Abstract
The magnitude and duration of the abruptly occurring increases in cytosolic Ca2+ in human neutrophils following activation with PAF (20 and 200 nM) and FMLP (1 μM), have been compared and related to alterations in NADPH oxidase activity, membrane potential and intracellular cyclic AMP.
Cytosolic Ca2+ and membrane potential were measured by spectrofluorimetry, transmembrane fluxes of Ca2+ by radiometric procedures, and NADPH oxidase activity and cyclic AMP by chemiluminescence and radioimmunoassay respectively.
Activation of neutrophils with both PAF (200 nM) and FMLP (1 μM) was accompanied by an abrupt increase in cytosolic Ca2+, which was of similar magnitude for each activator (393±9 and 378±17 nM respectively). Unlike FMLP-activated cells in which Ca2+ was rapidly removed from the cytosol, peak levels of cytosolic Ca2+ were sustained for longer (0.14±0.02 vs 1.16±0.04 min, P⩽0.0001) and declined at a slower rate in PAF-treated neutrophils.
The prolonged elevation of cytosolic Ca2+ in PAF-treated cells was due to accelerated store-operated influx of extracellular cation and was attenuated by dibutyryl cyclic AMP (4 mM), the Ca2+-chelator, EGTA (5 mM), and SKF 96365 (10 μM). In contrast to FMLP, basal levels of superoxide production and cyclic AMP were unaltered in PAF-activated neutrophils, while only moderate membrane depolarization was detected.
These observations demonstrate that mechanisms which restore Ca2+ homeostasis to FMLP-activated neutrophils, viz. activation of NADPH oxidase and adenylate cyclase, are not operative in PAF-treated cells, presenting the potential hazard of Ca2+ overload and hyperactivity.
Keywords: Calcium, CGS 21680, cyclic AMP, membrane depolarization, dibutyryl cyclic AMP, elastase, FMLP, neutrophils, PAF, superoxide
Introduction
Platelet-activating factor (PAF; 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) is arguably the most potent of the pro-inflammatory, bioactive phospholipids (Zimmerman et al., 1992; Kravchenko et al., 1995) with the potential to reproduce all of the clinical manifestations of bronchial asthma (Barnes & Chung, 1987). Receptor-mediated pro-inflammatory activities of PAF include pro-adhesive and chemotactic activities for eosinophils and neutrophils, as well as sensitization (‘priming') of these cells for increased production of reactive oxidants and release of granule constituents on subsequent exposure to chemoattractants and other activators (Peplow, 1999). PAF has also been reported to sensitize monocytes/macrophages for increased production of leukotriene B4 (Shindo et al., 1998) and interleukin-8 (Hilger et al., 1996; Arbabi et al., 1999) and to activate the proinflammatory, cytosolic nuclear transcription factor NF-κB in a variety of cell types (Kravchenko et al., 1995).
Although many of these pro-inflammatory activities of PAF are believed to be achieved through receptor-mediated increases in cytosolic Ca2+ in target cells, relatively little is known about the sites (extracellular or intracellular) from which the cation is mobilized, or the mechanisms used by PAF-activated cells to restore Ca2+ homeostasis. In the case of neutrophils activated with chemoattractants such as N-formyl-L-methionyl-L-leucyl-L-phenylalanine (FMLP), the plasma membrane and endo-membrane Ca2+-ATPases, which are regulated by calmodulin and adenosine 3′,5′ cyclic monophosphate (cyclic AMP)-dependent protein kinase respectively (Lagast et al., 1984; Tao et al., 1992), promote clearance of cytosolic Ca2+ by efflux and resequestration of the cation (Anderson et al., 1998; Pettit & Hallett, 2000). The efficiency of these Ca2+-clearance pumps is enhanced by the membrane depolarizing actions of NADPH oxidase which restrict the uptake of extracellular Ca2+ (di virgilio et al., 1987; Geiszt et al., 1997; Tintinger et al., 2001). In contrast to FMLP-activated neutrophils, neither NADPH oxidase (Nick et al., 1997) nor adenylate cyclase (Ali et al., 1998) undergoes significant activation on exposure of neutrophils to PAF, suggesting that Ca2+ handling by cells activated with this bioactive phospholipid is likely to differ from that of cells exposed to the chemotactic tripeptide.
In the current study we have compared the magnitude and duration of the changes in cytosolic Ca2+ concentrations which accompany activation of human neutrophils with PAF and FMLP, as well as the origins of the cytosolic cation mobilized by both activators. In addition, we have investigated the potential of the cell-permeable cyclic AMP-analogue, dibutyryl cylic AMP, the type 4 phosphodiesterase inhibitor, rolipram and the subtype A2A receptor agonist, 2(4-[(2- carboxyethyl)phenyl]ethylamino) -5′- N - ethylcarboxamido adenosine (CGS 21680), to restore Ca2+ homeostasis to PAF-activated neutrophils.
Methods
Neutrophils
Neutrophils were prepared from heparinized (5 U of preservative-free heparin ml−1) venous blood taken from healthy, adult volunteers. The heparinized blood was fractionated by centrifugation (400 g for 25 min) on Histopaque®-1077 (Sigma Diagnostics) cushions and the resultant neutrophil fraction removed by sequential sedimentation with 3% gelatin and selective lysis with 0.84% ammonium chloride (Anderson et al., 1998). The neutrophils, which were routinely of high purity (>90%) and viability (>95%) were resuspended to a concentration of 107 ml−1 in phosphate-buffered saline (PBS) and held on ice until use.
Spectrofluorimetric measurement of Ca2+-fluxes
Fura-2/AM was used as the fluorescent, Ca2+-sensitive indicator for these experiments (Grynkiewicz et al., 1985). Neutrophils (107 ml−1) were pre-loaded with fura-2 (2 μM) for 30 min at 37°C in PBS (0.15 M, pH 7.4), washed twice and resuspended in indicator-free Hanks balanced salt solution (HBSS, pH 7.4) containing 1.25 mM CaCl2 (Ca2+-replete HBSS). The fura-2 loaded cells (2×106 ml−1) were then pre-incubated for 10 min at 37°C, after which they were transferred to cuvettes, which were maintained at 37°C in a Hitachi 650 10S fluorescence spectrophotometer with excitation and emission wavelengths set at 340 nm and 500 nm, respectively. After a stable baseline was obtained (±1 min), PAF (2 pmol – 200 nM) or FMLP (1 μM) was added to the neutrophils and the subsequent increase in fura-2 fluorescence intensity was monitored over a 5 min period. The final volume in each cuvette was 3 ml containing a total of 6×106 neutrophils. Based on the results of these experiments, PAF was used at 20 and/or 200 nM in subsequent investigations since at these concentrations the phospholipid caused elevations in neutrophil cytosolic Ca2+ equivalent to those induced by FMLP at 1 μM, a concentration of this agent which was found in preliminary experiments to be maximally effective with respect to Ca2+ mobilization, membrane depolarization and superoxide generation (concentrations of 0.001, 0.01, 0.1 and 1 μM were evaluated). The values shown in the text and tables are those for the peak increments observed within 30 s of addition of FMLP or PAF to the cells and were calculated as described previously (Grynkiewicz et al., 1985).
The effects of the extracellular Ca2+-chelating agent ethylene glycol-bis(β-aminoethyl ether) N,N,N′,N′-tetraacetic acid (EGTA, 5 mM), (1-[β-)3-[4-methoxyphenyl]-1H-imidazole hydrochloride (SKF 96365, 10 μM) a selective inhibitor of store-operated Ca2+ influx (Merritt et al., 1990), (1-[6-([17() - 3 - methoxyestra -1,3,5(10)-trien-17-yl]amino)hexyl]-1H-pyrrole-2,5-dione (U 73122, 10 μM) a phospholipase C (PLC) inhibitor (Tatrai et al., 1994), the cell permeable analogue of cyclic AMP, dibutyryl cyclic AMP (4 mM), the type 4 phosphodiesterase inhibitor, rolipram (0.5 μM), the subtype A2A receptor agonist, CGS 21680 (1 μM) and the protein kinase C activator, phorbol 12-myristate 13-acetate (PMA, 40 nM) on the PAF (200 μM)-activated fura-2 fluorescence responses of neutrophils were also investigated.
Mn2+-quenching of fura-2 fluorescence
Cells loaded with fura-2/AM as described above were activated with FMLP (1 μM) or PAF (20 and 200 nM) in HBSS supplemented with 300 μM MnCl2 (added 5 min prior to FMLP or PAF) and fluorescence quenching, as a measure of Ca2+ influx, was determined at an excitation wavelength of 360 nm, which is an isosbestic wavelength, and at an emission wavelength of 500 nm (Geiszt et al., 1997). This procedure was also used to investigate the effects of CGS 21680 (1 μM), dibutyryl cyclic AMP (4 mM), rolipram (0.5 μM) and SKF 96365 (10 μM) on the influx of Ca2+ into PAF (200 nM)-activated neutrophils.
Radiometric assessment of Ca2+ fluxes
45Ca2+ (Calcium-45 chloride, specific activity 18.53 mCi mg−1, Du Pont NEN Research Products, Boston, MA, U.S.A.) was used as tracer to lable the intracellular Ca2+ pool and to monitor Ca2+ fluxes in resting and PAF-stimulated neutrophils. In the assays of Ca2+ influx and efflux described below, the radiolabelled cation was used at a fixed, final concentration of 2 μCi ml−1, containing 50 nM cold carrier Ca2+ (as CaCl2) and the final assay volumes were 5 ml containing a total of 1×107 neutrophils. The standardization of the procedures used to load the cells with 45Ca2+, as well as a comparison with oil-based methods for the separation of labelled neutrophils from unbound isotope, have been described (Anderson & Goolam Mohamed, 1997).
Efflux of 45Ca2+ from neutrophils exposed to PAF
To measure net efflux of 45Ca2+ from neutrophils uncomplicated by concomitant influx of the radiolabelled cation, the cells (107 ml−1) were loaded with 45Ca2+ (2 μCi ml−1) for 20 min at 37°C in HBSS. The neutrophils were then pelleted by centrifugation, washed once with, and resuspended in Ca2+-replete HBSS. The 45Ca2+-loaded neutrophils (2×106 ml−1) were then preincubated for 10 min at 37°C followed by activation with FMLP (1 μM) or PAF (20 and 200 nM). In additional experiments, neutrophils were preincubated for 10 min at 37°C in the presence and absence of dibutyryl cyclic AMP (4 mM), CGS 21680 (1 μM), rolipram (0.5 μM) or SKF 96365 (10 μM) followed by addition of PAF (20 and 200 nM) and measurement of the net efflux of 45Ca2+ over a 60 s time-course.
Reactions were stopped by adding 10 ml ice-cold Ca2+-replete HBSS to the tubes, which were then transferred immediately to an ice-bath. The cells were then pelleted by centrifugation at 400 g for 5 min followed by washing with ice-cold Ca2+-replete HBSS and the cell pellets finally dissolved in 0.5 ml of Triton X-100/0.05 M NaOH and the radioactivity assayed in a scintillation spectrometer.
Influx of 45Ca2+ into PAF-exposed neutrophils
To measure net influx of 45Ca2+ from neutrophils, uncomplicated by concomitant efflux of the radiolabelled cation, the cells (107 ml−1) were suspended in Ca2+-replete HBSS and incubated for 15 min at 37°C, after which they were pelleted by centrifugation, then washed once and resuspended in ice-cold Ca2+-free HBSS and held on ice until use. Preloading with cold Ca2+ was undertaken to minimize spontaneous uptake of 45Ca2+ in the efflux assay. The efficiency of this loading procedure was demonstrated by measurement of the FMLP-activated fura-2 responses of the Ca2+-loaded neutrophils, which did not differ from those of neutrophils maintained in Ca2+-replete HBSS (Anderson & Goolam Mahomed, 1997). The Ca2+-loaded neutrophils (2×106 ml−1) were then incubated for 10 min at 37°C followed by simultaneous addition of PAF (200 nM) and 45Ca2+ (2 μCi ml−1) or FMLP (1 μM) and 45Ca2+, or by 45Ca2+ only to control systems. Influx of 45Ca2+ into FMLP- or PAF-activated neutrophils was then monitored over a 5 min period, after which influx is complete (Anderson & Goolam Mohamed, 1997), and compared with the uptake of the radiolabelled cation by identically processed, unstimulated cells. The effects of CGS 21680 (1 μM), dibutyryl cyclic AMP (4 mM), rolipram (0.5 μM) and SKF 96365 (10 μM) on PAF-activated influx of 45Ca2+ into neutrophils were also investigated.
Reactions were stopped by adding 10 ml ice-cold Ca2+-replete HBSS to the tubes. The cells were then pelleted by centrifugation at 400 g for 5 min followed by washing with ice-cold Ca2+-replete HBSS and the cell pellets finally dissolved in 0.5 ml of Triton X-100/0.05 M NaOH and the radioactivity assayed in a scintillation spectrometer.
Superoxide production
This was measured by use of a lucigenin (bis-N-methylacridinium nitrate)-enhanced chemiluminescence (LECL) method (Minkenberg & Ferber, 1984). Neutrophils (106 ml−1) were preincubated for 15 min at 37°C in 900 μl HBSS (pH 7.4, 1.25 mM CaCl2) containing 0.2 mM lucigenin after which PAF (20 and 200 nM) or FMLP (1 μM) was added. LECL responses were measured using a model 2010R Biocounter (Lumac Systems, Titusville, Flo, U.S.A.) and the results expressed as relative light units (r.l.u.).
Elastase release
Neutrophil degranulation was measured according to the extent of release of the primary granule-derived enzyme, elastase. PAF (200 nM) was added to neutrophils which were preincubated at a concentration of 2×106 ml−1 in HBSS for 10 min at 37°C. FMLP, (0.1 μM final) in combination with a submaximal concentration of cytochalasin B (CB, 1 μM), was then added to the cells, which were incubated for a further 15 min at 37°C. The tubes were then transferred to an ice bath, followed by centrifugation at 400 g for 5 min to pellet the cells. The neutrophil-free supernatants were then decanted and assayed for elastase activity using a micromodification of a standard colourimetric procedure (Beatty et al., 1982). Briefly, 125 μl of supernatant were added to an equal volume of the elastase substrate N-succinyl-L-alanyl-L-alanyl-L-alanine-p-nitroanilide (3 mM) in 0.05 M Tris-HCl (pH 8.0) and elastase activity monitored at a wavelength of 405 nm.
The effects of dibutyryl cyclic AMP (4 mM), CGS 21680 (1 μM) and rolipram (0.5 μM) on the release of elastase from neutrophils exposed to PAF (200 nM) were also investigated. These agents were added to the neutrophils 5 min prior to addition of PAF.
Membrane potential
The potential sensitive fluorescent dye, dipentyloxacarbocyanine (di-O-C5(3)) was used to measure changes in membrane potential in activated neutrophils (Seligmann & Gallin, 1980). The cells (106 ml−1) were preincubated for 10 min at 37°C in HBSS containing 80 nM di-O-C5(3), after which they were transferred to disposable reaction cuvettes which were maintained at 37°C in a Hitachi 650 10S fluorescence spectrophotometer with excitation and emission wavelengths set at 460 nm and 510 nm respectively. The neutrophils were then activated with FMLP (1 μM) or PAF (20 and 200 nM) and subsequent alterations in fluorescence intensity monitored over a 5 – 10 min period.
Measurement of Intracellular cyclic AMP
Neutrophils (107 ml−1) in HBSS were preincubated for 10 min at 37°C with and without the phosphodiesterase inhibitor, rolipram (0.5 μM). Following preincubation, the cells were activated with PAF (20 and 200 nM), in a final volume of 1 ml. The reactions were terminated and the cyclic AMP extracted by the addition of ice-cold ethanol (65% v v−1) at 0, 10 and 30 s and 1, 3 and 5 min after addition of the PAF. The resultant precipitates were washed twice (2000 g for 15 min at 4°C) with ice-cold ethanol and the supernatants pooled and evaporated at 60°C under a stream of air. The dried extracts were reconstituted in assay buffer (0.05 M acetate buffer, pH 5.8) and assayed for cyclic AMP using the Biotrak cyclic AMP [125I] scintillation proximity assay system (Amersham International plc.), which is a competitive binding radioimmunoassay procedure. These results are expressed as pmoles cyclic AMP 10−7 neutrophils.
Drugs and reagents
CGS 21680 and rolipram were kindly provided by Dr Malcolm Johnson (Glaxo Smith Kline, Stockley Park West, London, U.K.), while all other chemicals and reagents were purchased from the Sigma Chemical Co. Dimethyl sulphoxide (DMSO) was used as the solvent for PAF, rolipram, SKF 96365 and U 73122 and appropriate solvent control systems were included for these agents.
Statistical analysis
The results of each series of experiments are expressed as the mean value±the standard error of the mean (s.e.mean). Where appropriate, levels of statistical significance were calculated using the Mann-Whitney test. P values of ⩽0.05 were considered significant.
Results
Fura-2 responses of neutrophils
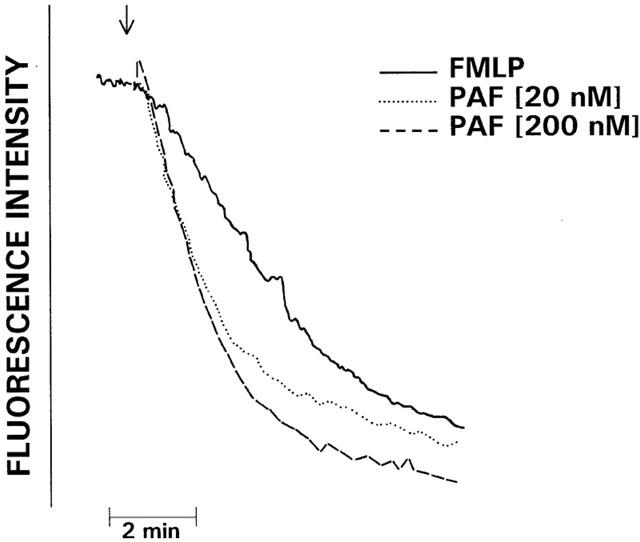
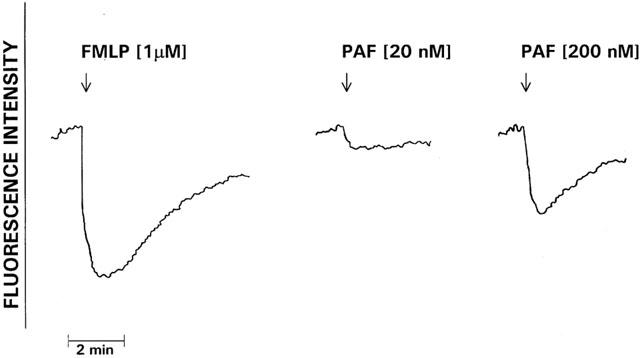
In preliminary experiments, PAF at concentrations of 20 pmol and higher caused dose-related, transient, increases in neutrophil cytosolic Ca2+ which were maximal at 20 nM. Concentrations of 20 and 200 nM were used in all subsequent experiments. The traces shown in Figure 1 are representative of the FMLP (1 μM)- and PAF (20 and 200 nM)-activated fura-2 responses of neutrophils. Addition of FMLP or PAF to neutrophils was accompanied by an abrupt increase in fura-2 fluorescence intensity due to elevations in the cytosolic concentrations of Ca2+, which were of similar magnitude for each activator. In the case of FMLP, peak fluorescence intensity occurred at around 10 s and was followed by an initially linear decrease in fluorescence intensity, reflecting clearance of Ca2+ from the cytosol, which reached baseline values approximately 5 min after addition of FMLP. However, in the case of PAF-treated neutrophils, peak cytosolic Ca2+ concentrations were either sustained for longer (200 nM PAF) or subsided briefly to be followed by a second peak (20 nM PAF). In both cases, the prolonged peaks (relative to FMLP) in fluorescence intensity were followed by an initially linear decrease in fluorescence. The effects of EGTA (5 mM), SKF 96365, PMA and U 73122 on the fura-2 responses of PAF (200 nM)-activated neutrophils are shown in Figure 2. EGTA, SKF 96365 and PMA did not affect the peak increases in cytosolic Ca2+ in PAF-activated neutrophils. However, all three agents abolished the prolongation in peak fluorescence intensity, suggesting that this is due to influx of extracellular Ca2+. Treatment of neutrophils with U 73122 completely abrogated the PAF-activated increase in cytosolic Ca2+.
Figure 1.
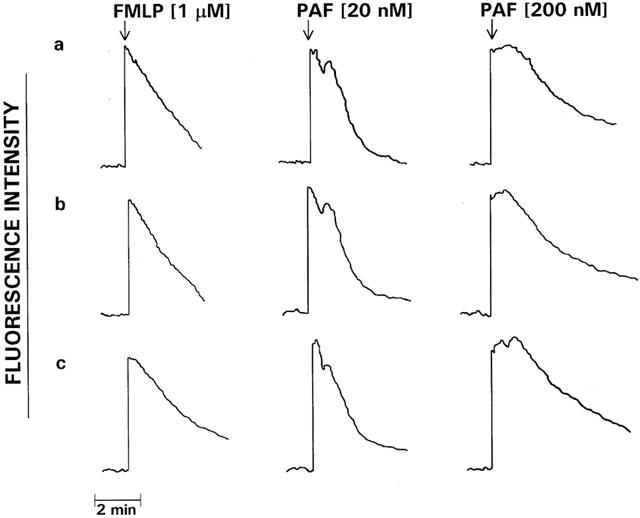
Fura-2 fluorescence responses of FMLP (1 μM)- and PAF (20 and 200 nM)-activated neutrophils. Following loading with fura-2, the cells were preincubated for 10 min at 37°C prior to the addition of FMLP or PAF and subsequent alterations in fluorescence intensity monitored over a 5 min period. The results shown are three typical sets of traces of 15 obtained. The arrow (↓) denotes addition of FMLP or PAF.
Figure 2.
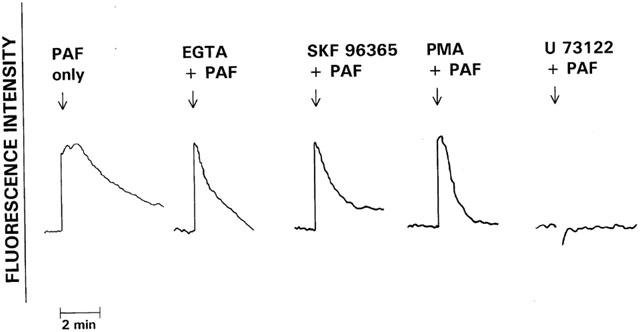
The effects of pretreatment with EGTA (5 mM), SKF 96365 (10 μM), PMA (40 nM) and U 73122 (10 μM) on the fura-2 responses of PAF (200 nM)-activated neutrophils. The responses of cells treated with these various agents were compared with those of the corresponding control system activated with PAF in the absence of these agents. The results shown are representative traces of between three and six sets of results obtained. The arrow (↓) denotes addition of PAF.
EGTA and SKF 96365 also dramatically hastened the return to basal fura-2 fluorescence intensity in FMLP-activated neutrophils without affecting the magnitude of peak fluorescence intensity, while in the case of cells treated with U 73122 there was complete attenuation of the fura-2 fluorescence response (not shown).
The effects of dibutyryl cyclic AMP (4 mM), rolipram (0.5 μM) and CGS 21680 (1 μM) on the fura-2 fluorescence responses of PAF (200 nM)-activated neutrophils are shown in Figure 3. Although none of the test agents affected the peak changes in fluorescence, all three agents, in particular dibutyryl cyclic AMP and CGS 21680, hastened the rate of decline in peak fluorescence intensity, indicative of accelerated clearance of Ca2+ from the cytosol.
Figure 3.
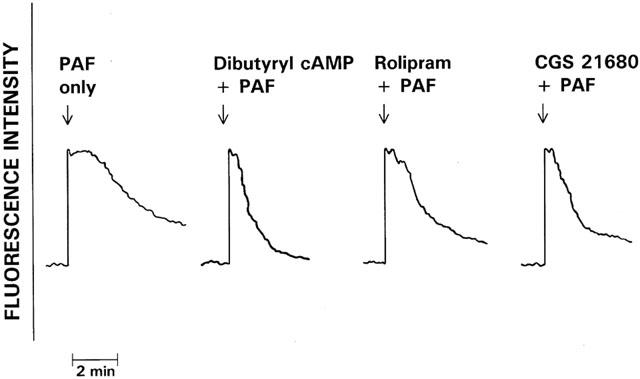
The effects of pretreatment with dibutyryl cyclic AMP (4 mM), rolipram (0.5 μM) and CGS 21680 (1 μM) on the fura-2 responses of PAF (200 nM)-activated neutrophils. The responses of cells treated with these various agents were compared with those of the corresponding control system activated with PAF in the absence of these agents. The results shown are representative traces of 12 sets of results obtained. The arrow (↓) denotes addition of PAF.
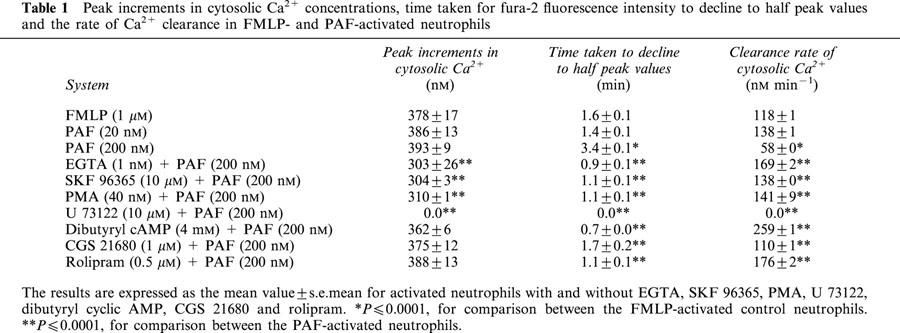
Peak increments in cytosolic Ca2+ concentrations, time taken for fluorescence intensity to decline to half peak values, as well as the rate of Ca2+ clearance for neutrophils from eight different individuals activated with FMLP or PAF (200 nM), in the absence or presence of EGTA, SKF 96365, PMA, U 73122, CGS 21680, dibutyryl cyclic AMP and rolipram, are shown in Table 1.
Table 1.
Peak increments in cytosolic Ca2+ concentrations, time taken for fura-2 fluorescence intensity to decline to half peak values and the rate of Ca2+ clearance in FMLP- and PAF-activated neutrophils
Influx of Ca2+ using Mn2+ quenching of fura-2 fluorescence
The results of the indirect measurement of Ca2+ influx into FMLP- and PAF-activated neutrophils using Mn2+ quenching of fluorescence are shown in Figure 4. In the case of FMLP-activated cells, addition of the chemoattractant was accompanied by a lag phase of about 20 – 30 s duration followed by an almost linear decrease, indicative of influx of Ca2+, over 3 – 4 min. In the case of PAF-activated neutrophils, the decrease in fluorescence intensity occurred almost immediately and proceeded at a much faster rate than that observed in FMLP-activated cells.
Figure 4.
FMLP (1 μM)- and PAF (20 and 200 nM)-activated Mn2+-quenching of the fura-2 responses of neutrophils. A direct relationship exists between the magnitude of Mn2+-quenching of fura-2 fluorescence and the extent of store-operated influx of Ca2+ into the cells. The results shown are representative traces of five sets of results obtained. The arrow (↓) denotes addition of FMLP or PAF.
Efflux of 45Ca2+
The kinetics of efflux of Ca2+ from PAF (20 and 200 nM)- and FMLP (1 μM)-activated neutrophils over a 60 s time-course are shown in Figure 5. No significant loss of 45Ca2+ was observed in the unstimulated, control neutrophils over the 60 s incubation period during which efflux was measured. Exposure of the neutrophils to PAF and FMLP resulted in rapid efflux of the radiolabelled cation from the neutrophils in the first 10 s incubation period, followed by a slow efflux of Ca2+ to a maximum loss of 43, 54 and 42% of cell-associated cation for 20 nM, 200 nM PAF and 1 μM FMLP respectively at the end of the 60 s time-course of the experiment.
Figure 5.
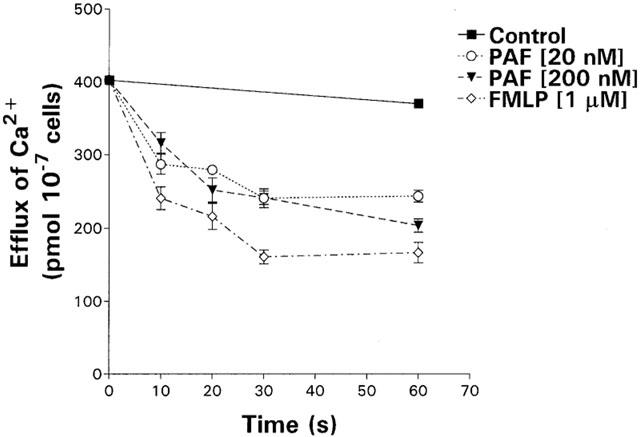
Kinetics of efflux of 45Ca2+ from unstimulated neutrophils and from neutrophils activated with PAF (20 and 200 nM) or FMLP (1 μM) over a 60 s time-course. The results of three different experiments are expressed as the mean amount of cell-associated 45Ca2+ (pmol 10−7 cells) with vertical lines showing s.e.mean.
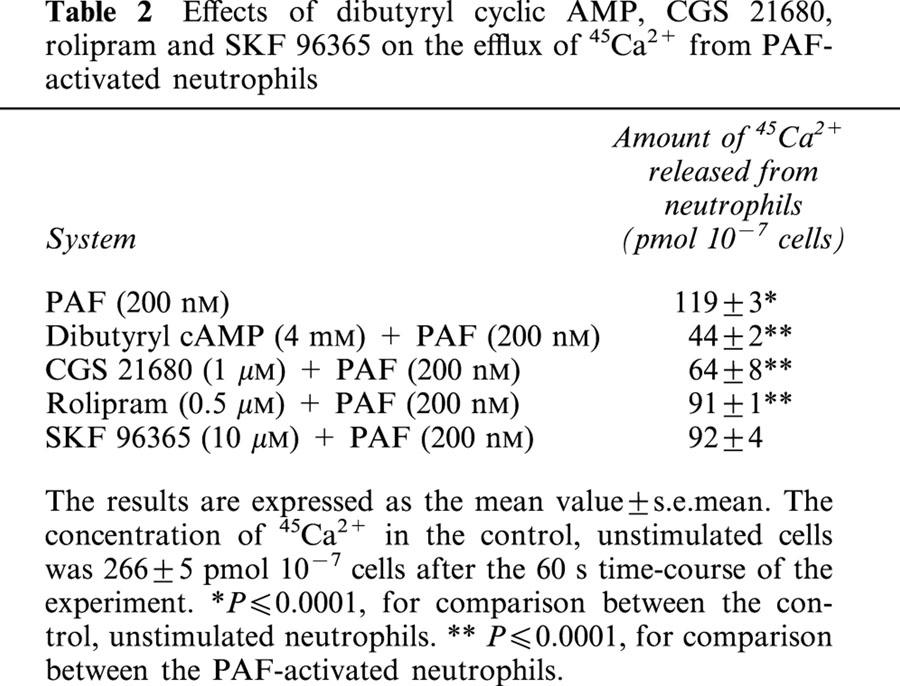
The effects of dibutyryl cyclic AMP, CGS 21680, rolipram and SKF 96365 on the efflux of 45Ca2+ from PAF-activated neutrophils are shown in Table 2. Pre-treatment of the neutrophils with dibutyryl cyclic AMP, CGS 21680 or rolipram significantly reduced the extent of PAF-mediated efflux of 45Ca2+ from the cells, while the discharge of the cation was not significantly affected by SKF 96365.
Table 2.
Effects of dibutyryl cyclic AMP, CGS 21680, rolipram and SKF 96365 on the efflux of 45Ca2+ from PAF-activated neutrophils
Influx of 45Ca2+
The kinetics of influx of 45Ca2+ into PAF- and FMLP-activated neutrophils are shown in Figure 6. Modest influx of 45Ca2+ (64 pmol 10−7 cells) was observed in control neutrophils over the 5 min time-course while, in the case of FMLP- and PAF-activated neutrophils, significant influx of Ca2+ above this level was noted (P⩽0.0001). The net influx of Ca2+ into FMLP- and PAF (20 and 200 nM)-activated neutrophils was 275±7 pmol 10−7 cells, 175±27 pmol 10−7 cells and 293±62 pmol 10−7 cells, respectively.
Figure 6.
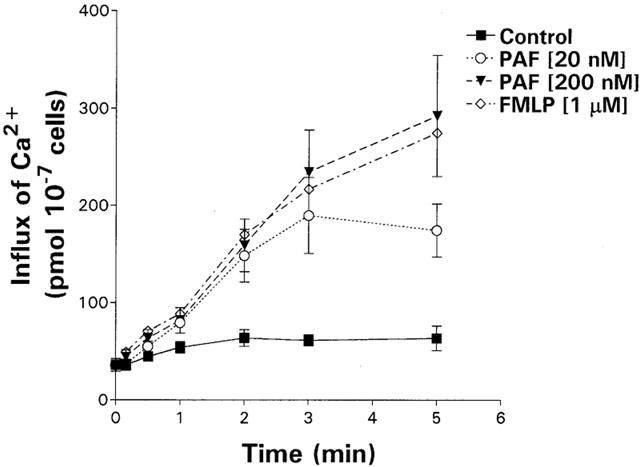
Kinetics of influx of 45Ca2+ into unstimulated neutrophils and into cells activated with PAF (20 and 200 nM) or FMLP (1 μM) over a 5 min time-course. The results of four different experiments are expressed as the mean amount of cell-associated 45Ca2+ (pmol 10−7 cells) with vertical lines showing s.e.mean.
The effects of CGS 21680, dibutyryl cyclic AMP, rolipram and SKF 96365 on influx of 45Ca2+ into PAF-activated neutrophils are shown in Table 3. Influx of 45Ca2+ into PAF-activated neutrophils was almost completely attenuated by SKF 96365, and partly attenuated by dibutyryl cyclic AMP, rolipram and CGS 21680. These findings were confirmed using the Mn2+ quenching of fura-2 fluorescence procedure (not shown).
Table 3.
Effects of dibutyryl cyclicAMP, CGS 21680, rolipram and SKF 96365 on the influx of 45Ca2+ into PAF-activated neutrophils
Superoxide generation
PAF, at the concentrations used in this study, did not activate oxidant production by neutrophils. The absolute peak values±s.e.mean for superoxide production observed over a 5 min time-course for the control system (in the absence of FMLP or PAF) and FMLP (1 μM)-activated neutrophils were 3929±245 and 16066±857 r.l.u., while those for PAF at concentrations of 20 and 200 nM were 3766±287 and 3853±297 r.l.u., respectively.
Elastase release
Although PAF per se did not activate the release of elastase from neutrophils, this phospholipid, at a concentration of 200 nM, caused significant enhancement of elastase release from FMLP/CB-activated neutrophils (P⩽0.001). The effects of pre-incubation of neutrophils with dibutyryl cyclic AMP, CGS 21680 and rolipram on the release of elastase from PAF (200 nM)-sensitised, FMLP/CB-activated neutrophils are shown in Table 4. All three of these agents caused significant (P⩽0.001), but incomplete, attenuation of the sensitising effects of PAF on elastase release from neutrophils.
Table 4.
Effects of dibutyryl cyclic AMP, CGS 21680 and rolipram on the release of elastase by PAF-treated FMLP/CB-activated neutrophils
Membrane potential
FMLP- and PAF-activated alterations in neutrophil membrane potential are shown in Figure 7. Exposure of neutrophils to FMLP resulted in rapid membrane depolarization which terminated at around 30 s and was followed approximately 30 – 60 s later by gradual membrane repolarization which was complete by 5 min, but which did not recover to preactivation values. In contrast, the PAF-activated decrease in membrane potential was considerably less (averages of 16±1% and 46±5% of the FMLP response for 20 nM and 200 nM PAF respectively).
Figure 7.
Alterations in neutrophil membrane potential following activation of the cells with PAF (20 and 200 nM) or FMLP (1 μM). The neutrophils were preincubated for 10 min at 37°C with the potential-sensitive, fluorescent dye di-O-C5(3) followed by addition of PAF or FMLP (denoted by ↓) and measurement of alterations in fluorescence intensity. Membrane depolarization is documented as a decrease in fluorescence intensity, while recovery towards pre-activation values demonstrates repolarization. The results shown are representative traces from one of five separate experiments.
Intracellular cyclic AMP levels
Intracellular cyclic AMP levels in control and rolipram (0.5 μM)-treated unstimulated neutrophils were 122±8 and 321±28 pmol 10−7 cells immediately prior to the addition of PAF (200 nM) to the cells and were not significantly altered throughout the subsequent 5 min time-course of the experiment, the maximum values for PAF-treated cells in the absence and presence of rolipram being 135±8 and 351±38 pmol 10−7 cells respectively (data from five experiments).
Discussion
Although both FMLP and PAF activate neutrophils via binding to members of the seven trans-membrane spanning G-protein coupled receptor family, the repertoire of functional responses elicited by these chemoattractants is distinctly different (Nick et al., 1997). Activation of neutrophils with FMLP elicits a broad range of responses including Ca2+ mobilization, membrane depolarization, actin assembly, adhesion to vascular endothelium, chemotaxis, superoxide generation, granule enzyme release and synthesis of cyclic AMP (Snyderman & Uhing, 1992). Exposure of neutrophils to PAF, on the other hand, is accompanied by receptor-mediated activation of a more limited range of responses, with no meaningful alterations in superoxide generation, granule enzyme release or intracellular cyclic AMP (Nick et al., 1997; Ali et al., 1998).
These differences in the profiles of the proinflammatory responses of human neutrophils activated by FMLP and PAF have been attributed to differential utilization of G proteins by the two chemoattractants. FMLP utilizes a receptor-associated, pertussis-sensitive G-protein which initiates a series of events leading to activation of p38 and p42/44 mitogen-activated protein kinases (MAPk), while the PAF receptor is coupled to a G protein, which is at least partially pertussis insensitive and results in predominant activation of p38 MAPk (Nick et al., 1997; Ali et al., 1998).
The inability of PAF to cause meaningful receptor-mediated activation of NADPH oxidase and adenylate cyclase suggests that the mechanisms used by neutrophils to restore Ca2+ homeostasis following exposure to this proinflammatory phospholipid may differ from those utilized by FMLP-activated cells. FMLP-activation of neutrophils is accompanied by activation of phospholipase C and inositol triphosphate-mediated mobilization of Ca2+ from intracellular stores (Prentki et al., 1984). Ca2+ overload is prevented by the combined action of the calmodulin-dependent plasma membrane Ca2+-ATPase (Lagast et al., 1984) and the cyclic AMP-dependent protein kinase-upregulated Ca2+-ATPase (Tao et al., 1992) which promote efflux and resequestration of cytosolic Ca2+ respectively. The efficiency of these cytosolic Ca2+ clearance pumps is potentiated by the electrogenic, membrane depolarizing action of NADPH oxidase, which restricts the influx of extracellular cation by abolishing the electrical component of the electrochemical gradient for Ca2+ (di virgilio et al., 1987; Geiszt et al., 1997; Tintinger et al., 2001).
In the current study, activation of neutrophils with FMLP (1 μM) resulted in the characteristic, immediate increase in cytosolic Ca2+ which was completely abolished by pre-treatment of the cells with U 73122, underscoring the dependence of this event on activation of phospholipase C. As reported previously, the peak concentration of cytosolic Ca2+ in FMLP-activated neutrophils was short-lived (of about 10 s duration) and subsided rapidly, reaching basal levels within several minutes due to efflux and resequestration of the cation (Anderson & Goolam Mahomed, 1997). Net influx of the cation was only detectable at around 30 – 60 s, proceeded over a 5 min time-course coincident with membrane repolarization as reported previously (Anderson & Goolam Mahomed, 1997; Tintinger et al., 2001), and was abolished by treatment of the cells with SKF 96365, all of which support a store-operated uptake mechanism. Although the effects of EGTA and PMA were not investigated in the current study, both agents have been reported to attenuate the store-operated influx of Ca2+ into FMLP-activated neutrophils, underscoring the respective requirements for extracellular Ca2+ and membrane repolarization in this process (di virgilio et al., 1987; Geiszt et al., 1997).
Although not shown in the current study, we have observed that activation of neutrophils with a lower concentration of FMLP (0.01 μM), which did not activate superoxide generation and caused only modest membrane depolarization, resulted in accelerated influx of the Ca2+ relative to the corresponding responses of cells activated with 1 μM of the chemoattractant. These observations appear to support the proposed relationship between activation of NADPH oxidase resulting in membrane depolarization and restricted influx of extracellular Ca2+. Activation of neutrophils with PAF (20 and 200 nM) also resulted in U 73122-inhibitable mobilization of Ca2+ from intracellular stores, the peak cytosolic concentrations of the cation being similar in magnitude to those observed with FMLP. However, in contrast to FMLP, peak levels of cytosolic Ca2+ were sustained for about 1 min in PAF-activated neutrophils. This relative prolongation of the peak cytosolic Ca2+ response in PAF-activated neutrophils was not a consequence of delayed/decreased extrusion of the cation since the magnitude and kinetics of efflux of Ca2+ were similar to those observed with FMLP-activated neutrophils. Accelerated influx of the cation as an alternative mechanism of prolongation of the peak cytosolic Ca2+ concentration was supported by kinetic data from Ca2+ influx experiments using both the radiometric and Mn2+ quenching of fura-2 fluorescence procedures. This contention was also supported by observations that the sustained elevations in cytosolic Ca2+ in PAF-activated neutrophils were attenuated by pre-treatment of the cells with SKF 96365, or by inclusion of EGTA in the cell-suspending medium.
PAF, at the highest concentration used (200 nM), depolarized the neutrophil membrane. However, the magnitude of the depolarization response was considerably less (<50%) than that observed with FMLP-activated neutrophils, being insufficient to prevent early influx of extracellular Ca2+. Moreover, exposure of neutrophils to PAF was not accompanied by detectable superoxide production by the cells, suggesting the existence of a mechanistic relationship between failure of the phospholipid to activate NADPH oxidase (with consequent inadequate membrane depolarization) and accelerated influx of Ca2+. This was supported by data from experiments in which neutrophils were coactivated with PAF and PMA, the latter being a potent activator of NADPH oxidase which does not cause fluctuations in cytosolic Ca2+ (Naccache et al., 1985; Goolam Mahomed & Anderson, 2000). PMA, which caused membrane depolarization equivalent to or greater than that mediated by FMLP, did not affect the PAF-mediated abrupt increase in cytosolic Ca2+, but did attenuate the prolongation of peak levels of the cation.
The mechanism of the membrane depolarization response observed in PAF-activated neutrophils remains to be established, but does not appear to be due to trivial activation of NADPH oxidase since it was unaffected by treatment of the cells with staurosporine, a potent inhibitor of superoxide production by phagocytes, nor was it attenuated in PAF-treated neutrophils from two individuals with chronic granulomatous disease (results not included).
The failure of PAF to activate adenylate cyclase in neutrophils, as previously described by others (Ali et al., 1998), is in contrast to the well-documented transient increase in cyclic AMP observed in FMLP-activated cells (Iannone et al., 1989; Goolam Mahomed et al., 1998). This transient increase in cyclic AMP has been linked to deactivation of the proinflammatory activities of neutrophils as a consequence of up-regulation of the activity of the endo-membrane Ca2+-ATPase (Anderson et al., 1998) and/or inactivation of phospholipase C (Ali et al., 1998). We reasoned that the inability of PAF to activate adenylate cyclase may also contribute to prolonged Ca2+ transients in neutrophils through failure to up-regulate the endo-membrane Ca2+-ATPase. This contention was supported by observations, from fura-2 fluorescence experiments, that dibutyryl cyclic AMP, rolipram and CGS 21680 hastened the clearance of Ca2+ from the cytosol of PAF-activated neutrophils, without affecting release from stores, as has previously been reported for FMLP-activated neutrophils (Anderson et al., 1998, 2000). Using the radiometric procedure, all three agents were found to reduce the magnitude of both the PAF-activated efflux and influx of Ca2+. These observations are compatible with up-regulation of the endo-membrane Ca2+-ATPase, which in turn favours re-sequestration of cytosolic Ca2+ with a corresponding reduction in magnitude of both efflux and store-operated influx of the cation (Anderson et al., 1998, 2000). The lesser activity of rolipram, relative to that of CGS 21680, which activates adenylate cyclase via subtype A2A receptors, and dibutyryl cyclic AMP, in potentiating the clearance of Ca2+ from the cytosol of PAF-activated neutrophils, may be explained by the inability of the phospholipid to activate synthesis of cyclic AMP. It is noteworthy, however, that cyclic AMP-dependent protein kinase has been reported to inhibit phospholipase C (Yue et al., 1998), a mechanism, which if operative in activated neutrophils, may also contribute to the reduction in cytosolic Ca2+ concentrations in neutrophils treated with dibutyryl cyclic AMP, CGS 21680 or rolipram.
The functional consequences of the altered handling of Ca2+ by PAF-activated neutrophils (relative to FMLP and possibly other chemoattractants) remain to be conclusively established, but may result in hyper-adhesion of the cells to vascular endothelium (Ruchaud-Sparagano et al., 2000) or ‘priming' of other pro-inflammatory activities of the cells such as oxidant production and release of granule enzymes (Zimmerman et al., 1992). A possible relationship between PAF-mediated mobilization of Ca2+ and ‘priming' of neutrophils for activation with a second stimulus is supported by the observations that dibutyryl cyclic AMP, CGS 21680 and rolipram all attenuated the sensitizing effects of PAF on FMLP/CB-activated release of elastase by the cells. These findings also suggest that cyclic AMP-elevating agents, presumably by enhancement of clearance of Ca2+ from the cytosol of activated neutrophils, may represent a possible therapeutic alternative to PAF-receptor antagonists and -hydrolyzing enzymes (Kuijpers et al., 2001).
In conclusion, the results of the current study demonstrate that the efficiency of clearance of Ca2+ from the cytosol of PAF-activated human neutrophils is not optimal due to the failure of the proinflammatory phospholipid to activate adenylate cyclase and NADPH oxidase. The resultant prolongation of the peak cytosolic Ca2+ concentration, which is amenable to attenuation by cyclic AMP-elevating agents, may contribute to hyper-activity of the cells.
Abbreviations
- cyclic AMP
adenosine 3′,5′ cyclic monophosphate
- CB
cytochalasin B
- CGS 21680
2(4-[(2-carboxyethyl)phenyl]ethylamino)-5′-N-ethylcarboxamido adenosine
- Di-O-C5(3)
dipentyloxacarbocyanine
- DMSO
dimethyl sulphoxide
- EGTA
ethylene glycol-bis(β-aminoethyl ether) N,N,N′,N′-tetraacetic acid
- FMLP
N-formyl-L-methionyl-L-leucyl-L-phenylalanine
- HBSS
Hanks balanced salt solution
- LECL
lucigenin-enhanced chemiluminescence
- MAPk
mitogen-activated protein kinase
- PBS
phosphate-buffered saline
- PLC
phospholipase C
- PMA
phorbol 12-myristate 13-acetate
- r.l.u.
relative light units
- SKF 96365
(1-[β-)3-[4-methoxyphenyl]-1H-imidazole hydrochloride
- U 73122
(1-[6-([17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino) hexyl]-1H-pyrrole-2,5-dione
References
- ALI H., SOZZANI S., FISHER I., BARR A.J., RICHARDSON R.M., HARIBABU B., SNYDERMAN R. Differential regulation of formyl peptide and platelet-activating factor receptors: Role of phospholipase Cβ3 phosphorylation by protein kinase A. J. Biol. Chem. 1998;273:11012–11016. doi: 10.1074/jbc.273.18.11012. [DOI] [PubMed] [Google Scholar]
- ANDERSON R., GOOLAM MAHOMED A. Calcium efflux and influx in f-met-leu-phe (fMLP)-activated human neutrophils are chronologically distinct events. Clin. Exp. Immunol. 1997;110:132–138. doi: 10.1046/j.1365-2249.1997.5051403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON R., GOOLAM MAHOMED A., THERON A.J., RAMAFI G., FELDMAN C. Effect of rolipram and dibutyryl cyclic AMP on resequestration of cytosolic calcium in FMLP-activated human neutrophils. Br. J. Pharmacol. 1998;124:547–555. doi: 10.1038/sj.bjp.0701849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON R., VISSER S.S., RAMAFI G., THERONA J. Accelerated resequestration of cytosolic calcium and suppression of the pro-inflammatory activities of human neutrophils by CGS 21680 in vitro. Br. J. Pharmacol. 2000;130:717–724. doi: 10.1038/sj.bjp.0703344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARBABI S., ROSENGART M.R., GARCIA I., JELACIC S., MAIER R.V. Priming interleukin 8 production. Role of platelet-activating factor and p38. Arch. Surg. 1999;134:1348–1353. doi: 10.1001/archsurg.134.12.1348. [DOI] [PubMed] [Google Scholar]
- BARNES P.J., CHUNG K.F. PAF closely mimics the pathology of asthma. Trends Pharmacol. Sci. 1987;8:285–288. [Google Scholar]
- BEATTY K., ROBERTIE P., SENIOR R.M., TRAVIS J. Determination of oxidized alpha-1-proteinase inhibitor in serum. J. Lab. Clin. Med. 1982;100:186–192. [PubMed] [Google Scholar]
- DI VIRGILIO F., LEW P.D., ANDERSSONT T., POZZAN T. Plasma membrane potential modulates chemotactic peptide-stimulated cytosolic free Ca2+ changes in human neutrophils. J. Biol. Chem. 1987;262:4574–4579. [PubMed] [Google Scholar]
- GEISZT M., KAPUS A., NÉMET K., FARKAS L., LIGETI E. Regulation of capacitative Ca2+ influx in human neutrophil granulocytes. Alterations in chronic granulomatous disease. J. Biol. Chem. 1997;272:26471–26478. doi: 10.1074/jbc.272.42.26471. [DOI] [PubMed] [Google Scholar]
- GOOLAM MAHOMED A., ANDERSON R. Activation of human neutrophils with chemotactic peptide, opsonized zymosan and the calcium ionophore A23187, but not with a phorbol ester, is accompanied by efflux and store-operated influx of calcium. Inflammation. 2000;24:559–569. doi: 10.1023/a:1007029524141. [DOI] [PubMed] [Google Scholar]
- GOOLAM MAHOMED A., THERON A.J., ANDERSON R., FELDMAN C. Anti-oxidative effects of theophylline on human neutrophils involve cyclic nucleotides and protein kinase A. Inflammation. 1998;22:545–557. doi: 10.1023/a:1022306328960. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HILGER R.A., KOLLER M., KONIG W. Inhibition of leukotriene formation and IL-8 release by the PAF-receptor antagonist SM-12502. Inflammation. 1996;20:57–70. doi: 10.1007/BF01487745. [DOI] [PubMed] [Google Scholar]
- IANNONE M.A., WOLBERG G., ZIMMERMAN T.P. Chemotactic peptide induces cAMP elevation in human neutrophils by amplification of the adenylate cyclase response to endogenously produced adenosine. J. Biol. Chem. 1989;264:20177–20180. [PubMed] [Google Scholar]
- KRAVCHENKO V.V., PAN Z., HAN J., HERBERT J-M., ULEVITCH R.J., YE R.D. Platelet-activating factor induces NF-κB activation through a G protein-coupled pathway. J. Biol. Chem. 1995;270:14928–14934. doi: 10.1074/jbc.270.25.14928. [DOI] [PubMed] [Google Scholar]
- KUIJPERS T.W., VAN DEN BERG J.M., TOOL A.T., ROOS D. The impact of platelet-activating factor (PAF)-like mediators on the functional activity of neutrophils: anti-inflammatory effects of human PAF-acetylhydrolase. Clin. Exp. Immunol. 2001;123:412–420. doi: 10.1046/j.1365-2249.2001.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAGAST H., LEW D.P., WALDVOGEL F.A. Adenosine triphosphatase-dependent calcium pump in the plasma membrane of guinea pig and human neutrophils. J. Clin. Invest. 1984;73:107–115. doi: 10.1172/JCI111180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERRITT J.E., ARMSTRONG W.P., BENHAM C.D., HALLAM T.J., JACOB R., JAXA-CHAMIEC A., LEIGH B.K., MCCARTHY S.A., MOORES K.E., RINK T.J. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem. J. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINKENBERG I., FERBER E. Lucigenin-dependent chemiluminescence as a new assay for NADPH-oxidase activity in particulate fractions of human polymorphonuclear leukocytes. J. Immunol. Methods. 1984;71:61–67. doi: 10.1016/0022-1759(84)90206-0. [DOI] [PubMed] [Google Scholar]
- NACCACHE P.H., MOLSKI T.F.P., BORGEAT P., WHITE J.R., SHA'AFI R.I. Phorbol esters inhibit the f-met-leu-phe and leukotriene B4-stimulated calcium mobilization and enzyme secretion in rabbit neutrophils. J. Biol. Chem. 1985;260:2125–2131. [PubMed] [Google Scholar]
- NICK J.A., AVDI N.J., YOUNG S.K., KNALL C., GERWINS P., JOHNSON G.L., WORTHEN G.S. Common and distinct intracellular signalling pathways in human neutrophils utilized by platelet activating factor and FMLP. J. Clin. Invest. 1997;99:975–986. doi: 10.1172/JCI119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEPLOW P.V. Regulation of platelet-activating factor (PAF) activity in human diseases by phospholipase A2 inhibitors, PAF acetylhydrolases, PAF receptor antagonists and free radical scavengers. Prostaglandins Leukot. Essent. Fatty Acids. 1999;61:65–82. doi: 10.1054/plef.1999.0038. [DOI] [PubMed] [Google Scholar]
- PETTIT E.J., HALLETT M.B. Nonuniform distribution of Ca(2+) uptake sites within human neutrophils. Biochem. Biophys. Res. Commun. 2000;279:337–340. doi: 10.1006/bbrc.2000.3954. [DOI] [PubMed] [Google Scholar]
- PRENTKI M., WOLLHEIM C.B., LEW D.P. Ca2+ homeostasis in permeabilized human neutrophils: characterisation of Ca2+ sequestering pools and the action of inositol 1,4,5-triphosphate. J .Biol. Chem. 1984;259:13777–13782. [PubMed] [Google Scholar]
- RUCHAUD-SPARAGANO M-H., WALKER T.R., ROSSI A.G., HASLETT C., DRANSFIELD I. Soluble E-selectin acts in synergy with platelet-activating factor to activate neutrophil β2-integrins. J. Biol. Chem. 2000;275:15758–15764. doi: 10.1074/jbc.M907390199. [DOI] [PubMed] [Google Scholar]
- SELIGMANN B.E., GALLIN J.I. Use of lipophilic probes of membrane potential to assess human neutrophil activation. Abnormality in chronic granulomatous disease. J. Clin. Invest. 1980;66:493–503. doi: 10.1172/JCI109880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHINDO K., KOIDE K., FUKUMURA M. Platelet-activating factor increases leukotriene B4 release in stimulated alveolar macrophages from asthmatic patients. Eur. Respir. J. 1998;11:1098–1104. doi: 10.1183/09031936.98.11051098. [DOI] [PubMed] [Google Scholar]
- SNYDERMAN R., UHING R.J.Chemoattractant stimulus-response coupling Inflammation: Basic Principles and Clinical Correlates 1992New York: Raven Press; 421–439.2nd edn. Ed. Gallin, J.I., Goldstein, I.M. & Snyderman, R. pp [Google Scholar]
- TATRAI A., LEE S.K., STERN P.H. U-73122, a phospholipase C antagonist, inhibits effects of endothelin-1 and parathyroid hormone on signal transduction in UMR-106 osteoblastic cells. Biochim. Biophys. Acta. 1994;1224:575–582. doi: 10.1016/0167-4889(94)90296-8. [DOI] [PubMed] [Google Scholar]
- TAO J., JOHANSSON J.S., HAYNES D.H. Stimulation of dense tubular Ca2+ uptake in human platelets by cAMP. Biochim. Biophys. Acta. 1992;110:29–39. doi: 10.1016/0005-2736(92)90159-j. [DOI] [PubMed] [Google Scholar]
- TINTINGER G.R., THERON A.J., STEEL H.C., ANDERSON R. Accelerated calcium influx and hyperactivation of neutrophils in chronic granulomatous disease. Clin. Exp. Immunol. 2001;123:254–263. doi: 10.1046/j.1365-2249.2001.01447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUE C., DODGE K.L., WEBER G., SANBORN B.M. Phosphorylation of serine 1105 by protein kinase A inhibits phospholipase Cβ3 stimulation by Gαq. J. Biol. Chem. 1998;273:18023–18027. doi: 10.1074/jbc.273.29.18023. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN G. A., PRESCOTT S.M., MCINTYRE T.M.Platelet-activating factor: a fluid and cell-associated mediator of inflammation Inflammation: Basic Principles and Clinical Correlates 1992New York: Raven Press; 149–176.2nd edn. Ed. Gallin, J.I., Goldstein, I.M. & Snyderman, R. pp [Google Scholar]


