: 10.1016/0006-291x(92)91095-8. [DOI] [PubMed] [Google Scholar]
- CONLON J.M., TOSTIVINT H., VAUDRY H. Somatostatin- and urotensin II-related peptides: molecular diversity and evolutionary perspectives. Regul. Pept. 1997;69:95–103. doi: 10.1016/s0167-0115(97)02135-6. [DOI] [PubMed] [Google Scholar]
- CONLON J.M., YANO K., WAUGH D., HAZON N. Distribution and molecular forms of urotensin II and its role in cardiovascular regulation in vertebrates. J. Expl. Zool. 1996;275:226–238. [PubMed] [Google Scholar]
- COULOUARN Y., FERNEX C., JEGOU S., HENDERSON C.E., VAUDRY H., LIHRMANN I. Specific expression of the urotensin II gene in sacral motoneurons of developing rat spinal cord. Mech. Dev. 2001;101:187–190. doi: 10.1016/s0925-4773(00)00548-7. [DOI] [PubMed] [Google Scholar]
- COULOUARN Y., JEGOU S., TOSTIVINT H., VAUDRY H., LIHRMANN I. Cloning, sequence analysis and tissue distribution of the mouse and rat urotensin II precursors. FEBS Lett. 1999;457:28–32. doi: 10.1016/s0014-5793(99)01003-0. [DOI] [PubMed] [Google Scholar]
- COULOUARN Y., LIHRMANN I., JEGOU S., ANOUAR Y., TOSTIVINT H., BEAUVILLAIN J.C., CONLON J.M., BERN H.A., VAUDRY H. Cloning of the cDNA encoding the urotensin II precursor in frog and human reveals intense expression of the urotensin II gene in motoneurons of the spinal cord. Proc. Natl. Acad. Sci. 1998;95:15803–15808. doi: 10.1073/pnas.95.26.15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LECEA L., RUIZ-LOZANO P., DANIELSON P.E., PEELLE-KIRLEY J., FOYE P.E., FRANKEL W.N., SUTCLIFFE J.G. Cloning, mRNA expression, and chromosomal mapping of mouse and human preprocortistatin. Gemonics. 1997;42:499–506. doi: 10.1006/geno.1997.4763. [DOI] [PubMed] [Google Scholar]
- DEVEREUX J., HAEBERLI P., SMITHIES O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS S.A., AIYAR N.V., WILLETTE R.N. Human urotensin-II induced vasoconstriction is attenuated by inhibition of phospholipase c-mediated [Ca+2]-signaling and by PKC/calmodulin. Circulation. 2000a;102:II–113. [Google Scholar]
- DOUGLAS S.A., OHLSTEIN E.H. Human urotensin-II, the most potent mammalian vasoconstrictor identified to date, as a therapeutic target for the management of cardiovascular disease. Trends Cardiovasc. Med. 2000;10:229–237. doi: 10.1016/s1050-1738(00)00069-4. [DOI] [PubMed] [Google Scholar]
- DOUGLAS S.A., OHLSTEIN E.H.Urotensin Receptors The IUPHAR compendium of receptor characterization and classification 2001London: IUPHAR Media; 365–372.ed. Girdlestone, D. pp [Google Scholar]
- DOUGLAS S.A., SULPIZIO A.C., PIERCY V., SARAU H.M., AMES R.S., AIYAR N.V., OHLSTEIN E.H., WILLETTE R.N. Differential vasoconstrictor activity of human urotensin-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. Br. J. Pharmacol. 2000b;131:1262–1274. doi: 10.1038/sj.bjp.0703690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUN S.L., BRAILOIU G.C., YANG J, , CHANG J.K., DUN N.J. Urotensin II-immunoreactivity in the brainstem and spinal cord of the rat. Neurosci. Lett. 2001;305:9–12. doi: 10.1016/s0304-3940(01)01804-3. [DOI] [PubMed] [Google Scholar]
- ELSHOURBAGY N.A., ADAMOU J.E., SWIFT A.M., DISA J., MAO J., GANGULY S., BERGSMA D.J., AIYAR N. Molecular cloning and characterization of porcine calcitonin gene-related peptide receptor. Endocrinology. 1998;139:1678–1683. doi: 10.1210/endo.139.4.5860. [DOI] [PubMed] [Google Scholar]
- ELSHOURBAGY N.A., KORMAN D.R., HSIAO-LING W, , SYLVESTER D.R., LEE J.A., NUTHULAGANTI P., BERGSMA D.J., KUMAR C.S., NAMBI P. Molecular characterization and regulation of the human endothelin receptors. J. Biol. Chem. 1993;268:3873–3879. [PubMed] [Google Scholar]
- GARDINER S.M., MARCH J.E., KEMP P.A., DAVENPORT A.P., BENNETT T. Depressor and regionally-selective vasodilator effects of human and rat urotensin II in conscious rats. Br. J. Pharmacol. 2001;132:1625–1629. doi: 10.1038/sj.bjp.0704051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARTLON J., PARKER F., HARRISON D.C., DOUGLAS S.A., ASHMEADE T.E., RILEY G.J., HUGHES Z.A., TAYLOR S.G., MUNTON R.P., HAGAN J.J., HUNTER J.A., JONES D.N. Psychopharmacology. 2001. pp. 426–443. [DOI] [PubMed]
- GRAY G.A., JONES M.R., SHARIF I. Human urotensin II increases coronary perfusion pressure in the isolated rat heart: potentiation by nitric oxide synthase and cyclooxygenase inhibition. Life Sci. 2001;69:175–180. doi: 10.1016/s0024-3205(01)01101-8. [DOI] [PubMed] [Google Scholar]
- HABERT-ORTOLI E., AMIRANOFF B., LOQUET I., LABURTHE M., MAYAUX J.F. Molecular cloning of a functional human galanin receptor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9780–9783. doi: 10.1073/pnas.91.21.9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAY D.W., LUTTMANN M.A., DOUGLAS S.A. Human urotensin-II is a potent spasmogen of primate airway smooth muscle. Br. J. Pharmacol. 2000;131:847–852. doi: 10.1038/sj.bjp.0703533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITOH H., MCMASTER D., LEDERIS K. Functional receptors for fish neuropeptide neurotensin II in major rat arteries. Eur. J. Pharmacol. 1988;149:61–66. doi: 10.1016/0014-2999(88)90042-8. [DOI] [PubMed] [Google Scholar]
- KELSALL C.J., BALMENT R.J. Native urotensins influence cortisol secretion and plasma cortisol concentration in the euryhaline flounder, platichthys flesus. Gen. Comp. Endocrinol. 1998;112:210–219. doi: 10.1006/gcen.1998.7166. [DOI] [PubMed] [Google Scholar]
- LESLIE S.J. Human urotensin-II causes vasoconstriction in the human skin microcirculation. Circulation. 2000;102:II–113. [Google Scholar]
- LIU Q., PONG S.S., ZENG Z., ZHANG Q., HOWARD A.D., WILLIAMS D.L., DAVIDOFF M., WANG R., AUSTIN C.P., MCDONALD T.P., BAI C., GEORGE S.R., EVANS J.F., CASKEY C.T. Identification of urotensin II as the endogenous ligand for the orphan G-protein-coupled receptor GPR14. Biochem. Biophys. Res. Commun. 1999;266:174–178. doi: 10.1006/bbrc.1999.1796. [DOI] [PubMed] [Google Scholar]
- LYSKO P.G., WEBB C.L., YUE T., GU J., FEUERSTEIN G. Neuroprotective effects of tetrodotoxin as a Na+ channel modulator and glutamate release inhibitor in cultured rat cerebellar neurons and in gerbil global brain ischemia. Stroke. 1994;25:2476–2482. doi: 10.1161/01.str.25.12.2476. [DOI] [PubMed] [Google Scholar]
- MACLEAN M.R., ALEXANDER D., STIRRAT A., GALLAGHER M., DOUGLAS S.A., OHLSTEIN E.H., MORECROFT I., POLLARD K. Contractile responses to human urotensin-II in rat and human pulmonary arteries: effect of endothelial factors and chronic hypoxia in the rat. Br. J. Pharmacol. 2000;130:201–204. doi: 10.1038/sj.bjp.0703314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGUIRE J.J., KUC R.E., DAVENPORT A.P. Orphan-receptor ligand human urotensin II: receptor localization in human tissues and comparison of vasoconstrictor responses with endothelin-1. Br. J. Pharmacol. 2000;131:441–446. doi: 10.1038/sj.bjp.0703601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANSSON E., BARE L., YANG D. Isolation of a human kappa opioid receptor cDNA from placenta. Biochem. Biophys. Res. Commun. 1994;202:1431–1437. doi: 10.1006/bbrc.1994.2091. [DOI] [PubMed] [Google Scholar]
- MORI M., SUGO T., ABE M., SHIMOMURA Y., KURIHARA M., KITADA C., KIKUCHI K., SHINTANI Y., KUROKAWA T., ONDA H., NISHIMURA O., FUJINO M. Urotensin II is the endogenous ligand of a G-protein-coupled orphan receptor, SENR (GPR14) Biochem. Biophys. Res. Commun. 1999;265:123–129. doi: 10.1006/bbrc.1999.1640. [DOI] [PubMed] [Google Scholar]
- NOTHACKER H.P., WANG Z., MCNEILL A.M., SAITO Y., MERTEN S., O'DOWD B., DUCKLES S.P., CIVELLI O. Identification of the natural ligand of an orphan G-protein-coupled receptor involved in the regulation of vasoconstriction. Nat. Cell Biol. 1999;1:383–385. doi: 10.1038/14081. [DOI] [PubMed] [Google Scholar]
- OIKAWA S., IMAI M., UENO A., TANAKA S., NOGUCHI T., NAKAZATO H., KANGAWA K., FUKUDA A., MATSUO H. Cloning and sequence analysis of cDNA encoding a precursor for human atrial natriuretic polypeptide. Nature. 1984;309:724–726. doi: 10.1038/309724a0. [DOI] [PubMed] [Google Scholar]
- OPGAARD O.O., NOTHACKER H., EHLERT F.J., KRAUSE D.N. Human urotensin II mediates vasoconstriction via an increase in inositol phosphates. Eur. J. Pharmacol. 2000;406:265–271. doi: 10.1016/s0014-2999(00)00672-5. [DOI] [PubMed] [Google Scholar]
- PADGETT R.A., GRABOWSKI P.J., KONARSKA M.M., SEILER S., SHARP P.A. Splicing of messenger RNA precursors. Annu. Rev. Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- PEARSON D.S., SHIVELY J.E., CLARK B.R., GESCHWIND I, , BARKLEY M., NISHIOKA R.S., BERN H.A. Urotensin II: a somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc. Natl. Acad. Sci. 1980;77:5021–5024. doi: 10.1073/pnas.77.8.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROBST W.C., SNYDER L.A., SCHUSTER D.I., BROSIUS J., SEALFON S.C. Sequence alignment of the G-protein coupled receptor superfamily. DNA Cell Biol. 1992;1:1–20. doi: 10.1089/dna.1992.11.1. [DOI] [PubMed] [Google Scholar]
- PROTOPOPOV A., KASHUBA V., PODOWSKI R., GIZATULLIN R., SONNHAMMER E., WAHLESTEDT C., ZABAROVSKY E.R. Assignment of the GPR14 gene coding for the G-protein-coupled receptor 14 to human chromosome 17q25.3 by fluorescent in situ hybridization. Cytogenet Cell Genet. 2000;88:312–313. doi: 10.1159/000015516. [DOI] [PubMed] [Google Scholar]
- READ S.J., PARSONS A.A., HARRISON D.C., PHILPOTT K., O'BRIEN S., CLARK S., BRAWNER M., BATES S., GLOGER I., LEGOS J.J., BARONE F.C. Stroke Genomics: Approach to identify, validate, and understand ischemic stroke gene expression. J. Cereb. Blood Flow Metab. 2001;21:755–778. doi: 10.1097/00004647-200107000-00001. [DOI] [PubMed] [Google Scholar]
- ROHRER L., RAULF F., BRUNS C., BUETTNER R., HOFSTAEDTER F., SCHULE R. Cloning and characterization of a fourth human somatostatin receptor. Proc. Natl. Acad. Sci. U.S.A. 1993;90:4196–4200. doi: 10.1073/pnas.90.9.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSSELL F.D., MOLENAAR P., O'BRIEN D.M. Cardiostimulant effects of urotensin-II in human heart in vitro. Br. J. Pharmacol. 2001;132:5–9. doi: 10.1038/sj.bjp.0703811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAETRUM OPGAARD O.O., NOTHACKER H., EHLERT F.J., KRAUSE D.N. Human urotensin II mediates vasoconstriction via an increase in inositol phosphates. Eur. J. Pharmacol. 2000;406:265–271. doi: 10.1016/s0014-2999(00)00672-5. [DOI] [PubMed] [Google Scholar]
- SAUZEAU V., LE MELLIONNEC E., BERTOGLIO J., SCALBERT E., PACAUD P., LOIRAND G. Human urotensin II-induced contraction and arterial smooth muscle cell proliferation are mediated by RhoA and Rho-kinase. Circ. Res. 2001;88:1102–1104. doi: 10.1161/hh1101.092034. [DOI] [PubMed] [Google Scholar]
- SHERIDAN M.A., PLISETSKAYA E.M., BERN H.A., GORBMAN A. Effects of somatostatin-25 and urotensin II on lipid and carbohydrate metabolism of coho salmon, Oncorhynchus kisutch. Gen. Comp. Endocrinol. 1987;66:405–414. doi: 10.1016/0016-6480(87)90251-6. [DOI] [PubMed] [Google Scholar]
- SILVESTRE R.A., RODRIGUEZ-GALLARDO J., EGIDO E.M., MARCO J. Inhibition of insulin release by urotensin II – a study on the perfused rat pancreas. Horm. Metab. Res. 2001;33:379–381. doi: 10.1055/s-2001-15414. [DOI] [PubMed] [Google Scholar]
- STRIRRAT A., GALLAGHER M., DOUGLAS S.A., OHLSTEIN E.H., BERRY C., RICHARDSON M., MACLEAN M.R. Potent vasodilator responses to human urotensin-II in human pulmonary and abdominal resistance arteries. Am. J. Physiol. 2001;280:H925–H928. doi: 10.1152/ajpheart.2001.280.2.H925. [DOI] [PubMed] [Google Scholar]
- TZANIDIS A., HANNAN R.D., KRUM H. Urotensin-II stimulates collagen synthesis by cardiac fibroblasts in vitro: implications for myocardial remodeling. Eur. Heart J. 2000;221:72. [Google Scholar]
- WATANABE T., PAKALA R., KATAGIRI T., BENEDICT C.R. Synergistic effect of urotensin II with mildly oxidized LDL on DNA synthesis in vascular smooth muscle cells. Circulation. 2001;104:16–18. doi: 10.1161/hc2601.092848. [DOI] [PubMed] [Google Scholar]
- WINTER M.J., HUBBARD P.C., MCCROHAN C.R., BALMENT R.J. A homologous radioimmunoassay for the measurement of urotensin-II in the euryhaline flounder, Platichthys flesus. Gen. Comp. Endorcinol. 1999;114:249–256. doi: 10.1006/gcen.1998.7245. [DOI] [PubMed] [Google Scholar]
- YANO K., HICKS J.W., VAUDRY H., CONLON J.M. Cardiovascular actions of frog urotensin II in the frog, Rana catesbeiana. Gen. Comp. Endocrinol. 1995;97:103–110. doi: 10.1006/gcen.1995.1010. [DOI] [PubMed] [Google Scholar]
- YANO K., VAUDRY H., CONLON J.M. Spasmogenic actions of frog urotensin II on the bladder and ileum of the frog, Rana catesbeiana. Gen. Comp. Endocrinol. 1994;96:412–419. doi: 10.1006/gcen.1994.1197. [DOI] [PubMed] [Google Scholar]


