Abstract
The effect of the selective serotonin reuptake inhibitor fluoxetine was examined on the 5-HT4 receptor-mediated relaxation in the rat isolated ileum.
Fluoxetine unsurmountably antagonized the relaxation to exogenous 5-HT with abolition of the response at 10 μM. Fluoxetine (10 μM) also caused a gradual loss of the resting tension. These effects of fluoxetine were prevented by a prior addition of the 5-HT4 receptor selective antagonist GR113808 (100 nM), which itself caused a contraction of the tissues when administered alone.
Fluoxetine (10 μM) also failed to prevent the relaxation due to exogenous 5-HT and the 5-HT4 receptor agonist 5-methoxytryptamine in tissues taken from the rats treated with para-chlorophenylalanine (300 mg kg−1) for 3 and 6 days, which reduced the 5-HT level in the mucosa by 88 and 97.5% respectively.
The contraction of the tissues with GR113808 indicates the presence of an endogenous 5-HT tone at the 5-HT4 receptor in the rat ileum. It is hypothesized that in the presence of fluoxetine, the concentration of endogenous 5-HT at the receptor was increased sufficiently to reduce or abolish the relaxation to 5-HT added exogenously. The inability of fluoxetine to prevent the relaxation to 5-HT in the presence of GR113808 or after the p-CPA treatment supports this hypothesis.
Keywords: 5-hydroxytryptamine, 5-HT4 receptor, fluoxetine, paroxetine, GR113808
Introduction
5-HT4 receptors are located on the enteric neurones and smooth muscle cells of the gastrointestinal tract and are implicated in the control of motility and secretion in the gastrointestinal tract (Hoyer et al., 1994). The stimulation of the neural 5-HT4 receptor increases neurotransmitter release (Craig & Clarke, 1990) whilst activation of the postjunctional 5-HT4 receptor results in relaxation of the smooth muscle in the rat oesophagus (Baxter et al., 1991; Reeves et al., 1991), ileum (Tuladhar et al., 1996) and the human colon (Tam et al., 1994). The selective serotonin reuptake inhibitor (SSRI) fluoxetine has been shown to antagonise the 5-HT4 receptor-mediated responses in the guinea-pig ileum (Lucchelli et al., 1995) and the rat oesophagus (Lucchelli et al., 1999). However, the mechanisms underlying such interactions remain to be elucidated.
Fluoxetine and other SSRIs inhibit the reuptake of 5-HT in both the brain (Wong et al., 1975; Stanford, 1996) and gastrointestinal tract (Gershon & Jonakait, 1979; Wade et al., 1996). An accumulation of 5-HT at the receptor may then modify 5-HT receptor function. Although this has been realized in the brain (Raap & Van de Kar, 1999), no clear evidence of an accumulation of 5-HT has so far been reported at the 5-HT receptor sites in the gastrointestinal tract following treatment with fluoxetine or any other SSRI.
Fluoxetine is one of the most widely prescribed antidepressant drugs. Gastrointestinal effects have been reported in as many as 20 – 30% of patients treated with fluoxetine (Messiha, 1993) and the accumulation of endogenous 5-HT at the 5-HT4 or other 5-HT receptors located in the gastrointestinal tract may, in part, explain the gastrointestinal effects observed following treatment with fluoxetine and other SSRIs. In the present study we investigate the possibility that an enhanced endogenous 5-HT function following treatment with fluoxetine may modify the relaxation to 5-HT in the rat terminal ileum, a response mediated via the 5-HT4 receptor (Tuladhar et al., 1996).
Methods
Preparation of tissues and construction of concentration response curves
Female Hooded Lister rats (Bradford strain) weighing 200 – 300 g were stunned by a blow to the head and killed by cervical dislocation. Segments of the terminal ileum (up to four segments, 2 – 3 cm in length, 3 – 20 cm from the ileo-caecal junction) were carefully removed, the intraluminal content was flushed out and the tissues mounted in 10 ml organ baths containing Krebs – Henseleit solution (composition in mM: NaCl 118, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.5, NaHCO3 25.0, glucose 10.0) kept at 37°C and oxygenated with 95% O2 and 5% CO2. The Krebs – Henseleit solution routinely contained methysergide (1 or 10 μM) and atropine (0.1 μM) which enhance the relaxation and block the contractile response to 5-HT (Tuladhar et al., 1996). The tissues were washed and allowed to equilibrate for the first 45 min without tension with a washout every 15 min. Tension was then slowly applied to the tissues to a maximum of 1 g: the tension was increased or decreased as necessary, until a stable baseline tension of 0.6 – 0.75 g was obtained. GR113808 and/or fluoxetine was then added to the bath, if required, in the above sequence with at least 10 min between the additions of GR113808 and fluoxetine. Tension was readjusted to 0.6 – 0.75 g (tissues were allowed to equilibrate with no tension before the readjustment if spontaneous activity developed in the tissue) before the construction of the agonist concentration response curves. GR113808, fluoxetine and paroxetine were equilibrated for at least 30 min before the construction of the concentration response curves to 5-HT or 5-methoxytryptamine. Concentration response curves to the agonists were obtained by a cumulative addition of the agonist with a 2 min contact time. Changes in tissue tension were measured isometrically using Grass Force Displacement transducers (FT03C, Grass Instrument Co., Mass, U.S.A.) and recorded on a multi-channel Grass recorder.
In experiments where rats were treated with para-chlorophenylalanine (p-CPA) for depletion of endogenous 5-HT (Weber, 1970), eight to ten animals were divided into two groups and one group received p-CPA 300 mg kg−1 day−1 i.p. and another group received an injection of an equal volume of the vehicle for 3 or 6 days.
Biochemical measurement of 5-HT and5-hydroxyindoleacetic acid (5-HIAA) levels
The ileal mucosa
Small segments (about 1 cm) of the terminal ileum were removed from the treated animals (from which tissues were obtained for the pharmacological studies) and cut along the mesenteric border. The ileum was cleaned and blotted between folds of filter paper. Using curved forceps, about 20 mg of mucosa was carefully removed and placed in a pre-weighed polypropylene tube and stored in liquid nitrogen until further analysis. Triplicate samples of ileum were taken from each animal.
On the day of analysis, 200 μl of 0.2 M perchloric acid containing N-methyl-5-hydroxytryptamine (100 nM, added as internal standard) was added to the ice cold tissue which was homogenized using a sonic tissue disrupter (Soniprep 150, MSE, Crawley, U.K.) with 10 pulses each of 5 s duration. The preparation was centrifuged (5 min at 15400×g) and 5-HT and 5-HIAA were measured by high pressure liquid chromatography (HPLC) with electrochemical detection. The mobile phase for the HPLC consisted of a mixture of 0.12 M disodium hydrogen orthophosphate and 36 mM citric acid, pH 6.3 with 11% methanol and 2 mM tetraethylammonium chloride. The mobile phase was delivered at a rate of 1.4 ml min−1 to a Hypersil-ODS (250×4.6 mm, 5 μM particle size) analytical column (HPLC Technology, Macclesfield, U.K.) protected by a guard column (Guard-Pak precolumn module, Waters, Milford, U.S.A.). The column eluent passed over a single amperometric glassy carbon electrode held at a potential of 800 mV with respect to a silver/silver chloride reference electrode. The signal from the detector was recorded and analysed using a 3396A integrator (Hewlett Packard Co., Avondale, U.S.A.). 5 – 10 μl of test solutions were injected into the system and the amount of 5-HT/5-HIAA present in the injection was calculated by comparing the peak-heights of the sample with the standard and expressed as the amount present per unit wet-weight of the tissue.
The bathing fluid
The segments of the terminal ileum were mounted in the 10 ml organ baths as described above. A sample of the bathing fluid was taken from the organ bath 10 – 15 min after the application of the basal tension. Fluoxetine (10 μM) was then added to the organ bath and a second sample was taken 30 min after the addition of fluoxetine. The samples were centrifuged (5 min at 15,400×g) and 10 μl of the supernatant were injected into the HPLC system described above.
Analysis of results
Results are expressed as the arithmetic mean±s.e.m. or mean (95% confidence limits). The concentration response curves were analysed graphically. The potency of the agonists to relax the ileum was expressed as pEC50 values (relative to individual maxima). The apparent pKB values for antagonists were estimated from the equation:
where CR is the concentration ratio derived from the agonist EC50 values in the presence and absence of a concentration of antagonist (B), assuming a competitive interaction with a slope of 1, at this concentration of antagonist. The significance of differences between the values was determined at P<0.05 using Student's t-test (unpaired two tailed) or ANOVA followed by Bonferroni Dunnett's t-test.
Drugs
5-Hydroxytryptamine maleate (Sigma), 5-methoxytryptamine hydrochloride (Sigma), N-methyl-5-hydroxytryptamine (Sigma), 5-hydroxyindole-3-acetic acid (Sigma), atropine sulphate (Sigma), GR113808 ([1-[2-methylsulphonyl)amino]ethyl]-4-piperidinyl]methyl-1-methyl-1H-indole-3-carboxylate) maleate (Glaxo Wellcome), and fluoxetine hydrochloride (Sigma) were dissolved in distilled water and diluted in Krebs – Henseleit solution. Methysergide hydrogen maleate (Novartis) was dissolved in distilled water by sonication. Para-Chlorophenylalanine (Sigma) was suspended in 5% Tween 80 (Sigma).
Results
Effects of fluoxetine on the relaxation caused by 5-HT
In the presence of methysergide (1 μM) and atropine (0.1 μM), the cumulative addition of exogenous 5-HT (0.01 – 1 μM) produced a concentration related relaxation in tissues obtained from untreated rats. The relaxation to 5-HT was abolished in the presence of fluoxetine (10 μM). A concentration of 1 μM fluoxetine resulted in an unsurmountable antagonism of the 5-HT response and the use of a lower concentration of fluoxetine (0.1 μM) was ineffective (Figure 1a). Paroxetine (1 – 100 nM) also reduced the response to 5-HT in a concentration related fashion and abolished the relaxation to 5-HT at 1 μM (Figure 1b).
Figure 1.
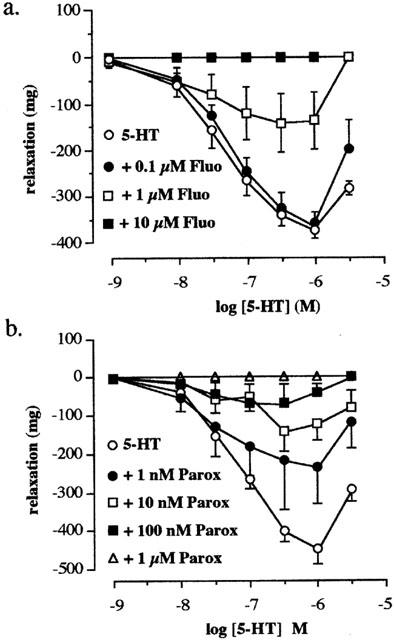
The effects of fluoxetine and paroxetine on the relaxation to 5-HT in the rat terminal ileum. Concentration response curves to 5-HT (a) in the absence (5-HT) and presence of 0.1 μM (+0.1 μM Fluo), 1 μM (+1 μM Fluo) and 10 μM (+10 μM Fluo) fluoxetine and (b) in the absence (5-HT) and presence of 1 nM (+1 nM Parox), 10 nM (+10 nM Parox), 100 nM (+100 nM Parox) and 1 μM (+1 μM Parox) of paroxetine. Values shown are the means with the s.e.m. shown by vertical bars in tissues taken from 4 – 6 animals. Methysergide (1 μM) and atropine (0.1 μM) were routinely included in the bathing medium.
The reversal of the effects of fluoxetine by the 5-HT4 receptor antagonist GR113808
Fluoxetine (10 μM) caused a gradual loss of tone in the tissues when added to the organ baths (n=4). The loss of tone started soon after the addition of fluoxetine and reached a maximum value of 400±64 mg (n=4) within 15 – 20 min. Such loss of tone with fluoxetine did not occur in any tissue (n=4) to which GR113808 had been added before the addition of fluoxetine. GR113808 (100 nM) increased the resting tone of the tissues by 327.5±90 mg (n=4): this effect was observed starting 2 – 3 min after the addition and had reached a higher stable baseline within 5 – 8 min (Figure 2a). A concentration dependent relaxation to 5-HT was observed in control tissues and those tissues which had received GR113808 before the addition of fluoxetine but not in tissues treated only with fluoxetine (Figure 2b,c).
Figure 2.
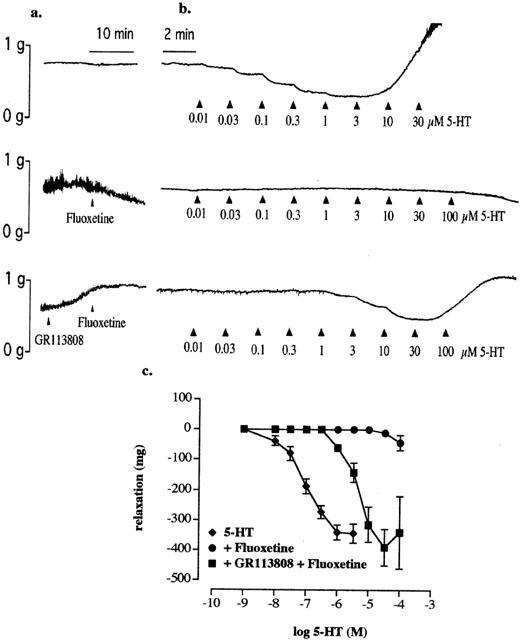
(a) Typical traces illustrating the loss of tone following treatment with fluoxetine (10 μM) and the increase in tone with GR113808 (100 nM), which also prevented the loss of tone due to fluoxetine. (b) Typical relaxation (or lack of it) on cumulative addition of 5-HT in the control tissues, tissues treated with fluoxetine alone and tissues treated with GR113808 (100 nM) followed by fluoxetine (10 μM). The tension was readjusted if required before the addition of 5-HT (c) Concentration-response curves to 5-HT in above tissues. Methysergide (10 μM) and atropine (0.1 μM) were routinely present in the buffer.
Effects of vehicle or p-CPA pre-treatment on the relaxation to 5-HT and the effects of fluoxetine
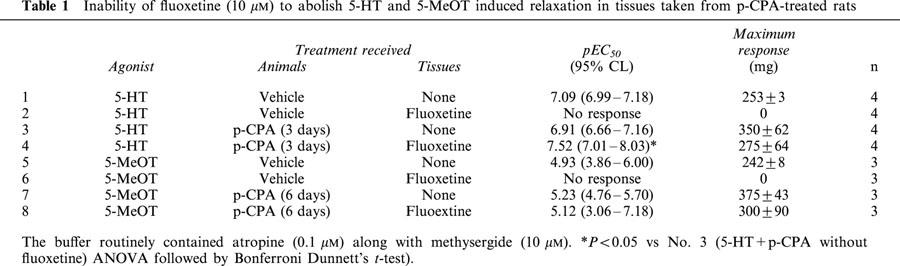
5-HT produced a relaxation in the tissues taken from both vehicle and p-CPA treated rats (3 days). Fluoxetine (10 μM) abolished the relaxation to 5-HT in tissues taken from vehicle-control animals but not in tissues taken from the p-CPA-treated animals (Figure 3). The profile of action of 5-HT in tissues taken from p-CPA treated rats and the interaction with fluoxetine was also found using the 5-HT4 receptor agonist 5-methoxytryptamine (5-MeOT) (Figure 4). Pre-exposure to 10 μM fluoxetine abolished the relaxation in the tissues obtained from the vehicle control rats. However, 5-MeOT maintained a concentration related relaxation in the tissues taken from the p-CPA treated rats (6 days) in the presence of 10 μM fluoxetine. The pEC50 values and the maximum response to 5-HT and 5-MeOT in these tissues are presented in Table 1. The results also show a significant shift to the left of the concentration response curve to 5-HT in the presence of fluoxetine.
Figure 3.
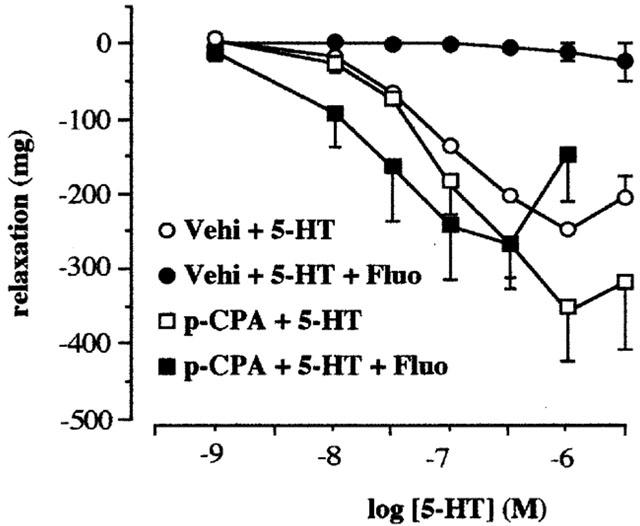
Concentration response curves to 5-HT in the absence (Vehi+5-HT) and presence (Vehi+5-HT+Fluo) of fluoxetine (10 μM) in the terminal ileum taken from vehicle treated rats and concentration response curves to 5-HT in the absence (p-CPA+5-HT) and presence (p-CPA+5-HT+Fluo) of fluoxetine (10 μM) in tissues taken from the p-CPA (300 mg kg−1 for 3 days) treated rats. Methysergide (10 μM) and atropine (0.1 μM) were routinely included in the bathing medium. Values shown are the means with the s.e.m. shown by vertical bars in tissues taken from four animals.
Figure 4.
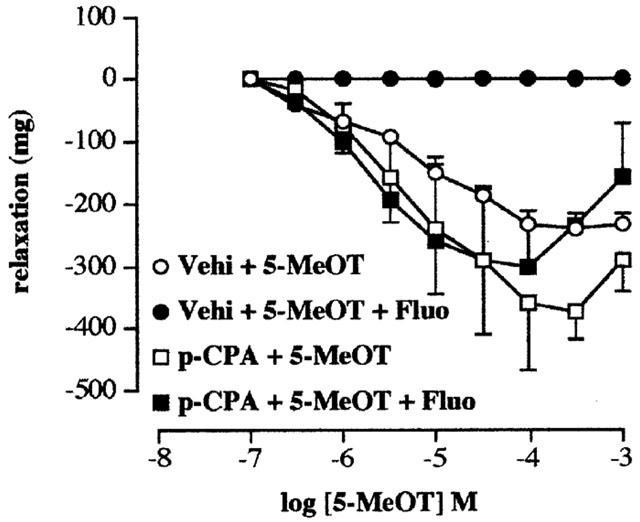
Concentration response curves to 5-methoxytryptamine in the absence (Vehi+5-MeOT) and presence (Vehi+5-MeOT+Fluo) of fluoxetine (10 μM) in terminal ileum taken from vehicle treated rats and concentration response curves to 5-methoxytryptamine in the absence (p-CPA+5-MeOT) and presence (p-CPA+5-MeOT+Fluo) of fluoxetine (10 μM) in tissues taken from the p-CPA (300 mg kg−1 for 6 days)-treated rats. Methysergide (10 μM) and atropine (0.1 μM) were routinely included in the bathing medium. Values shown are the means with the s.e.m. shown by vertical bars in tissues taken from three animals.
Table 1.
Inability of fluoxetine (10 μM) to abolish 5-HT and 5-MeOT induced relaxation in tissues taken from p-CPA-treated rats
Effects of p-CPA pre-treatment on activity of GR113808
The selective 5-HT4 receptor antagonist GR113808 (30 nM) when added to the tissue increased the basal tone of the tissues taken from all the vehicle control animals. The increase in tone caused by GR113808 was abolished in the tissues taken from the rats treated with p-CPA for 6 days (Table 2). GR113808 (30 nM) produced essentially parallel shifts of the concentration response curves to 5-HT in tissues taken from vehicle or p-CPA treated animals (for 3 days) (Figure 5). The apparent pKB estimations in tissues taken from vehicle and p-CPA treated animals were 8.95±0.13 and 8.74±0.18 (n=4, P>0.05). GR113808 (10 nM) also shifted to the right the concentration response curve to 5-MeOT in the presence of 10 μM fluoxetine in the tissues taken from the rats treated with p-CPA for 6 days (data not shown) from which an apparent pKB value of 8.74±0.38 (n=3) was calculated.
Table 2.
The failure of GR113808 to increase tone in tissues taken from rats treated with p-CPA (300 mg kg−1) for 6 days
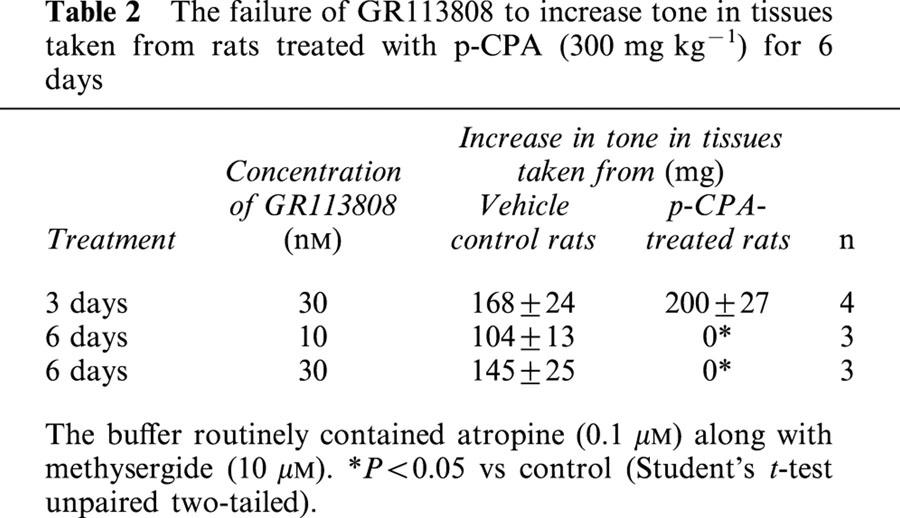
Figure 5.
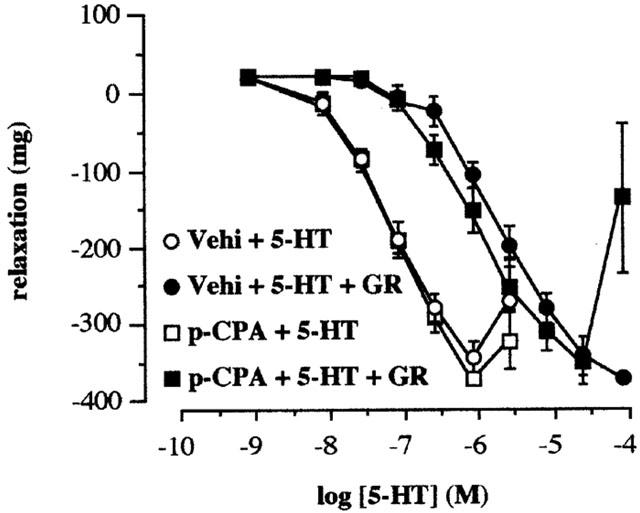
Antagonism by GR113808 of the relaxation to 5-HT in the terminal ileum taken from vehicle-control and p-CPA-treated rats (300 mg kg−1 for 3 days). Concentration response curves to 5-HT in the absence (Vehi+5-HT) and presence (Vehi+5-HT+GR) of GR113808 (30 nM) in the terminal ileum taken from vehicle treated rats and concentration response curves to 5-HT in the absence (p-CPA+5-HT) and presence (p-CPA+5-HT+GR) of GR113808 (30 nM) in tissues taken from the p-CPA treated rats. Methysergide (10 μM) and atropine (0.1 μM) were routinely included in the bathing medium. Values shown are the means with the s.e.m. shown by vertical bars in tissues taken from 4 – 6 animals.
Ex vivo effect of p-CPA on the mucosal 5-HT and 5-HIAA levels
The HPLC procedure produced a clear separation of 5-HT and 5-HIAA when either the standard solutions or the samples prepared from the tissue homogenates were injected onto the column. The sensitivity of the HPLC assay was approximately 2 pg μl−1. The levels of 5-HT and 5-HIAA in the mucosa are shown in Figure 6. p-CPA decreased the 5-HT level in the mucosa by 88 and 97.5% following 3 and 6 days treatment respectively. The level of 5-HIAA was also decreased by the p-CPA treatments by 77% following both 3 and 6 day treatments.
Figure 6.
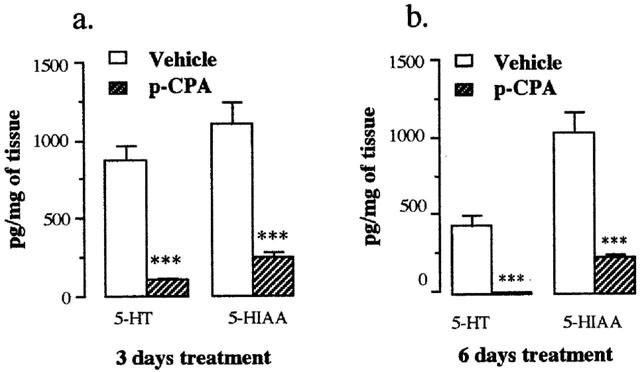
Effect of pretreatment of rats with vehicle (open bars) and parachlorophenylalanine 300 mg kg−1 (shaded bars) for (a) 3 days and (b) 6 days on the 5-HT and 5-HIAA level in the rat mucosa. Values shown are the means with the s.e.m. shown by vertical bars in tissues taken from 4 – 5 animals. ***P<0.001 compared with vehicle control.
Measurement of 5-HT and 5-HIAA in the bathing fluid
No 5-HT or 5-HIAA were detectable in any samples of the bathing fluid taken either before or after fluoxetine in tissues obtained from six animals.
Discussion
Exogenous 5-HT produces a reproducible relaxation in the presence of methysergide and atropine that we have previously shown to be mediated by a 5-HT4 receptor (Tuladhar et al., 1996). The parallel shifts of the concentration response curves to 5-HT and 5-methoxytryptamine with GR113808 observed in this study, with estimated apparent pKB values ranging from 8.7 – 8.9 in tissues taken from both control and p-CPA treated animals, confirm the 5-HT4 receptor involvement in the relaxation response. The main finding of the present study is the ability of the selective serotonin reuptake inhibitor fluoxetine to reduce and at a sufficient concentration abolish the relaxation to exogenous 5-HT in the rat ileum. Such effects of fluoxetine are unlikely to be a direct effect on the 5-HT4 receptor since its affinity for the 5-HT4 receptor is low (reported IC50 values for inhibition of specific [3H]-GR113808 binding to 5-HT4 receptor in pig striatal membranes 42.8 μM (Lucchelli et al., 1995)). The lack of a direct effect of fluoxetine on the 5-HT4 receptor in the present study is also indicated by the failure of fluoxetine (10 μM) to alter the extent of shift produced by GR113808 in tissues taken from either control or p-CPA treated rats.
Fluoxetine is a selective serotonin reuptake inhibitor that acts on the serotonin transporter to prevent the uptake of 5-HT in both the brain (Wong et al., 1975) and the gastrointestinal tract (Gershon & Jonakait, 1979; Wade et al., 1996). Such an effect of fluoxetine can be expected to increase the 5-HT concentration at the receptor sites. The accumulation of endogenous 5-HT at the receptor site is expected to reduce the effect of exogenously added agonist as exogenous agonist can only produce an effect above that already produced by the endogenous 5-HT. Indeed, if endogenous 5-HT concentration at the receptor site is already sufficient to produce a maximum response, the exogenous 5-HT would produce no further response or may even facilitate desensitisation.
Thus, the antagonism of the relaxation to 5-HT (and the 5-HT4 receptor agonist 5-methoxytryptamine) by fluoxetine is likely to be a consequence of an accumulation of the endogenous 5-HT at the 5-HT4 receptor. Indeed, the presence of a basal level of endogenous 5-HT tone mediating relaxation is indicated by the increase in the resting tone by the 5-HT4 receptor selective antagonists (Tuladhar et al., 1996 and the present study). The antagonism of the 5-HT response was not restricted to fluoxetine but was also observed with another selective 5-HT uptake inhibitor paroxetine, which further supports the above hypothesis.
The above hypothesis of an endogenous 5-HT interaction with the 5-HT4 receptors is further supported by the ability of GR113808 to prevent the effect of fluoxetine (abolition of the relaxation to 5-HT in the presence of fluoxetine). The concentration of GR113808 (100 nM) used in the present study is unlikely to be acting at any other site except at the 5-HT4 receptor (Gale et al., 1994). Furthermore, GR113808 increased the resting tone of the tissue in its own right, indicating blockade of the relaxant endogenous 5-HT tone. The finding that GR113808 prevented the loss of tone due to fluoxetine further supports the hypothesis of an endogenous 5-HT interaction with the 5-HT4 receptor.
An accumulation of endogenous 5-HT at the 5-HT4 receptor with fluoxetine is further supported by the findings of the experiments using p-CPA. p-CPA depletes 5-HT in both the brain and ileum (Kenneth & Weissman, 1966; Weber, 1970) by inhibition of 5-HT synthesis. In the present study, the ability of a prior p-CPA treatment to prevent the fluoxetine-induced response may again be explained by a decrease in endogenous 5-HT release, reducing the accumulation of endogenous 5-HT at the receptor site. A reduction in endogenous 5-HT by p-CPA treatment in the present study was evidenced by a decrease in 5-HT content of the mucosa, the site where the highest concentration of 5-HT is found in the body (Erspamer, 1954).
An ability of p-CPA treatment to decrease endogenous 5-HT tone at the 5-HT4 receptor is supported by the failure of the 5-HT4 receptor antagonist GR113808 to increase the basal tone in tissues taken from animals treated with p-CPA for 6 days. The inability of a shorter p-CPA treatment for 3 days to abolish the increase in the basal tone due to GR113808 probably reflects the smaller concentrations of 5-HT required for the endogenous tone as compared to that required to produce a maximum stimulation or desensitisation.
The failure to measure the 5-HT and 5-HIAA in the bathing fluid shows that the level in the bathing fluid is below 2 pg μl−1 (approximately 10 nM; the limit of sensitivity of the HPLC procedure employed) at any time. The failure to detect the 5-HT or 5-HIAA to support the functional data is understandable as the sites responsible for the release of 5-HT, the 5-HT4 receptors mediating relaxation and the uptake sites sensitive to SSRIs are likely to be nearer to each other. The dilution of the released 5-HT and 5-HIAA may contribute to the failure to detect 5-HT/5-HIAA in the bathing fluid. Although, it may have been possible to measure 5-HT and 5-HIAA by improving the methodology, such effort was not deemed to be worthwhile as it is unlikely that 5-HT is solely metabolized by the ‘cells' expressing the reuptake sites sensitive to SSRIs in the rat ileum, making it unlikely that the measurement of released 5-HT/5-HIAA profile in the bathing fluid will reflect the uptake or lack of it into the cells expressing the SSRI sensitive reuptake sites for 5-HT.
Fluoxetine has been shown to be a less potent 5-HT reuptake inhibitor in the ileum as compared to the brain synaptosomal preparations (Gershon & Jonakait, 1979) and the concentration of fluoxetine (1 – 10 μM) required to inhibit the relaxant effect of exogenous 5-HT in the present study is comparable to that required to inhibit 5-HT uptake in the guinea pig ileum (ID50 1.7 μM) (Gershon & Jonakait, 1979) and in the intestinal crypt epithelial cells in the rat intestinal mucosa (1 μM) (Wade et al., 1996). Data on the potency of paroxetine to inhibit reuptake in the gastrointestinal tissue is not available. However, paroxetine is approximately ten times more potent in blocking uptake of 5-HT in both a brain synaptosomal preparation (Stanford, 1996) and in CV1-cells transfected with the 5-HT transporter c-DNA isolated from the rat mast cells (Hoffman et al., 1991); the same potency ratio of fluoxetine and paroxetine was observed in the present study to abolish the 5-HT receptor mediated relaxation of the rat ileum.
Thus, the present data are interpreted to reflect on enhancement of endogenous 5-HT function at the 5-HT4 receptor with fluoxetine in the isolated preparation of the rat terminal ileum. It would be interesting to see whether an in vivo treatment with fluoxetine also results in an enhancement of the effects of endogenous 5-HT in the gastrointestinal tract, which may include a desensitization of the 5-HT receptors. Such possibilities could be predicted from the present study and may explain the gastrointestinal effects observed with fluoxetine and other SSRIs. The enhanced endogenous 5-HT tone at the receptor sites, and possibly the desensitisation of the 5-HT receptors in the brain, is the accepted pharmacological basis of the mechanism of action of fluoxetine (Stanford, 1996). It is also relevant to note that higher concentrations of fluoxetine (and paroxetine) (as compared to concentrations required to block uptake in the brain tissues) were required to antagonize the 5-HT response in the ileum, probably indicating a lower propensity of such compounds to cause gastrointestinal effects. However, the concentrations used in the present study are still relevant since plasma levels of fluoxetine are usually in the range of 0.5 – 1.5 μM (Altamura et al., 1994), and gastrointestinal side effects are common with such treatment (Messiha, 1993).
In conclusion, the present study has demonstrated the ability of fluoxetine to antagonize a 5-HT4 receptor mediated relaxation in the rat isolated ileum. The study supports the hypothesis that an increased endogenous 5-HT tone is responsible for such an effect. The findings may contribute to an understanding of the mechanism(s) of action of the 5-HT reuptake inhibitors, with particular reference to their effects on gut motility.
Acknowledgments
The authors thank the ORS Awards Scheme for providing an ORS award to B.R. Tuladhar, Glaxo Wellcome and Novartis for the gifts of drugs and Dr C.H.K. Cheng for her help in the HPLC analysis.
Abbreviations
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-MeOT
5-methoxytryptamine
- GR113808
([1-[2-methylsulphonyl)amino]methyl]-4-piperidinyl]methyl-1-methyl-1H-indole-3-carboxylate) maleate
- p-CPA
para-chlorophenylalanine
- SSRI
selective serotonin reuptake inhibitor
References
- ALTAMURA A.C., MORO A.R., PERCUDANI M. Clinical pharmacokinetics of fluoxetine. Clin. Pharmacokin. 1994;26:201–214. doi: 10.2165/00003088-199426030-00004. [DOI] [PubMed] [Google Scholar]
- BAXTER G.S., CRAIG D.A., CLARKE D.E. 5-Hydroxytryptamine4 receptors mediate relaxation of the rat oesophageal tunica muscularis mucosae. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;343:439–446. doi: 10.1007/BF00169544. [DOI] [PubMed] [Google Scholar]
- CRAIG D.A., CLARKE D.E. Pharmacological characterization of a neuronal receptor for 5-HT in guinea-pig ileum with properties similar to the 5-hydroxytryptamine4 receptor. J. Pharmacol. Exp. Ther. 1990;252:1378–1386. [PubMed] [Google Scholar]
- ERSPAMER V. Pharmacology of indolalkylamines. Pharmacol. Rev. 1954;6:425–487. [PubMed] [Google Scholar]
- GALE J.D., GROSSMAN C.J., WHITEHEAD J.W.F., OXFORD A.W., BUNCE K.T., HUMPHREY P.P.A. GR113808 – a novel, selective antagonist with high affinity at the 5-HT4 receptor. Br. J. Pharmacol. 1994;111:332–338. doi: 10.1111/j.1476-5381.1994.tb14064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSHON M.D., JONAKAIT G.M. Uptake and release of 5-hydroxytryptamine by enteric 5-hydroxytryptaminergic neurons: Effects of fluoxetine (Lilly 110140) and chlorimipramine. Br. J. Pharmacol. 1979;66:7–9. doi: 10.1111/j.1476-5381.1979.tb16089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN B.J., MEZEY E., BROWNSTEIN M.J. Cloning of a serotonin transporter affected by antidepressants. Science. 1991;254:579–580. doi: 10.1126/science.1948036. [DOI] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P.A. VII. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- KENNETH B., WEISSMAN A. p-Chlorophenylalanine: A specific depletor of brain serotonin. J. Pharmacol. Exp. Ther. 1966;154:499–516. [PubMed] [Google Scholar]
- LUCCHELLI A., SANTAGOSTINO-BARBONE M.G., BARBIERI A., CANDURA S.M., TONINI M. The interaction of antidepressant drugs with central and peripheral (enteric) 5-HT3 and 5-HT4 receptors. Br. J. Pharmacol. 1995;114:1017–1025. doi: 10.1111/j.1476-5381.1995.tb13307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCCHELLI A., SANTAGOSTINO-BARBONE M.G., MASOERO E., BAIARDI P., TONINI M. Influence of fluoxetine and litoxetine on 5-HT4 receptor-mediated relaxation in the rat isolated oesophagus. Fund. Clin. Pharmacol. 1999;13:330–336. doi: 10.1111/j.1472-8206.1999.tb00352.x. [DOI] [PubMed] [Google Scholar]
- MESSIHA F.S. Fluoxetine-Adverse effects and drug-drug interactions. Clin. Toxicol. 1993;31:603–630. doi: 10.3109/15563659309025765. [DOI] [PubMed] [Google Scholar]
- RAAP D.K., VAN DE KAR L.D. Selective serotonin reuptake inhibitors and neuroendocrine function. Life Sci. 1999;65:1217–1235. doi: 10.1016/s0024-3205(99)00169-1. [DOI] [PubMed] [Google Scholar]
- REEVES J.J., BUNCE K.T., HUMPHREY P.P.A. Investigation into the 5-hydroxytryptamine receptor mediating smooth muscle relaxation in the rat oesophagus. Br. J. Pharmacol. 1991;103:1067–1072. doi: 10.1111/j.1476-5381.1991.tb12301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANFORD S.C. Prozac: panacea or puzzle. Trends Pharmacol. Sci. 1996;17:150–154. doi: 10.1016/0165-6147(96)81591-4. [DOI] [PubMed] [Google Scholar]
- TAM F.S.F., HILLIER K., BUNCE K.T. Characterization of the 5-hydroxytryptamine receptor-type involved in inhibition of spontaneous activity of human isolated colonic circular muscle. Br. J. Pharmacol. 1994;113:143–150. doi: 10.1111/j.1476-5381.1994.tb16186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TULADHAR B.R., COSTALL B., NAYLOR R.J. Pharmacological characterization of the 5-hydroxytryptamine receptor mediating relaxation in the rat isolated ileum. Br. J. Pharmacol. 1996;119:303–310. doi: 10.1111/j.1476-5381.1996.tb15986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADE P.R., CHEN J., JAFFE B., KASSEM I.S., BLAKELY R.D., GERSHON M.D. Localization and function of a 5-HT transporter in crypt epthelia of the gastrointestinal tract. J. Neurosci. 1996;16:2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER W.J. p-chlorophenylalanine depletion of gastrointestinal 5-hydroxytryptamine. Biochem. Pharmacol. 1970;19:2169–2172. doi: 10.1016/0006-2952(70)90317-5. [DOI] [PubMed] [Google Scholar]
- WONG D.T., BYMASTER F.P., HORNG J.S., MOLLOY B.B. A new selective inhibitor for uptake of serotonin into synaptosomes of rat brain: 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine. J. Pharmacol. Exp. Therp. 1975;193:804–811. [PubMed] [Google Scholar]


