Abstract
The effects of sodium nitroprusside (SNP) and 8-bromo-guanosine 3′5′ cyclic monophosphate (8-Br-cyclic GMP) on nerve-mediated and acetylcholine (ACh)-evoked amylase secretion, tritiated choline ([3H]-choline) release and on intracellular free calcium concentration ([Ca2+]i) in the isolated rat pancreas were investigated.
Electrical field stimulation (EFS; 10 Hz) and ACh (1×10−5 M) caused large increases in amylase output from pancreatic segments. The response to ACh was blocked by atropine (1×10−5 M) whereas the EFS-evoked response was markedly reduced but not abolished. In contrast, pretreatment with tetrodotoxin (1×10−6 M) abolished the secretory effect of EFS.
Either SNP (1×10−3 M) or 8-Br-cyclic GMP (1×10−4 M) inhibited amylase secretion compared to basal. Combining either SNP or 8-Br-cyclic GMP with EFS resulted in a marked decrease in amylase output compared to EFS alone. In contrast, either SNP or 8-Br-cyclic GMP had no significant effect on the amylase response to ACh. When extracellular Ca2+ concentration ([Ca2+]o) was elevated from 2.56 mM to 5.12 mM, SNP failed to inhibit the response to EFS.
EFS stimulated the release of 3H from pancreatic segments preloaded with [3H]-choline. Either SNP or 8-Br-cyclic GMP had no effect on basal 3H release but significantly reduced the EFS-evoked response.
In fura-2 loaded acinar cells, SNP elicited a small decrease in [Ca2+]i compared to basal and had no effect on the ACh-induced [Ca2+]i peak response.
Nitric oxide may modulate the release of endogenous neural ACh in response to EFS in the rat pancreas.
Keywords: Pancreas, nerve stimulation, acetylcholine, neurotransmission, sodium nitroprusside, calcium, nitric oxide, amylase secretion
Introduction
Exocrine pancreatic secretion is regulated mainly by the autonomic nervous system (Richins, 1945; Lenninger, 1974; Pearson et al., 1984; Holst, 1993) and by the gut hormones cholecystokinin and secretin (Singh et al., 1992; Chey, 1993). There is also evidence that non-adrenergic, non-cholinergic nerves (Pearson et al., 1981), growth factors (Lajas et al., 1996) and the paracrine substance histamine (Singh et al., 1997) may also be involved with exocrine secretion. The different secretagogues utilize four distinct stimulus-secretion coupling pathways involving cellular Ca2+ mobilization, adenosine 3′5′-cyclic monophosphate (cyclic AMP) metabolism and activation of protein kinase C and tyrosine kinase in the regulation of exocrine secretion (Brown & McDonough, 1989; Berridge, 1993; Chey, 1993; Holst, 1993; Lajas et al., 1996).
Endogenous nitric oxide (NO) is generated from L-arginine (L-arg) by an enzymatic pathway (nitric oxide synthase, NOS) present in a variety of cell types and many of its biological actions are mediated via activation of guanylate cyclase, although NO may also participate in some other guanosine 3′5′-cyclic monophosphate (cyclic GMP)-independent functions (see Stuehr & Griffith, 1992; Yago et al., 2001 for review). Recently, attention has been focused on the role of NO in the control of exocrine pancreatic secretion employing donors such as sodium nitroprusside (SNP) and L-arg, and NOS inhibitors (L-NG-nitroarginine methyl ester, L-NG-nitroarginine) in the absence and presence of secretagogues (Yago et al., 2001). In isolated pancreatic acini, both exogenous NO (Konturek et al., 1993; Yoshida et al., 1997) and L-arg (Wrenn et al., 1994) have been shown to increase amylase secretion whereas NOS inhibitors seem to attenuate basal and secretagogue-evoked secretory responses (Wrenn et al., 1994; Ahn et al., 1998). Similarly, in the in vivo pancreas inhibition of NO synthesis is associated with decreases in both basal and secretagogue-evoked pancreatic juice secretion. These effects are believed to be associated with changes in pancreatic blood flow in response to NO (Konturek et al., 1993; Satoh et al., 1994). It is particularly noteworthy that much work has been done on NOS inhibitors in the exocrine pancreas, but the findings are still not clear cut (Konturek et al., 1993; Wrenn et al., 1994; Xu et al., 1994; Molero et al., 1995; Hernandez-Guijo et al., 1996; Ahn et al., 1998). Despite the expanding literature on the effects of NO in the exocrine pancreas, a possible role of this substance, via its donor SNP, in the control of nerve-mediated enzyme secretion has not yet been examined. This study was designed to investigate specifically the interaction of SNP with intrinsic nerve-mediated amylase secretion. Furthermore, and given the importance of the cholinergic pathway in terms of enzyme secretion, we have studied the efflux of radioactivity from pancreatic tissue preloaded with tritiated choline chloride ([3H]-choline chloride). SNP was chosen as NO donor and, since many actions of NO are mediated via the cyclic GMP system, the effects of SNP were compared with those of the cyclic GMP permeant analogue 8-bromo-guanosine 3′5′-cyclic monophosphate (8-Br-cyclic GMP). The effects of exogenous NO on acetylcholine (ACh)-evoked secretory responses and intracellular free Ca2+ concentration ([Ca2+]i) were also investigated for comparison.
Methods
All procedures were approved by Institutional (U.K.) Ethics Committees. The experiments which were undertaken in the U.K. are covered by the relevant Home Office Project Licence. Adult female Sprague-Dawley rats weighing 200 – 300 g were used throughout this study. Animals were killed by a blow to the head followed by cervical dislocation. The pancreas was quickly removed and placed into modified Krebs-Henseleit (KH) solution comprising (mM): NaCl, 103.0; KCl, 4.76; CaCl2, 2.56; MgCl2, 1.13; NaHCO3, 25.0; NaH2PO4, 1.15; D-glucose, 2.8; sodium pyruvate, 4.9; sodium fumarate, 2.7; sodium glutamate, 4.9. The solution was kept at pH 7.4 while being continuously gassed with a mixture of 95% O2-5% CO2 and maintained at 37°C.
Measurement of amylase output in pancreatic segments
Small segments (10 – 15 mg) of pancreas totalling 100 – 150 mg were placed in a Perspex flow chamber (volume, 1 ml) and superfused with oxygenated KH solution (37°C) at a flow rate of 1 ml min−1. The effluent from the chamber passed directly to an on-line automated fluorometric assay for the continuous measurement of amylase output. This method, which is a modification of the original technique described by Rinderknecht & Marbach (1970) depends essentially upon the liberation of dialyzable fluorogenic products from amylopectin anthranilate used as a substrate. Amylase output was expressed in units (u) ml−1 (100 mg tissue)−1 and alpha-amylase (Sigma type II-A) was used as standard for calibration.
The chamber contained silver electrodes for electrical field stimulation (EFS). EFS parameters were: amplitude, 50 V; frequency, 10 Hz; and pulse duration, 1 ms. The tissue was stimulated with either EFS, ACh or cholecystokinin octapeptide (CCK-8) for 6 min. In those experiments involving either atropine, tetrodotoxin (TTX), SNP or 8-Br-cyclic GMP, the tissue was pretreated with them for 15 min and subsequently stimulated. All drugs were added directly to the superfusing solution in known concentrations. When the tissue was superfused with KH solution containing different CaCl2 concentrations, the appropriate amount of NaCl was substituted in order to maintain a constant osmolarity.
[3H]-choline efflux experiments
Tritium (3H) loading and release were investigated using established methods (Singh & Pearson, 1984; Jennings et al., 1996). Rat pancreatic segments weighing a total of 100 – 150 mg were loaded with [methyl-3H]-choline chloride (740 kBq ml−1) in oxygenated KH solution for 60 min at 37°C in a shaking water bath. Preliminary experiments established that uptake of [methyl-3H]-choline chloride was complete after this time. After the loading period the segments were transferred to a Perspex flow chamber (volume, 1 ml) and superfused (1 ml min−1) with oxygenated label-free KH solution at 37°C for 105 min prior to the collection of effluent samples. Initial time-course experiments showed that this time was adequate for 3H efflux to attain a steady-state level. Following equilibration, effluent samples were collected at 2-min intervals directly into glass scintillation vials. The experimental protocol took place over 50 min broken into 10 min periods. In the first 10 min nothing was added to the superfusate. This was designated the initial basal period. Next, the tissue was pretreated with either SNP (1×10−3 M) or 8-Br-cyclic GMP (1×10−4 M) for 10 min and subsequently stimulated electrically (EFS; 50 V, 20 Hz, 1 ms) for another 10 min in the continuous presence of the drugs. After a further 10 min of unstimulated collection in SNP/8-Br-cyclic GMP KH solution, the tissue was superfused with normal KH. Separate control experiments in the absence of SNP and 8-Br-cyclic GMP were also performed. At the end of each experiment, the tissue was blotted and weighed. [3H]-choline activity in effluent samples was determined by liquid scintillation analysis (Singh & Pearson, 1984; Jennings et al., 1996) using a Beckman LS 5801 scintillation counter (Beckman, U.K.) and values were expressed as pmol (100 mg tissue)−1.
Measurement of [Ca2+]i in pancreatic acini and acinar cells
The pancreas was dissociated into acinar cells with collagenase in two stages totalling 75 min by established methods (Streb & Schulz, 1983; Francis et al., 1990; Francis & Singh, 1990). Pancreatic acinar cells were loaded with fura-2 acetoxymethyl ester (fura-2 AM; 2 μM) by a method described previously (Lennard & Singh, 1992). Aliquots of the cell suspension (2 ml) were added to quartz cuvettes in an LS50 spectrofluorimeter (Perkin Elmer, Beaconsfield, Bucks, U.K.), continuously stirred and maintained at 37°C. An aliquot from newly prepared batch of cells was subjected to excitation scans from 300 to 400 nm. Emissions were measured at 510 nm. Scans were repeated in the presence of digitonin (5 μM) plus ethylene bis (oxonitrilo) tetraacetic acid (EGTA; 10 mM). These scans showed the optimal excitation wavelengths to use in subsequent experiments and gave some indication of the success of dye loading. Batches of cells which showed poor loading or perverse excitation scans were discarded (Lennard & Singh, 1991). Batches of cells found to be acceptable were then excited at 340 nm and 380 nm and emission measured at 510 nm. Either SNP (1×10−3 M), ACh (1×10−5 M) or SNP plus ACh were added directly to the cell suspension in the cuvette. After each experiment the maximum and minimum fluorescence values were measured following addition of, respectively, digitonin (5 μM) and EGTA (10 mM). Final [Ca2+]i levels were calculated using the method described by Grynkiewicz et al. (1985).
Analysis of data
All data are expressed as mean±s.e.mean. Values were compared using Student's t-test and one way ANOVA test where appropriate, and values of P<0.05 were accepted as significant.
Chemicals
All chemicals were obtained from either Sigma (Poole, U.K.), Molecular Probes Ltd. (U.S.A.) or Amersham International (Slough, U.K.).
Results
Characterization of amylase secretory responses to nerve stimulation
Basal amylase output in this series of experiments was 5.21±0.80 u ml−1 (100 mg tissue)−1 (n=35) and 4.21±0.61 u ml−1 (100 mg tissue)−1 (n=10) in the absence and presence, respectively, of the nerve blocking drug TTX (1×10−6 M). As shown in Figure 1, both EFS (10 Hz) and ACh (1×10−5 M) elicited marked increases in amylase output from superfused pancreatic segments. Pretreatment with TTX (1×10−6 M) completely abolished the EFS-evoked amylase secretion, indicating that EFS is activating intrinsic secretomotor nerves.
Figure 1.
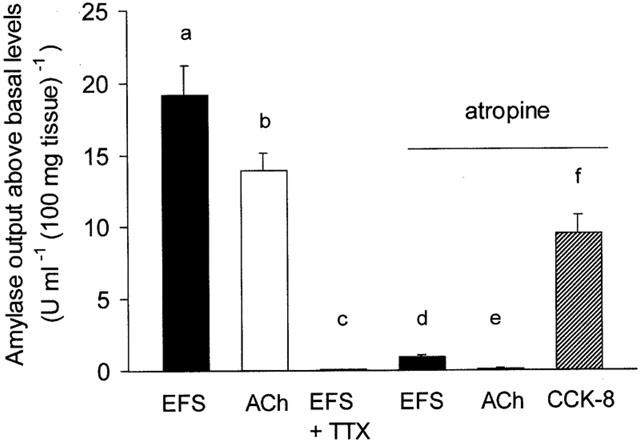
Characterization of amylase secretory responses to nerve stimulation. The bar chart shows the peak amylase output above basal levels following stimulation with electrical field stimulation (EFS; 10 Hz) alone, ACh (1×10−5 M) alone, EFS (10 Hz) in the presence of tetrodotoxin (TTX; 1×10−6 M), and EFS (10 Hz), ACh (1×10−5 M) and CCK-8 (1×10−8 M), all in the continuous presence of atropine (1×10−5 M). The tissue was pretreated with either TTX or atropine for 15 min and subsequently stimulated for 6 min. Values represent means±s.e.mean of 8 – 12 experiments. P<0.005 for (a) compared with (c) and (d), and for (b) compared with (e).
The presence of atropine (1×10−5 M) resulted in a large and significant (P<0.005) decrease in the EFS-induced amylase output compared to EFS alone. In contrast, the muscarinic antagonist completely abolished the response to exogenous ACh. In the continuous presence of atropine, CCK-8 (1×10−8 M) evoked a large increase in amylase secretion. This observation indicated that atropine is indeed blocking the secretory effect of ACh. These results confirm that, together with a small non-cholinergic neural component, nerve-mediated exocrine pancreatic secretion is controlled predominantly by intrinsic cholinergic nerves.
Effects of SNP on amylase secretion
Basal amylase output (mean±s.e.mean) from superfused pancreatic segments in low (1.25 mM), normal (2.56 mM) and elevated (5.12 mM) extracellular Ca2+ ([Ca2+]o) was 2.50±0.2 (n=8), 6.94±0.4 (n=40) and 12.50±1.0 (n=8) u ml−1 (100 mg tissue)−1, respectively. Figure 2 shows original chart recordings of amylase output from superfused pancreatic segments during stimulation with either EFS (10 Hz) or ACh (1×10−5 M) in the absence and presence of SNP (1×10−3 M). Figure 3 shows the mean (±s.e.mean) peak amylase output either above or below basal levels in the absence and presence of SNP (1×10−3 M) but during perturbation of [Ca2+]o. The results show that electrical field stimulation of rat pancreatic segments can result in marked increases in amylase output during perturbation of [Ca2+]o. However, the EFS-evoked secretory response in elevated [Ca2+]o was much larger compared to low [Ca2+]o. In a normal Ca2+ medium, SNP (1×10−3 M) alone inhibited basal amylase output from pancreatic segments (Figures 2 and 3). When combined with EFS (10 Hz), SNP induced a significant (P<0.05) attenuation in amylase output compared with the response obtained with EFS alone but only in low and normal [Ca2+]o. In elevated [Ca2+]o , SNP failed to inhibit the secretory effect of EFS (Figure 3). The effect of SNP on amylase release evoked by exogenous application of the cholinergic agonist ACh was also examined for comparison. As shown in Figures 2 and 3, ACh (1×10−5 M) had a clear secretagogue effect on the rat pancreas when a normal Ca2+ concentration was present in the superfusate and SNP not only failed to inhibit this response but even produced a slight elevation in the ACh-evoked amylase secretion.
Figure 2.
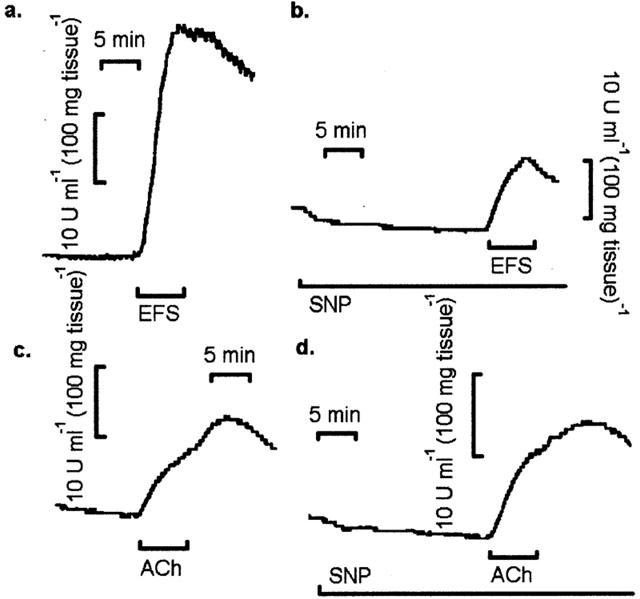
Effects of SNP on amylase output from rat pancreatic segments in response to EFS and ACh. Original chart recordings of amylase output during (a) EFS (10 Hz), (b) EFS (10 Hz) in the presence of SNP (1×10−3 M), (c) ACh (1×10−5 M) and (d) ACh (1×10−5 M) in the presence of SNP (1×10−3 M). The tissue was pretreated with SNP for 15 min and subsequently stimulated for 6 min with either EFS or ACh. Traces are typical of 10 – 12 such experiments for each application.
Figure 3.
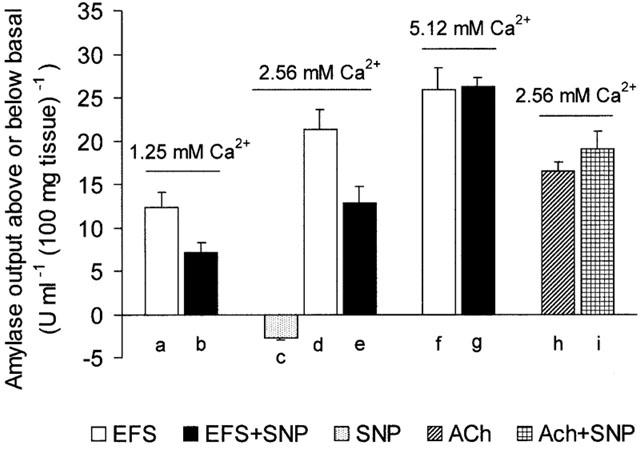
Effects of SNP on amylase secretion from rat pancreatic segments. The bar chart shows peak amylase output either above or below basal level following stimulation with EFS (10 Hz) in the absence or presence of SNP (1×10−3 M) either in a low (1.25 mM), normal (2.56 mM) or high (5.12 mM) Ca2+ medium. The effects of SNP (1×10−3 M) on basal (SNP alone) and on ACh-evoked amylase release, all in normal (2.56 mM) Ca2+ media, are also shown. The tissue was pretreated with SNP for 15 min and subsequently stimulated for 6 min with either EFS or ACh. Values represent means±s.e.mean of 10 – 12 such experiments for each application. P<0.05 for (a) compared with (b) and for (d) compared with (e). It is worth noting that (f) is not significantly different from (g) and neither (h) from (i).
Effects of 8-Br-cyclic GMP on amylase secretion
So far, the results have demonstrated that exogenous NO affects the nerve-mediated secretory response of rat exocrine pancreas. Since many of the actions of NO are attributed to its ability to activate guanylate cyclase in target cells to form cyclic GMP, it was important to ascertain whether a permeant cyclic GMP analogue such as 8-Br-cyclic GMP could mimic the above effects of SNP in pancreatic segments. Figure 4 shows that, like SNP, 8-Br-cyclic GMP (1×10−4 M) elicited a decrease in amylase output compared to basal. Combining 8-Br-cyclic GMP with EFS (10 Hz) resulted in a significant (P<0.05) decrease in the EFS-evoked amylase output compared with the response to EFS alone. However, combining 8-Br-cyclic GMP with exogenous ACh (1×10−5 M) resulted in a clear increase in amylase secretion. All together, our results indicate that exogenous NO can inhibit nerve-mediated amylase secretion from the exocrine pancreas in vitro and this action does not seem to be exerted by antagonizing the cholinergic muscarinic receptor on the acinar cell. In an attempt to understand better the mechanism of action of NO, it was then decided to study the [3H]-choline efflux as an indicator of neuronal ACh release from preloaded pancreatic segments (Jennings et al., 1996).
Figure 4.
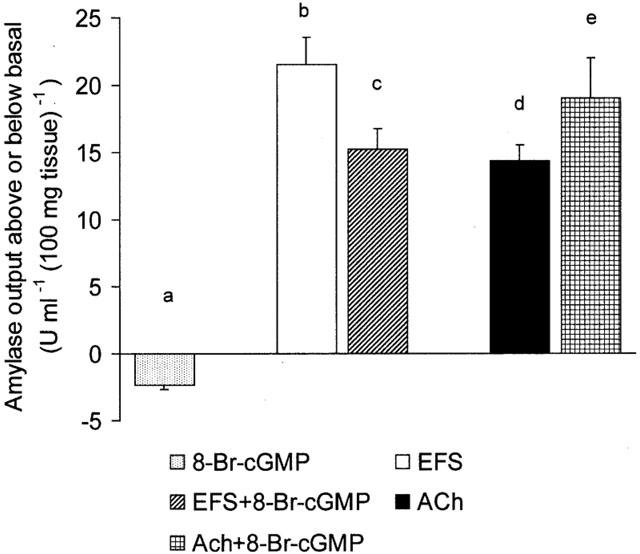
Effects of 8-Br-cyclic GMP on amylase secretion from rat pancreatic segments. The bar chart shows peak amylase output either above or below basal level after the application of 8-Br-cyclic GMP (1×10−4 M) alone and following stimulation with either EFS (10 Hz) or ACh (1×10−5 M) in the absence and presence of 8-Br-cyclic GMP (1×10−4 M), all in normal Ca2+ media. The tissue was pretreated with 8-Br-cyclic GMP for 15 min and subsequently stimulated for 6 min with either EFS or ACh. Values represent means±s.e.mean of 8 – 10 such experiments for each application. P<0.05 for (b) compared with (c) and for (d) compared with (e).
Effects of SNP and 8-Br-cyclic GMP on EFS-evoked [3H]-choline efflux from pancreatic segments
In normal [Ca2+]o, electrical field stimulation (EFS, 20 Hz) of preloaded pancreatic segments resulted in a significant (P<0.05) elevation in 3H release compared with basal level. Addition of either SNP (1×10−3 M) or 8-Br-cyclic GMP (1×10−4 M) to the medium had no detectable effect on basal tritium release (Figure 5). However, in the continuous presence of either SNP or 8-Br-cyclic GMP, the EFS-evoked 3H efflux was significantly (P<0.05) attenuated compared to the response obtained with EFS alone.
Figure 5.
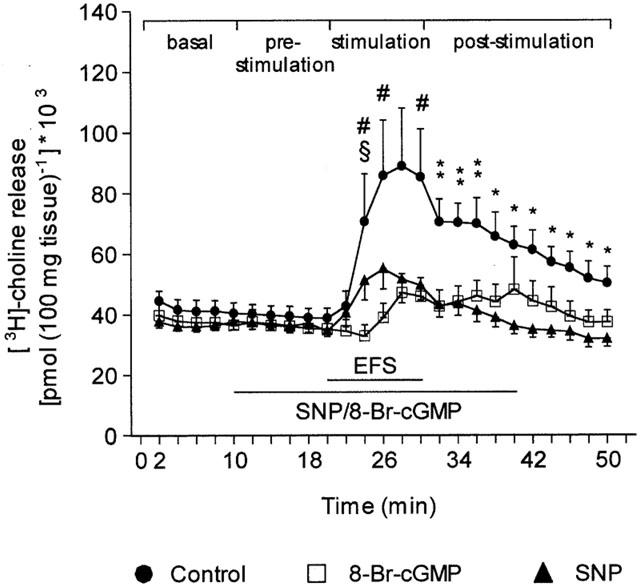
Effects of SNP and 8-Br-cyclic GMP on the time-course changes in [3H]-choline release induced by electrical field stimulation (EFS) of rat pancreatic segments. EFS (20 Hz, 50 V, 1 ms) was applied to [3H]-choline preloaded segments in the absence (control) or presence of either SNP (1×10−3 M) or 8-Br-cyclic GMP (1×10−4 M). The horizontal bars denote the duration of the EFS stimulation and the perfusion with either SNP or 8-Br-cyclic GMP. Each point represents the mean±s.e.mean of six experiments. *P<0.05 for control compared with SNP; #P<0.05 for control compared with 8-Br-cyclic GMP; **P<0.05 for control compared with both SNP and 8-Br-cyclic GMP; §P<0.05 for SNP compared with 8-Br-cyclic GMP.
Accordingly, when [3H]-choline release was expressed as the area under the curve (AUC), we found no difference during superfusion with either normal KH solution, SNP or 8-Br-cyclic GMP prior to the application of EFS to the tissue (pre-stimulation period, Figure 6A). In contrast, the marked increase in 3H release evoked by EFS stimulation in control conditions resulted in a combined stimulation plus post-stimulation AUC significantly (P<0.05) higher than the areas obtained when EFS was applied in the presence of either SNP or 8-Br-cyclic GMP (Figure 6B).
Figure 6.
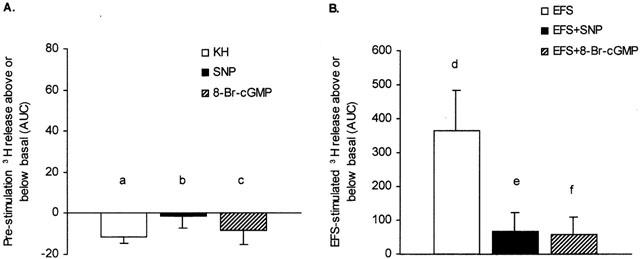
Effects of SNP and 8-Br-cyclic GMP on [3H]-choline release evoked by electrical field stimulation (EFS) of rat pancreatic segments. The bar charts show the area under the curve (AUC, see Figure 5) for [3H]-choline release from preloaded segments during (A) 10 min pre-stimulation in the absence (KH solution alone) and presence of either SNP (1×10−3 M) or 8-Br-cyclic GMP (1×10−4 M) and (B) combined 10 min EFS stimulation (20 Hz, 50 V, 1 ms) plus post-stimulation (20 min) in the absence and presence of either SNP (1×10−3 M) or 8-Br-cyclic GMP (1×10−4 M). Data were obtained by substracting the averaged basal values (first 0 – 10 min) from either the pre-stimulation values (10 – 20 min) or the combined stimulated (20 – 30 min) plus post-stimulated (30 – 50 min) values. Each point is mean±s.e.mean, n=6. P<0.05 for (d) compared with (e) and (f), but (a), (b) and (c) are not significantly different from one another.
Measurement of [Ca2+]i
Since SNP can inhibit amylase output and attenuate the EFS-evoked secretory response, it was pertinent to understand the mechanism by which SNP can exert its effect. Ca2+ is a major intracellular mediator in the action of secretagogues and, thus, it is necessary to measure the levels of [Ca2+]i during the interaction of SNP with ACh. Mean(±s.e.mean) basal [Ca2+]i in this series of experiments was 119.6±5.8 nM (n=50). Addition of ACh (1×10−5 M) to the cell suspension in the cuvette induced a marked transient increase in [Ca2+]i reaching a peak after 10 – 25 s. Thereafter, it declined gradually to around basal level (Figure 7A, upper panel). In contrast, SNP (1×10−3 M) application resulted in a small decrease in [Ca2+]i compared to basal (Figure 7A, lower panel). When the cells were stimulated with ACh in the continuous presence of SNP there was a small but not significant decrease in the [Ca2+]i peak response compared to ACh alone (Figure 7A,B).
Figure 7.
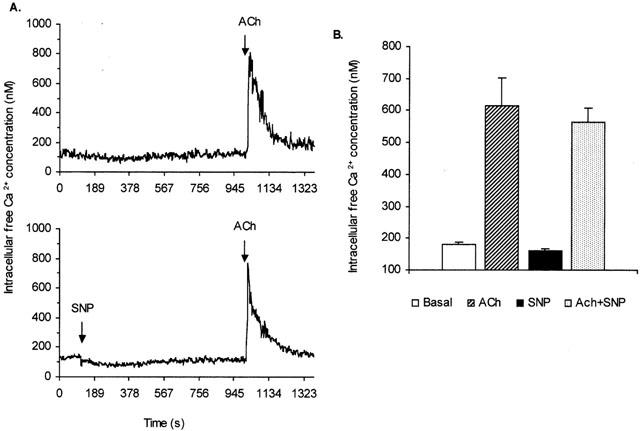
Effect of SNP on Ca2+ mobilization in isolated pancreatic acinar cells. (A) Original recordings showing the effect of ACh (1×10−5 M) on intracellular free Ca2+ concentration ([Ca2+]i) in a suspension of fura-2 loaded pancreatic acinar cells in the absence (upper panel) and presence of SNP (1×10−3 M, lower panel). The arrows indicate the addition of the drugs. Traces are typical of 8 – 10 such experiments. (B) Bar charts showing [Ca2+]i during basal condition and following the addition of ACh (1×10−5 M), SNP (1×10−3 M) or ACh (1×10−5 M) in the presence of SNP. Measurements were made at the peak (20 – 25 s) of the response. Each point is mean±s.e.mean (n=8 – 10).
Discussion
The technique of electrical field stimulation (EFS) is a useful and effective physiological tool to activate intrinsic secretomotor nerves in the isolated pancreas to release endogenous neurotransmitter(s) which in turn can stimulate pancreatic secretion (Davison et al., 1980; Pearson et al., 1984; Wisdom et al., 1994). In this study, EFS of intrinsic nerves of the in vitro pancreas resulted in marked increases in amylase secretion and 3H release. The nerve-mediated amylase secretion was sensitive to the nerve-blocking agent tetrodotoxin (TTX) confirming that EFS effects were truly nerve-mediated, since TTX is a blocker of voltage dependent Na+ channels (Narahashi, 1974). In contrast, basal amylase output was not completely abolished by TTX but only partially reduced, indicating that basal secretion is controlled only in part by nerves. The high level of basal amylase output obtained in the presence of TTX may be due to the damage exerted to the tissues when they were cut into small segments (Pearson et al., 1984). The parasympathetic neurotransmitter ACh strongly mimicked the effects of EFS on amylase output. The secretory effect of ACh was completely abolished by the muscarinic receptor antagonist atropine. In contrast, atropine markedly reduced but did not abolish completely the EFS-evoked amylase output, indicating that the neurotransmitter(s) released during EFS is predominantly ACh. In addition, there is another endogenous substance which elicited a small, but detectable, increase in amylase secretion. It has been known for several years that the exocrine pancreas is extensively innervated by cholinergic postganglionic fibres of the vagus (Richins, 1945). In previous studies, it was demonstrated that in the rat pancreas, EFS can elicit the release of both autonomic neurotransmitters ACh and noradrenaline (Pearson et al., 1984; Singh & Pearson, 1984; Wisdom et al., 1994). The small non-cholinergic EFS-evoked amylase secretion seen in this study may be due mainly to the release of noradrenaline rather than either CCK or peptidergic neurotransmitters since the response was completely abolished by β1 and β2 adrenergic receptor antagonists (Pearson et al., 1984).
In contrast to either EFS or ACh, the results of this study have also shown that either the NO donor, SNP, or the membrane permeant analogue, 8-Br-cyclic GMP, can induce a small but clear reduction in amylase output compared to basal value. Combining either SNP or 8-Br-cyclic GMP with EFS resulted in a significant reduction in amylase secretion compared to the response obtained with EFS alone, indicating that both SNP and 8-Br-cyclic GMP may be acting to exert their inhibitory effects either by antagonizing the cholinergic muscarinic receptor on the acinar cell plasma membrane or by controlling endogenous neurotransmitter(s) release at presynaptic sites. When either SNP or 8-Br-cyclic GMP was combined with ACh there was no attenuation in amylase output compared to the effect of ACh. In fact, both SNP and 8-Br-cyclic GMP seemed to enhance slightly the secretory effect of ACh. This enhancement of amylase secretion may be due to some form of interaction between ACh and SNP/cyclic GMP distal to receptor activation, possibly via cellular Ca2+ mobilization. Interestingly, the results of this study have also shown that the attenuating effect of SNP on EFS-evoked amylase secretion was Ca2+-dependent. SNP can reduce the effect of EFS at both low (1.25 mM) and normal (2.56 mM) extracellular Ca2+ ([Ca2+]o) but not during elevated (5.12 mM) [Ca2+]o. It is now known that the release of endogenous neurotransmitter is dependent upon [Ca2+]o. Low [Ca2+]o is associated with reduced release of neurotransmitters whereas elevated [Ca2+]o seems to have the opposite effect (Kirpekar & Misu, 1967; Singh & Pearson, 1984; Poli et al., 1994; Hebeiss & Kilbinger, 1996; Singh et al., 1997).
The literature regarding the secretory effects of the NO donors SNP and hydroxylamine, and the NOS substrate L-arg is not clear, and especially confusing with regard to the employment of pancreatic acini and acinar cell suspensions to study amylase secretion. Some authors have shown that neither SNP nor hydroxylamine had any detectable effect on amylase secretion from pancreatic acini (Hernandez-Guijo et al., 1996) whereas others have demonstrated small increases in amylase output in response to either exogenous NO (Konturek et al., 1993; Yoshida et al., 1997) or L-arg (Wrenn et al., 1994). The effects of both L-arg and exogenous NO (SNP) are associated with increased levels of endogenous cyclic GMP (Wrenn et al., 1994; Yoshida et al., 1997). However, it is clear and consistent from the literature and the present study that when NO is combined with either ACh or CCK-8, it has no effect on secretagogue-evoked secretory responses from both pancreatic segments and acinar cells (Konturek et al., 1993; Molero et al., 1995; Hernandez-Guijo et al., 1996; Yoshida et al., 1997).
Taken together, the present results and those obtained by other authors (Wrenn et al., 1994; Hernandez-Guijo et al., 1996; Yoshida et al., 1997) suggest that neither SNP nor other donors are acting as receptor antagonists to either ACh, carbachol or CCK-8, and therefore, the effect of SNP in the current study is most likely exerted at presynaptic sites. In order to test this interesting hypothesis, it was pertinent to investigate the effect of either SNP or 8-Br-cyclic GMP on EFS-evoked 3H release from preloaded pancreatic segments. Similar studies have been employed previously in the pancreas to determine the nature and release of endogenous neurotransmitters (Pearson et al., 1984; Singh & Pearson, 1984; Jennings et al., 1996). The present results have shown that nerve stimulation caused a marked increase in 3H efflux from pancreatic preparations preincubated with [3H]-choline chloride. Combining either SNP or 8-Br-cyclic GMP with EFS resulted in significant decreases in 3H release. These results corroborate our findings of the effect of either SNP or 8-Br-cyclic GMP on nerve-mediated amylase secretion in the pancreas. It is also noteworthy that previous studies in our laboratory have demonstrated both Ca2+ dependency and TTX sensitivity of EFS-evoked 3H release from [3H]-choline preloaded pancreatic segments (Pearson et al., 1984; Singh & Pearson, 1984; Jennings et al., 1996; Singh et al., 1997). In addition, several studies, employing a number of different tissue types, have demonstrated that SNP and other NO donors can inhibit electrically-evoked release of [3H]-acetylcholine (Wiklund et al., 1993; Hebeiss & Kilbinger, 1996; Hanania & Johnson, 1998) whereas NOS inhibitors have the opposite effects (for review see Lefebvre, 1995).
Turning now to the effect on [Ca2+]i the present study has shown that addition of SNP (1×10−3 M) to a suspension of fura-2 loaded pancreatic acinar cells results in a small decrease in [Ca2+]i compared to basal value. This result is in contrast to the effect of SNP in single pancreatic acinar cells in which a similar concentration induces a small but detectable increase in [Ca2+]i (Yoshida et al., 1997). This discrepancy may be due to the action of SNP on single acinar cells compared to cell suspensions or difference in gender. In the present study we have employed female rats whereas in the single cell study the authors have used male rats. Unlike SNP, ACh elicits a marked transient increase in [Ca2+]i. However, when SNP was combined with ACh there was no significant change in the [Ca2+]i response compared to the effect of ACh alone. These results indicate that Ca2+ mobilization in pancreatic acinar cells is unlikely to be associated with the inhibitory effect of SNP on nerve-mediated amylase secretion. Similarly, in a previous study (Yoshida et al., 1997) it was shown that the NO/cyclic GMP system was not associated with either the Ca2+ signalling or amylase secretion induced by carbachol and CCK-8. Taken together, these observations further suggest that the most likely site of action of SNP is at the presynaptic level to modulate endogenous neurotransmitter release.
In conclusion, the results of this study have demonstrated that the exogenous NO donor SNP can inhibit nerve-mediated enzyme secretion and 3H release from the in vitro pancreas. This inhibitory effect of SNP on the nerve-mediated response is not expressed at the Ca2+ signalling phase of the stimulus-secretion process in acinar cells, but its most likely action is to modulate presynaptic cholinergic neurotransmission, probably via a mechanism related to cyclic GMP level in the pancreas.
Acknowledgments
Dr Maria D. Yago was supported by the Spanish Ministry of Education and Culture through a post-doctoral research grant.
Abbreviations
- 8-Br-cyclic GMP
8-bromo-guanosine 3′5′-cyclic monophosphate
- ACh
acetylcholine
- AUC
area under the curve
- [Ca2+]i
intracellular free Ca2+ concentration
- [Ca2+]o
extracellular Ca2+ concentration
- CCK-8
cholecystokinin octapeptide
- EFS
electrical field stimulation
- KH
Krebs-Henseleit
- L-arg
L-arginine
- NO
nitric oxide
- NOS
nitric oxide synthase
- SNP
sodium nitroprusside
- TTX
tetrodotoxin
References
- AHN S.H., SEO D.W., KO Y.K., SUNG D.S., BAE G.U., YOON J.W., HONG S.Y., HAN J.W., LEE H.W. No/cGMP pathway is involved in exocrine secretion from rat pancreatic acinar cells. Arch. Pharmacol. Res. 1998;21:657–663. doi: 10.1007/BF02976753. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- BROWN J.S., MCDONOUGH P.M.Muscarinic cholinergic receptors of inositol phospholipid metabolism and calcium mobilisation The Muscarinic Receptors 1989Clifton, New Jersey: Humana Press Inc; 259–307.ed. Brown, J.H. pp [Google Scholar]
- CHEY W.Y.Hormonal control of pancreatic exocrine secretion The Pancreas: Biology, Pathobiology and Disease 1993New York: Raven Press; 403–424.ed. Go, V.L.W., Dimagno, E.P., Gardner, J.D., Lebenthal, E. Reber, H.A., Scheele, G.A. pp [Google Scholar]
- DAVISON J.S., PEARSON G.T., PETERSEN O.H. Mouse pancreatic acinar cells: Effect of electrical field stimulation on membrane potential and resistance. J. Physiol. 1980;301:295–305. doi: 10.1113/jphysiol.1980.sp013206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCIS L.P., LENNARD R., SINGH J. Mechanism of action of magnesium on acetylcholine-evoked secretory responses in isolated rat pancreas. Exp. Physiol. 1990;75:669–688. doi: 10.1113/expphysiol.1990.sp003445. [DOI] [PubMed] [Google Scholar]
- FRANCIS L.P., SINGH J. Effects of phorbol ester and related compounds on acetylcholine-evoked [3H]leucine-labelled protein secretion in permeabilized rat pancreatic acini. Exp. Physiol. 1990;75:537–546. doi: 10.1113/expphysiol.1990.sp003430. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HANANIA T., JOHNSON K.M. Regulation of neurotransmitter release by endogenous nitric oxide in striatal slices. Eur. J. Pharmacol. 1998;359:111–117. doi: 10.1016/s0014-2999(98)00636-0. [DOI] [PubMed] [Google Scholar]
- HEBEISS K., KILBINGER H. Differential effects of nitric oxide donors on basal and electrically evoked release of acetylcholine from guinea-pig myenteric neurones. Br. J. Pharmacol. 1996;118:2073–2078. doi: 10.1111/j.1476-5381.1996.tb15646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNANDEZ-GUIJO J.M., ACOSTA J.J., GARCIA-BENITO M., LOPEZ M.A., SAN ROMAN J.I., CALVO J.J. Nitric oxide synthesis modulates amylase secretion after cholecystokinin stimulation in perfused pancreatic segments of the rat. Digestion. 1996;57:236. [Google Scholar]
- HOLST J.J.Neural regulation of pancreatic exocrine function The Pancreas: Biology, Pathology and Disease 1993New York: Raven Press; 381–402.ed. Go, V.L.W., Dimagno, E.P., Gardner, J.D., Lebenthal, E., Reber, H.A., Scheele, G.A. pp [Google Scholar]
- JENNINGS L.J., SALIDO G.M., PARIENTE J.A., DAVISON J.S., SINGH J., SHARKEY K.A. Control of exocrine secretion in the guinea-pig pancreas by histamine H3 receptors. Can. J. Physiol. Pharmacol. 1996;74:744–752. [PubMed] [Google Scholar]
- KIRPEKAR S.M., MISU Y. Release of noradrenaline by splenic nerve stimulation and its dependence on calcium. J. Physiol. 1967;188:219–234. doi: 10.1113/jphysiol.1967.sp008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONTUREK S.J., BILSKI J., KONTUREK P.K., CIESZKOWSKI M., PAWLIK W. Role of endogenous nitric oxide in the control of canine pancreatic secretion and blood flow. Gastroenterology. 1993;104:896–902. doi: 10.1016/0016-5085(93)91028-g. [DOI] [PubMed] [Google Scholar]
- LAJAS A.I., POZO M.J., SALIDO G.M., SINGH J., PARIENTE J.A. Secretory activity and trophic effects of epidermal growth factor in the rat pancreas. Arch. Physiol. Biochem. 1996;104:293–299. doi: 10.1076/apab.104.3.293.12909. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE R.A. Nitric oxide in the peripheral nervous system. Ann. Med. 1995;27:379–388. doi: 10.3109/07853899509002591. [DOI] [PubMed] [Google Scholar]
- LENNARD R., SINGH J. Secretagogue-evoked changes in intracellular free magnesium concentrations in rat pancreatic acinar cells. J. Physiol. 1991;435:483–492. doi: 10.1113/jphysiol.1991.sp018520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNARD R., SINGH J. Effects of secretagogues on intracellular free calcium and magnesium concentrations in rat pancreatic acinar cells. Gen. Pharmacol. 1992;23:903–908. doi: 10.1016/0306-3623(92)90244-e. [DOI] [PubMed] [Google Scholar]
- LENNINGER S. The autonomic innervation of the exocrine pancreas. Med. Clin. North Am. 1974;58:1311–1318. doi: 10.1016/s0025-7125(16)32073-9. [DOI] [PubMed] [Google Scholar]
- MOLERO X., GUARNER F., SALAS A., MOURELLE M., PUIG V., MALAGELADA J.R. Nitric oxide modulates pancreatic basal secretion and response to caerulein in the rat: Effects in acute pancreatitis. Gastroenterology. 1995;108:1855–1862. doi: 10.1016/0016-5085(95)90150-7. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T. Chemicals as tools in the study of excitable membranes. Physiol. Rev. 1974;54:813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- PEARSON G.T., SINGH J., DAOUD M.S., DAVISON J.S., PETERSEN O.H. Control of pancreatic cyclic nucleotide levels and amylase secretion by noncholinergic, nonadrenergic nerves. A study employing electrical field stimulation of guinea pig segment. J. Biol. Chem. 1981;256:11025–11031. [PubMed] [Google Scholar]
- PEARSON G.T., SINGH J., PETERSEN O.H. Adrenergic nervous control of cAMP-mediated amylase secretion in the rat pancreas. Am. J. Physiol. 1984;246:G563–G573. doi: 10.1152/ajpgi.1984.246.5.G563. [DOI] [PubMed] [Google Scholar]
- POLI E., POZZOLI C., CORUZZI G., BERTACCINI G. Signal transducing mechanisms coupled to histamine H3 receptors and alpha-2 adrenoceptors in the guinea pig duodenum: possible involvement of N-type Ca++ channels. J. Pharmacol. Exp. Ther. 1994;270:788–794. [PubMed] [Google Scholar]
- RICHINS C.A. The innervation of the pancreas. J. Comp. Neurol. 1945;83:223–236. doi: 10.1002/cne.900830303. [DOI] [PubMed] [Google Scholar]
- RINDERKNECHT J., MARBACH E.P. A new automated method for the determination of serum alpha-amylase. Clin. Chim. Acta. 1970;29:107–110. doi: 10.1016/0009-8981(70)90230-5. [DOI] [PubMed] [Google Scholar]
- SATOH A., SHIMOSEGAWA T., ABE T., KIKUCHI Y., ABE R., KOIZUMI M., TOYOTA T. Role of nitric oxide in the pancreatic blood flow response to caerulein. Pancreas. 1994;9:574–579. doi: 10.1097/00006676-199409000-00006. [DOI] [PubMed] [Google Scholar]
- SINGH J., LENNARD R., SALIDO G.M., WISDOM D., RENDER C.L., POZO M.J., PARIENTE J.A., CAMELLO P.J. Interaction between secretin and cholecystokinin-octapeptide in the exocrine rat pancreas in vivo and in vitro. Exp. Physiol. 1992;77:191–204. doi: 10.1113/expphysiol.1992.sp003573. [DOI] [PubMed] [Google Scholar]
- SINGH J., PARIENTE J.A., SALIDO G.M. The physiological role of histamine in the exocrine pancreas. Inflamm. Res. 1997;46:159–165. doi: 10.1007/s000110050156. [DOI] [PubMed] [Google Scholar]
- SINGH J., PEARSON G.T. Effects of nerve stimulation on enzyme secretion from the in vitro rat pancreas and 3H release after preincubation with catecholamines. Naunyn Schmiedebergs Arch. Pharmacol. 1984;327:228–233. doi: 10.1007/BF00502454. [DOI] [PubMed] [Google Scholar]
- STREB H., SCHULZ I. Regulation of cytosolic free calcium concentration in acinar cells of rat pancreas. Am. J. Physiol. 1983;245:G347–G357. doi: 10.1152/ajpgi.1983.245.3.G347. [DOI] [PubMed] [Google Scholar]
- STUEHR D.J., GRIFFITH O.W. Mammalian nitric oxide synthases. Adv. Enzymol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- WIKLUND C.U., OLGART C., WIKLUND N.P., GUSTAFSSON L.E. Modulation of cholinergic and substance P-like neurotransmission by nitric oxide in the guinea-pig ileum. Br. J. Pharmacol. 1993;110:833–839. doi: 10.1111/j.1476-5381.1993.tb13888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISDOM D.M., CAMELLO P.J., SALIDO G.M., SINGH J. Interaction between secretin and nerve-mediated amylase secretion in the isolated rat pancreas. Exp. Physiol. 1994;79:851–863. doi: 10.1113/expphysiol.1994.sp003813. [DOI] [PubMed] [Google Scholar]
- WRENN R.W., CURRIE M.G., HERMAN L.E. Nitric oxide participates in the regulation of acinar cell secretion. Life Sci. 1994;55:511–518. doi: 10.1016/0024-3205(94)00743-8. [DOI] [PubMed] [Google Scholar]
- XU X., STAR R.A., TORTORICI G., MUALLEM S. Depletion of intracellular calcium stores activates nitric oxide synthase to generate cGMP and regulate calcium influx. J. Biol. Chem. 1994;269:12645–12653. [PubMed] [Google Scholar]
- YAGO M.D., MAÑAS M., EMBER Z.S., SINGH J. Nitric oxide and the pancreas: Morphological base and role in the control of the exocrine pancreatic secretion. Mol. Cell. Biochem. 2001;219:107–120. doi: 10.1023/a:1010834611480. [DOI] [PubMed] [Google Scholar]
- YOSHIDA H., TSUNODA Y., OWYANG C. Effect of uncoupling NO/cGMP pathways on carbachol- and CCK-stimulated Ca2+ entry and amylase secretion from the rat pancreas. Eur. J. Physiol. 1997;434:25–37. doi: 10.1007/s004240050359. [DOI] [PubMed] [Google Scholar]


