Abstract
Release of CGRP during migraine may produce harmful dilatation of cranial arteries, thereby possibly causing pain. We have compared the antagonism by BIBN4096BS and CGRP(8-37) of the relaxant effects of α-CGRP on rings of human temporal artery.
α-CGRP relaxed the arteries precontracted with 9 – 24 mM KCl (−logEC50=9.4) nearly as efficaciously as sodium nitroprusside (10 μM).
BIBN4096BS (0.1 – 100 nM) antagonized the effects of α-CGRP in surmountable manner with slopes of Schild-plots not different from unity. −LogKB values of 10.1 and 10.4 were estimated for BIBN4096BS when administered before or during the KCl-contracture respectively.
BIBN4096BS (1 μM) did not modify the relaxant effects of papaverine and sodium nitroprusside.
CGRP(8-37) (1 – 10 μM) antagonized the effects of α-CGRP in a surmountable manner with slopes of Schild-plots not different from unity. −LogKB values of 6.6 and 6.7 were estimated for CGRP(8-37) administered before or during the KCl-contracture respectively.
The high affinity of BIBN4096BS for CGRP receptors of human temporal artery makes it an excellent tool to explore the hypothesis of CGRP-evoked cerebral vasodilation in migraine.
Keywords: Human temporal artery, α-CGRP-evoked relaxation, BIBN4096BS, CGRP(8-37), migraine
Introduction
The temporal artery can dilate during migraine, thereby possibly contributing to pain (Graham & Wolff, 1938; Lance, 1992). One candidate for vasodilatation of cranial and extracranial arteries is CGRP, which can be released from trigeminal nerves in man (Goadsby et al., 1988) and which has been found to be elevated in the jugular blood of patients during migraine (Goadsby et al., 1990). The human temporal artery appears to be surrounded by nerve fibres containing CGRP (Uddman et al., 1986) and CGRP relaxes contractions induced by prostaglandin F2α (Jansen et al., 1986; Jansen-Olesen et al., 1995), consistent with the existence of receptors for CGRP. In order to gain insight into the CGRP-receptor characteristics we have now studied the α-CGRP-evoked relaxation of rings from human temporal artery and compared the mode of blocking action and affinity of two blocking agents.
A non-peptide CGRP receptor antagonist, BIBN4096BS, was recently reported to possess picomolar affinity for recombinant CGRP receptors and to potently inhibit the facial vasodilatation induced by trigeminal stimulation in marmoset monkeys (Doods et al., 2000). We investigated the mode of antagonism by BIBN4096BS of the relaxant effects of α-CGRP in the human temporal artery and provide the first analysis of the mode of interaction of BIBN4096BS with human vascular CGRP receptors. For comparison we also used the peptidic fragment of human α-CGRP, CGRP(8-37), as antagonist (Chiba et al., 1989) of the effects of α-CGRP. Both blockers were competitive antagonists, BIBN4096BS being 3000∼5000 times more potent than CGRP(8-37). A progress report of this work has been communicated (Verheggen et al., 2001).
Methods
Patients
The experiments were approved by the Ethics Committee of the University of Göttingen. Temporal arteries were obtained from 22 patients (11 males, 11 females; age range 18 – 67 years) undergoing neurosurgery. To prevent arterial damage, care was taken to avoid electrocoagulation as described (Verheggen et al., 1996). Patients were operated on for the following diseases: brain tumours (meningiomas eight, metastasis three, B-cell lymphona one), aneurysms four, orbital tumours two, head injury four. Anaesthesia was induced and maintained with midazolam, flunitrazepam, propofol, fentanyl, alfentanyl, thiopental or ketamine. Muscle relaxation was obtained with pancuronium and/or rocuronium. Until and during surgery patients received some of the following premedication: dexamethasone, heparin, midazolam, mannitol, calcium channel antagonists, carbamazepine, antibitiotics and angiotensin II receptor antagonist.
Isolated arterial rings
All arteries were free of macroscopic atheroma. Arterial segments of 400 – 700 μm outer diameter, obtained during neurosurgery, were placed in oxygenated Ringer solution containing (mM): Na+ 147, K+ 4, Ca2+ 2.3, Cl− 155.6, at room temperature, transferred immediately to the laboratory, dissected and set up in a physiological solution containing (mM): Na+ 142, Cl− 126, K+ 5.84, HCO3− 25, Ca2+ 2.5, H2PO4− 1.175, SO42− 1.175, glucose 5.56. The arterial segments were cleaned of adhering fat and connective tissue and cut into up to 10 rings of 3 – 4 mm in length. Each ring was mounted on an L-shaped brace in an organ bath containing 10 ml of physiological solution. The solution was gassed with 20% O2 in 75% N2 and 5% CO2 at 37°C. The rings were stretched once to 10 mN and left at that length thereafter. Changes in isometric arterial tension were transformed by a mechanoelectrical transducer (TF6V5-Fleck, Mainz, Germany) and recorded continuously on a multichannel recorder (Linearcorder Mark VII WR 3310-Graphtec, Tokyo, Japan). Tissues were allowed to equilibrate for at least 2 h.
To decrease endothelial function, we used a procedure with Triton X-100, previously applied to rabbit pial arteries (Verrecchia et al., 1986; Hamel et al., 1987) and to human cranial arteries (Jansen-Olesen et al., 1997). Physiological solution, containing 0.1% Triton X-100, was flushed for 12 s through one half of the temporal artery, followed immediately by further flushing without the detergent. This method produces oedema and vacuolization of endothelial cells (Verheggen, 2000).
Experimental protocol
To obtain a reference standard, experiments were initiated with a contraction to 3 μM 5-HT. After observing a maximal contraction to 5-HT, the functional integrity of the endothelium was assessed with the relaxation caused by 3 μM acetylcholine followed by washout of the agonists (Figure 1). To enhance arterial tone, rings were exposed to increasing concentrations of KCl (9 – 24 mM), titrated to achieve a contractile tension equivalent to approximately 30 – 45% of the maximum tension induced with 5-HT (Figures 1 and 2). After a stable contractile plateau had been ensured, a cumulative concentration-effect curve for α-CGRP was determined followed by a maximum relaxant concentration of sodium nitroprusside (10 μM). An antagonist was either administered before or during the KCl-contracture. When administrated during the KCl-contracture, the blocking agent was pre-incubated 1 h before a concentration-effect curve for α-CGRP was begun. When administered before the KCl-contracture, the blocking agent was pre-incubated 2 h. To verify the integrity of the arterial function, drugs were washed out and the experiment terminated with a contracture evoked by 100 mM KCl (Figure 1). Only a single concentration-effect curve for α-CGRP, in the absence or presence of a blocking agent, was determined on each arterial ring.
Figure 1.
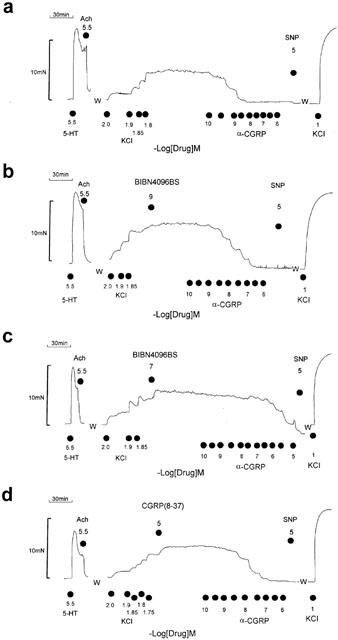
Antagonism of the relaxant effects of α-CGRP by BIBN4096BS and CGRP(8-37). Original tracings from the temporal artery of a 29 year old male patient with epidural haemorrhage. Each panel represents a recording from a separate ring. After a challenge with 5-HT followed by acetylcholine and washout (W), KCl was titrated to obtain a contracture equivalent to ∼30 – 45% of the 5-HT-evoked contraction. After a stable KCl-contracture was observed, a cumulative concentration-effect curve for α-CGRP was determined followed by SNP administration. Shown are experiments in the absence (a) and presence of BIBN4096BS (b,c) and CGRP(8-37) (d).
Figure 2.
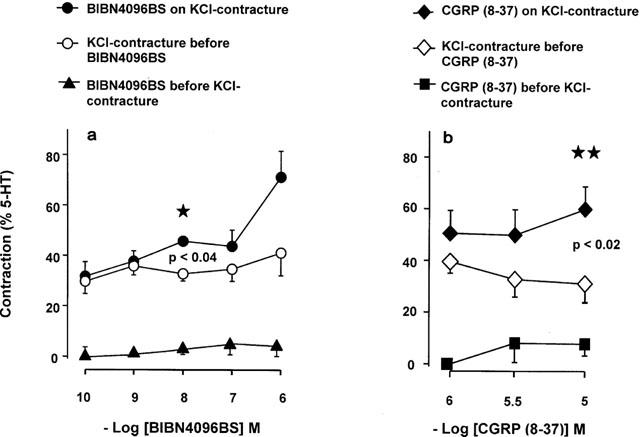
Marginal contractions elicited by BIBN4096BS (a) and CGRP (8-37) (b). Data from 12 patients (a) and six patients (b). Only the effects of 10 nM BIBN4096BS and 10 μM CGRP (8-37), administered during the KCl-contracture, were statistically significant.
To assess whether pathways unrelated to CGRP receptors were affected, we also investigated the effects of BIBN4096BS on the relaxation elicited by SNP and papaverine. SNP was used as a tool for effects mediated via a cyclic GMP pathway (Böhme et al., 1978). Papaverine was used as a vascular relaxant through a cyclic AMP-dependent pathway (Demesy-Waeldele & Stoclet, 1977; Kaneda et al., 1998), presumably through phosphodiesterase inhibition (Chasin & Harris, 1976), and cyclic AMP-unrelated pathways (Fujioka, 1984). A single concentration-effect curve for SNP or papaverine was determined in the absence or presence of 1 μM BIBN4096BS on arterial rings obtained from the same patient. After a challenge to 5-HT followed by acetylcholine and washout, BIBN4096BS was administered before the titration curve to KCl and the curve for SNP or papaverine begun 2 h thereafter. Concentration-effect curves for the relaxant effects of SNP and papaverine were expressed as percentage decreases of the KCl-contracture.
Data analysis and statistics
Concentration-effect curves of α-CGRP were expressed as percentage of the relaxation produced by sodium nitroprusside. −LogEC50 values for α-CGRP were estimated by interpolation from individual concentration-effect curves. The blocking potency of the antagonists against α-CGRP was estimated by analysis with EC50 concentration-ratios (CR; Arunlakshana & Schild, 1959). The error of CR was estimated by using log forms (−logEC50=pD2; −logEC50 in the presence of blocking agent=pD2B; Kaumann, 1990):
pKB values for the antagonists were calculated assuming slopes of one of the Schild-plot.
All data are expressed as mean±s.e.mean. Concentration-effect curves were fitted as hyperbolae with variable slope. Unless stated otherwise, number of experiments refer to number of patients. Statistical significance was determined by Student t-test, ANOVA and Mann – Whitney rank test. Differences were considered significant at P<0.05.
Drugs
5-Hydroxytryptamine creatine sulphate (5-HT) and Triton X-100 were purchased from Aldrich (Steinheim, Germany). Acetylcholine hydrochloride was obtained from Merck (Darmstadt, Germany). α-CGRP and CGRP(8-37) were purchased from Bachem (Heidelberg, Germany). 5-HT, SNP and papaverine hydrochloride were purchased from Sigma (Taufkirchen, Germany). BIBN4096BS was a gift of Dr Henri Doods (Boehringer Ingelheim, Biberach Germany).
Results
α-CGRP-evoked relaxations
All arterial rings studied relaxed with acetylcholine (Figure 1), consistent with functional integrity of the endothelium regardless of patients age, and with the absence of macroscopic atheroma lesions. α-CGRP was a potent relaxant (−logEC50=9.37±0.10, n=17 patients) and nearly as efficacious as SNP (Figures 1, 3 and 4). We inquired whether or not the endothelium participated in the relaxant effects of α-CGRP. The acetylcholine-induced relaxation of rings obtained from the Triton X-100-treated half was nearly abolished (13.1±14.0% decrease of the 5-HT-evoked contraction, five rings from three patients) compared to four rings from the untreated half (104.5±11.5% decrease of the 5-HT response). The −logEC50 values for α-CGRP did not differ significantly in the two groups (9.01±0.30 Triton X-100 vs 8.87±0.34 no Triton X-100). These results are consistent with the concept that in human temporal artery the effects of α-CGRP are mediated through receptors located at the membrane of the arterial smooth muscle and agree with a previous report (Jansen-Olesen et al., 1995).
Figure 3.
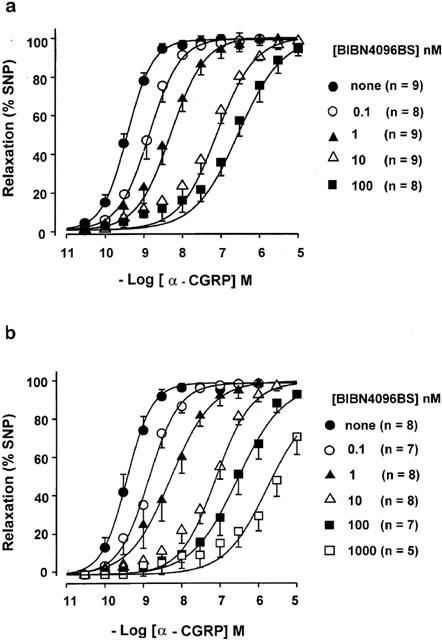
Antagonism of the relaxant effects of α-CGRP by BIBN4096BS, administered before (a) or during the KCl-contracture (b). Concentration-effect curves for α-CGRP are shown in the absence and presence of the indicated concentrations of BIBN4096BS. n=number of arterial rings. Data from 14 patients.
Figure 4.
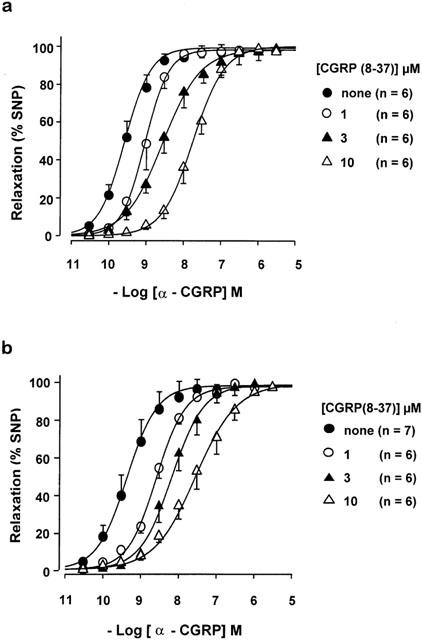
Antagonism of the relaxant effects of α-CGRP by CGRP(8-37) administered before (a) or during the KCl-contracture (b). Concentration-effect curves for α-CGRP are shown in the absence and presence of the indicated concentrations of CGRP (8-37). n=number of arterial rings. Data from 12 patients.
Antagonism of the effects of α-CGRP by BIBN4096BS and CGRP(8-37)
Higher concentrations of the blocking agents tended to produce small and inconsistent contractions, when administered before or during the KCl-contracture (Figures 1 and 2). Only 10 nM BIBN4096BS and 10 μM CGRP(8-37), administered during the KCl-contracture, enhanced significantly the KCl-contracture (Figure 2). The pH (7.40) of the solution remained unaltered during the contractions produced by the two blocking agents. The nature of the marginal contractions produced by BIBN4096BS and CGRP(8-37) is still unknown, but could be related to blockade of the relaxant effects of small amounts of CGRP released from the arterial nerve endings.
BIBN4096BS (0.1 – 100 nM) and CGRP(8-37) (1 – 10 μM) antagonized the effects of α-CGRP in surmountable manner, regardless of whether administered before or during (Figures 1, 3 and 4) the KCl-contracture. Although 1 μM BIBN4096BS caused additional blockade, it was partially insurmountable (Figure 3), conceivably due to insufficient dissociation of BIBN4096BS from the receptors. Therefore data with 1 μM BIBN4096BS were not included in the Schild-plot. Schild-plots for BIBN4096BS and CGRP(8-37) had slopes not significantly different from slope one under the two conditions (Figure 5, Table 1). The blocking potency of CGRP(8-37) was not different, while for unknown reasons BIBN4096BS appeared twice as potent (P<0.05, Student t-test between pKB values) when administered during the KCl-contracture than before the KCl-contracture (Figure 5). pKB values (Table 1) revealed that BIBN4096BS was a 3000 to 5000 times more potent antagonist than CGRP(8-37).
Figure 5.
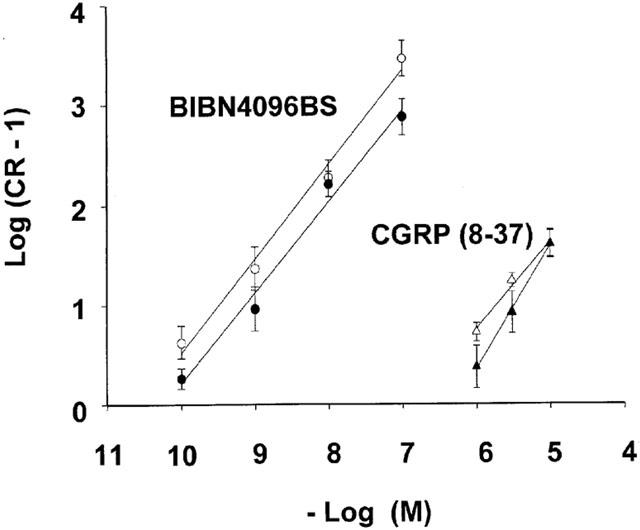
Schild-plots for BIBN4096BS and CGRP(8-37), administered before (solid circle) and during the KCl-contractures (open circle). For slopes and pKB values see Table 1.
Table 1.
Schild-plot analysis of BIBN4096BS and CGRP (8-37)
BIBN4096BS does not antagonize relaxations evoked by papaverine and SNP
BIBN4096BS (1 μM) failed to affect arterial relaxations produced by papaverine and SNP (Figure 6). The −logIC50 values for papaverine were 6.42±0.05 and 6.48±0.07 in the absence and presence of 1 μM BIBN4096BS respectively. The −logIC50 values for SNP were 7.77±0.16 and 7.62±0.16 in the absence and presence of 1 μM BIBN4096BS respectively.
Figure 6.
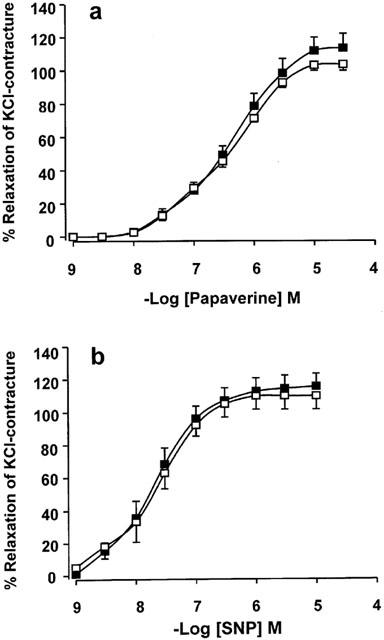
Lack of effects of BIBN4096BS (1 μM) on relaxations caused by papaverine (a) and SNP (b). Shown are curves obtained in the absence (solid square) and presence of BIBN4096BS (open square). Data from three patients with 5 – 6 arterial rings per group.
Discussion
BIBN4096BS is a potent competitive antagonist
BIBN4096BS is the first non-peptide blocker that antagonizes with high potency the relaxant effects of α-CGRP on rings of human temporal artery. The surmountable antagonism of the effects of CGRP caused by 0.1 – 100 nM BIBN4096BS and linear Schild-plots with slopes not different from one suggest specific interactions with CGRP receptors. The specificity is further supported by the failure of BIBN4096BS to antagonize the relaxant effects of papaverine and SNP, at a concentration (1 μM) four orders of magnitude greater than its KB for the CGRP receptors of the human temporal artery.
Most recently, a non-peptide compound chemically related to BIBN4096BS (patent number WO 98/11128) was reported to antagonize the relaxant effects of CGRP on human lenticulostriatal arteries, obtained >27 h after death (Edvinsson et al., 2001). However, the Schild-plot of data from that report yielded a slope of 0.67 and the affinity estimate for that compound (KB=40 nM) was 500 to 1000 times lower than the affinity estimated for BIBN4096BS from our data of competitive inhibition of CGRP-evoked relaxation of human temporal artery.
Our affinity estimate of BIBN4096BS for the CGRP-receptors of human temporal artery (KB= 40 – 90 pM) was somewhat lower than its affinity estimated from binding (Ki=14 pM) to CGRP-receptors of the SK-N-MC human neuroblastoma cell line (Doods et al., 2000). Reasons for this discrepancy are unknown but it could be that BIBN4096BS did not equilibrate completely with the CGRP-receptor population. This is unlikely though, because our affinity estimate was actually slightly lower with the longer BIBN4096BS incubation (2 h) before the KCl-contracture compared to the incubation (1 h) during the KCl-contracture.
Also for unknown reasons, CGRP(8-37) has been reported to bind with considerably higher affinity for CGRP receptors of the SK-N-MC cell line (Ki=3.6 nM, Doods et al., 2000; Ki=1.3 nM, Edvinsson et al., 2001) than estimated from its affinity for CGRP receptors of the human temporal artery (KB= 200 – 280 nM – this work), human middle meningeal artery (KB=150 nM, Edvinsson et al., 1998) and larger branches of lenticulostriate arteries obtained 24 h postmortem (KB∼300 nM, Sams et al., 2000). Unlike these discrepancies, Edvinsson et al. (2001) found that the affinity of CGRP(8-37) as antagonist of the relaxant effects of CGRP on lenticulostriatal arteries (KB=15 nM) agreed with its affinity as antagonist of CGRP-evoked increases in cyclic AMP in SK-N-MC cells (KB=16 nM). However, it is not clear why Edvinsson et al. (2001) estimated a 12 times higher affinity of CGRP(8-37) from binding assays than from cyclic AMP assays. Futhermore, it also unclear why Edvinsson et al. (2001) reported a KB=15 nM for CGRP(8-37) on lenticulostriatal arteries while they estimated a 20 times lower affinity (KB∼300 nM) on large branches (550 – 1727 μm diameter) of these arteries (Sams et al., 2000). One important difference that could also account for differences between the blocking potencies of CGRP(8-37) in arteries, is that the temporal arteries of our study were freshly obtained from patients undergoing surgery, whereas the lenticulostriatal arteries of Edvinsson et al. (2001) were obtained 27 – 32 h after death.
The relaxant potency of α-CGRP on the human temporal artery (EC50∼400 pM) was also lower than its binding affinity on the neuroblastoma cells (Ki=32 pM, Doods et al., 2000; 15 pM Edvinsson et al., 2001). It may be argued that the relaxant potency of α-CGRP found by us for the temporal artery was relatively low due to our experimental conditions of partially depolarized membranes produced by small KCl elevations. However, the relaxant potency α-CGRP against KCl-precontracted arterial rings agrees with that found for human temporal artery preparations precontracted with the prostaglandin F2α (EC50∼400 pM, Jansen-Olesen et al., 1995). Nanomolar concentrations of CGRP hyperpolarize the membrane of arterial smooth muscle cells (Nelson et al., 1990) through activation of ATP-dependent K+ current (Quayle et al., 1994) and this mechanism contributes to vascular relaxation. Large-conductance, Ca2+-activated K+ (BKCa) channels hyperpolarize the membrane of smooth muscle cells, when activated through phosphorylation by cyclic AMP-dependent protein kinase (PKA) (Schubert & Nelson, 2001). CGRP appears to produce PKA-mediated BKCa channel activation in porcine coronary arterial cells (Miyoshi & Nakaya, 1995), thereby contributing to relaxation, and we suggest that this pathway may also play a role in human temporal artery. The lower arterial relaxant potency than neuroblastoma binding affinity of CGRP could be related to the partial depolarization of the vascular cells under our conditions.
Both PKA and cyclic GMP-dependent protein kinase (GPK) phosphorylate BKCa channels, thereby causing sensitization to Ca2+-induced activation, hyperpolarization and relaxation of vascular smooth cells (Schubert & Nelson, 2001). It is likely that papaverine and SNP relax the temporal artery at least in part through activation of PKA and GPK respectively, via BKCa activation. Because BIBN4096BS (1 μM) failed to modify the relaxations produced by both papaverine and SNP, the antagonist does not interfere with the PKA and PKG pathways. Furthermore, the partially insurmountable antagonism of the CGRP effects by 1 μM BIBN4096BS is therefore unlikely to be due to non-specific depression of the CGRP effects, but possibly related to the slow dissociation of BIBN4096BS from the CGRP receptors.
The lower blocking potency of the two antagonists and lower agonist potency of α-CGRP on the temporal artery compared to the corresponding binding affinities in neuroblastoma cells, could also be due to differences in CGRP receptors of the two systems or different regulation by endogenous modulators. Receptor activity modifying proteins, RAMPs, are modulators that determine the pharmacology of CGRP and other peptides (McLatchie et al., 1998). In addition, an accessory protein, CGRP-receptor component protein (RCP), has been found to facilitate coupling of the receptor to the Gs-protein/cyclic AMP pathway (Evans et al., 2000). We speculate that RAMP modulation of the receptor that interacts with α-CGRP and/or RCP modulation of its coupling could be different in human temporal artery and neuroblastoma cells.
Possible therapeutic relevance
Up to one third of migraine sufferers undergo dilatation of extracranial arteries (Lance, 1992), such as the temporal artery. Our results, showing that BIBN4096BS is a potent antagonist of the relaxant effects of α-CGRP in the temporal artery suggest that CGRP can be involved in migraine. CGRP, released from the sensory nerves of the temporal artery, would interact with CGRP receptors, enhance cyclic AMP levels in the smooth muscle (Jansen-Olesen et al., 1996) thereby inducing vasodilatation that in turn activates trigeminal fibres and migraine pain (Uddman et al., 1986). Blockade of the CGRP receptors by BIBN4096BS would be expected to prevent the harmful effects of CGRP. Clinical trials will be required to test this hypothesis.
We conclude that BIBN4096BS is the first potent non-peptide and competitive antagonist of CGRP-evoked relaxation of a human extracranial artery. The ability of BIBN4096BS to block CGRP-evoked vasodilatation in a human extracranial artery might lead to therapeutic benefits.
Abbreviations
- Ach
acetylcholine
- BIBN4096BS
1-Piperidinecarboxamide, N-[2-[[5-amino-1-[[4-(4-pyridinyl)-1-piperazinyl]carbonyl]pentyl]amino]-1-[(3,5-dibromo-4-hydroxyphenyl)methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo -3(2H) -quinazolinyl)-, [R-(R*,S*)]-
- α-CGRP
α-calcitonin gene-related peptide
- 5-HT
5-hydroxytryptamine
- SNP
sodium nitroprusside
References
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÖHME E., GRAF H., SCHULTZ G. Effects of sodium nitroprusside and other smooth muscle relaxants on cyclic GMP formation in smooth muscle and platelets. Advances Cyclic Nucleotide Research. 1978;9:131–143. [PubMed] [Google Scholar]
- CHASIN M., HARRIS D.N. Inhibitors and activators of cyclic nucleotide phosphodiesterases. Advances Cyclic Nucleotide Research. 1976;7:223–264. [PubMed] [Google Scholar]
- CHIBA T., YAMAGUCHI A., YAMATANI T., NAKAMURA A., MORISHITA T., INUI T., FUKASE M., NODA T., FUJITA T.Calcitonin gene-related peptide receptor antagonist human CGRP-(8-37) Am. J. Physiol. 1989256E331–E335.(Endocrinol. Metab. 19 [DOI] [PubMed] [Google Scholar]
- DEMESY-WAELDELE F., STOCLET J.C. Effect of papaverine on cyclic nucleotide levels in the isolated rat aorta. Eur. J. Pharmacol. 1977;46:63–66. doi: 10.1016/0014-2999(77)90145-5. [DOI] [PubMed] [Google Scholar]
- DOODS H., HALLERMAYER G., WU D., ENTZEROTH M., RUDOLF K., ENGEL W., EBERLEIN W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br. J. Pharmacol. 2000;129:429–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDVINSSON L., GULBENKIAN S., BARROSO C., CUNHA E SÁ M., POLAK J. M., MORTENSEN A., JØRGENSEN L., JANSEN-OLESEN I. Innervation of the human middle meningeal artery: immunohistochemistry, ultrastructure, and role of endothelium for vasomotility. Peptides. 1998;19:1213–1225. doi: 10.1016/s0196-9781(98)00066-7. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., SAMS A., JANSEN-OLESEN I., TAJTI J., KANE S.A., RUTLEDGE R.Z., KOBLAN K.S., HILL R.G., LONGMORE J. Characterisation of the effects of a non-peptide CGRP receptor antagonist in SK-N-MC cells and isolated human cerebral arteries. Eur. J. Pharmacol. 2001;415:39–44. doi: 10.1016/s0014-2999(00)00934-1. [DOI] [PubMed] [Google Scholar]
- EVANS B.N., ROSENBLATT M. I., MNAYER L. O., OLIVER K.R., DICKERSON I.M. CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J. Biol. Chem. 2000;275:31438–31443. doi: 10.1074/jbc.M005604200. [DOI] [PubMed] [Google Scholar]
- FUJIOKA M. Lack of causal relationship between the vasodilator effect of papaverine and cyclic AMP production in the dog basilar artery. Br. J. Pharmacol. 1984;83:113–124. doi: 10.1111/j.1476-5381.1984.tb10125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L., EKMAN R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann. Neurol. 1988;23:193–196. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L., EKMAN R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- GRAHAM J.P., WOLFF H.G. Mechanism of migraine headache and the action of ergotamine tartrate. Arch. Neurol. Psychiatry. 1938;39:737–763. [PubMed] [Google Scholar]
- HAMEL E., ASSUMEL-LURDIN C., EDVINSSON L., FAGE D., MACKENZIE E.T. Neuronal versus endothelial origin of vasoactive acetylcholine in pial vessels. Brain Research. 1987;420:391–396. doi: 10.1016/0006-8993(87)91263-7. [DOI] [PubMed] [Google Scholar]
- JANSEN I., UDDMAN R., HOCHERMAN M., EKMAN R., JENSEN K., OLESEN J., STIERNHOLM P., EDVINSSON L. Localization and effects of neuropeptide Y, vasoactive intestinal polypeptide, substance P, and calcitonin gene-related peptide in human temporal arteries. Ann. Neurol. 1986;20:496–501. doi: 10.1002/ana.410200409. [DOI] [PubMed] [Google Scholar]
- JANSEN-OLESEN I., GULBENKIAN S., VALENÇA A., ANTUNES J.L., WHARTON J., POLAK J.M., EDVINSSON L. The peptidergic innervation of the human superficial temporal artery: immunohistochemistry, ultrastructure, and vasomotility. Peptides. 1995;16:275–287. doi: 10.1016/0196-9781(94)00165-0. [DOI] [PubMed] [Google Scholar]
- JANSEN-OLESEN I., MORTENSEN A., EDVINSSON L. Calcitonin gene-related peptide is released from capsaicin-sensitive nerve fibres and induces vasodilatation of human cerebral arteries concomitant with activation with adenylyl cyclase. Cephalalgia. 1996;16:310–316. doi: 10.1046/j.1468-2982.1996.1605310.x. [DOI] [PubMed] [Google Scholar]
- JANSEN-OLESEN I., OTTOSSON A., CANTERA L., STRUCK S., LASSEN L.H., OLESEN J., MORTENSEN A., ENGEL U., EDVINSSON L. Role of the endothelium and nitric acid in histamine-induced responses in human cranial arteries and detection of mRNA encoding H1- and H2-receptors by RT–PCR. Br. J. Pharmacol. 1997;121:41–48. doi: 10.1038/sj.bjp.0701097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANEDA T., SHIMIZU K., NAKAJYO S., URAKAWA N. The difference in the inhibitory mechanisms of papaverine on vascular and intestinal smooth muscles. Eur. J. Pharmacol. 1998;355:149–157. doi: 10.1016/s0014-2999(98)00479-8. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J. Piglet sinoatrial 5-HT receptors resemble human atrial 5-HT4-like receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1990;342:619–622. doi: 10.1007/BF00169055. [DOI] [PubMed] [Google Scholar]
- LANCE J.W.History of involvement of 5-HT in primary headaches 5-Hydroxytryptamine Mechanisms in Primary Headaches 1992New York: Raven Press; 19–28.Vol 2., ed. Olesen, J., Saxena, P.R. pp [Google Scholar]
- MCLATCHIE L.M., FRASER N.J., MAIN M.J., WISE A., BROWN J., THOMPSON N., SOLARI R., LEE M.G., FOORD S.M. RAMPs regulate the transport and ligand specificity of the calcitonin receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- MIYOSHI H., NAKAYA Y. Calcitonin gene-related peptide activates the K+ channels of vascular smooth muscle cells via adenylate cyclase. Basic Res. Cardiol. 1995;90:332–336. doi: 10.1007/BF00797911. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., HUANG Y., BRAYDEN J.E., HESCHELER J., STANDEN N.B. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990;344:770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- QUAYLE J.M., BONEV A.D., BRAYDEN J.E., NELSON M.T. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J. Physiol. 1994;475:9–13. doi: 10.1113/jphysiol.1994.sp020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMS A., KNYIHAR-CSILLIK E., ENGBERG J., SZOK D., TAJTI J., BODI I., EDVINSSON L., VECSEI L., JANSEN-OLESEN I. CGRP and adrenomedullin receptor populations in human cerebral arteries: in vitro pharmacological and molecular investigations in different artery sizes. Eur. J. Pharmacol. 2000;408:183–193. doi: 10.1016/s0014-2999(00)00781-0. [DOI] [PubMed] [Google Scholar]
- SCHUBERT R., NELSON M.T. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacological Sciences. 2001;22:505–512. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- UDDMAN R., EDVINSSON L., JANSEN I., STIERNHOLM P., JENSEN K., OLESEN J., SUNDLER F. Peptide-containing nerve fibres in human extracranial tissue: a morphological basis for neuropeptide involvement in extracranial pain. Pain. 1986;27:391–399. doi: 10.1016/0304-3959(86)90162-4. [DOI] [PubMed] [Google Scholar]
- VERHEGGEN R.Untersuchungen an der 5-HT-induzierten Kontraktion beteiligten Serotoninrezeptoren bei menschlichen Temporalarterien 2000. ISBN 3-8265-8074-5 Shaker-Verlag, Aachen
- VERHEGGEN R., BUMANN K., WEBEL M., MEIER A., KAUMANN A.J. BIBN4096BS is a potent antagonist of the relaxant effects of CGRP on human temporal artery. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:R36. [Google Scholar]
- VERHEGGEN R., FREUDENTHALER S., MEYER-DULHEUER F., KAUMANN A.J. Participation of 5-HT1-like and 5-HT2A receptors in the contraction of human temporal artery by 5-hydroxytryptamine and related drugs. Br. J. Pharmacol. 1996;117:283–292. doi: 10.1111/j.1476-5381.1996.tb15188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERRECCHIA C., SERCOMBE V., PHILIPSON V., SEYLAZ J. Functional destruction of cerebral vascular endothelium by Triton X-100. Blood Vessels. 1986;23:106. doi: 10.1159/000158632. [DOI] [PubMed] [Google Scholar]


