Abstract
From its original description as solely an intracellular molecular chaperone, heat shock proteins have now been shown to function as initiators of the host's immune response. Although the exact mechanism by which intracellular heat shock proteins leave cells is still incompletely understood, recent work from several labs suggest that heat shock proteins are released by both passive (necrotic) and active (physiological) mechanisms. Binding to specific surface receptors is a prerequisite for the initiation of an immune response. To date, several cell surface proteins have been described as the receptor for seventy kilo-Dalton heat shock protein (Hsp70) including Toll-like receptors 2 and 4 with their cofactor CD14, the scavenger receptor CD36, the low-density lipoprotein receptor-related protein CD91, the C-type lectin receptor LOX-1, and another member of the scavenger super-family SR-A plus the co-stimulatory molecule, CD40. Binding of Hsp70 to these surface receptors specifically activates intracellular signaling cascades, which in turn exert immunoregulatory effector functions; a process known as the chaperokine activity of Hsp70. This review will highlight recent advances in understanding the mechanism by which Hsp70 initiates the host's immune response.
Keywords: Chaperokine; exosomes; exercise, heat shock proteins; inflammation; lipid rafts; protein transport
Introduction
Heat shock proteins (Hsp) are highly conserved proteins found in all prokaryotes and eukaryotes. In response to a wide variety of stressful stimuli, there is a marked increase in total Hsp synthesis (38), known as the cellular stress response. The stress response is designed to enhance the ability of the cell to cope with increasing concentrations of unfolded or denatured proteins. Of all heat shock proteins, the Hsp70 family constitutes the most conserved and best-studied class. This family consists of the constitutively expressed Hsp70 (Hsc70; 73 kDa), the stress inducible Hsp70 (Hsp70; 72 kDa), the mitochondrial Hsp70 (Hsp75; 75 kDa), and the endoplasmic reticulum Hsp70 (Grp78; 78 kDa). The function of Hsp70 is exquisitely related to its structure. The Hsp70 family members all contain two major functional domains, including a N-terminal domain, also referred to as the ATPase domain which is composed of 45 kDa amino acids, and a C-terminal domain composed of a 15–18 kDa substrate-binding domain (SBD), and a 10 kDa carboxy-terminal domain of largely unknown function (18, 41). The Nand C-terminal domains have been shown to be critical in Hsp70 mediated cancer immunoediting by mediating the acquisition of cellular antigens and their delivery to immune effector cells (18, 37, 48, 62, 69).
The term cancer immunoediting has replaced the term cancer immunosurveillance. The immunoediting hypothesis states that the immune system function during tumor growth to select the tumor variants which are better suited to survive an immunologically intact environment (21). This hypothesis is based on the existence of two paradoxical phenomena that occur during tumor growth. First, the immune system is alerted to the presence of the growing tumor by the release of “danger signals” including Hsp's, cytokines and chemokines which attract leukocytes into the tumor site and activate specific CTL responses, a process known as an anti-tumor response. Second, the anti-tumor responses exerts tumor sculpting effects that result in the selection of the tumor variants which survive in immunologically intact hosts, thus escaping from immune-mediated killing (21).
The primary function ascribed to Hsp72 was as an intracellular molecular chaperone of naïve, aberrantly folded, or mutated proteins as well as in cytoprotection following the kinds of stressful stimuli including environmental (U. V. radiation, heat shock, heavy metals and amino acids), pathological (viral, bacterial, parasitic infections or fever, inflammation, malignancy or autoimmunity) or physiological stimuli (growth factors, cell differentiation, hormonal stimulation or tissue development), induces a marked increase in intracellular Hsp72 synthesis (38, 39), known as the stress response.
Reports indicating elevated levels of antibodies against the inducible form of Hsp70 in patients with autoimmune diseases (43) led our group to speculate that Hsp72 is found in the extracellular milieu and could exert chaperoning and regulatory effects on various immunocompetent cells. We later extended these findings and demonstrated that exogenously added Hsp72 possesses potent chaperone and cytokine activity, a term now referred to as the chaperokine ability of Hsp72 (5). This review will briefly underscore recent advances in understanding the role of this unique protein describing how it is released from cells to how it binds and stimulates the host's immune response.
Mechanisms of escape: Passive or active?
Initial studies from Gallucci and co-workers demonstrated that dendritic cells (DC) are stimulated by endogenous signals received from stressed, virally infected or necrosis-induced cells, but not by healthy cells or cells undergoing apoptosis (25). The Srivastava group subsequently demonstrated that necrotic but not apoptotic cell death leads to release of HSP's including gp96, calreticulin, Hsp90 and Hsp70 (8). These authors showed that exposure of DC to necrotic but not apoptotic cells resulted in the nuclear translocation of NF-κB and subsequent maturation of DC (8), for review see (60). Although additional studies for other laboratories clearly demonstrates that cellular necrosis induces the release of Hsp's from cells (12, 55). Necrotic cell death is highly unregulated and in the majority of cases only occurs during trauma. We hypothesized that other physiological mechanism must be at work.
Prior to these studies, Pockley and coworkers demonstrated the presence of soluble Hsp70 and antibodies against Hsp70 in the peripheral circulation of normal individuals (52). These and other authors later demonstrated elevated levels of circulating serum Hsp70 during a variety of disease including atherosclerosis (51), hypertension (50) and renal disease (70), sickle cell disease (1) and in aging (63). One might argue that released Hsp70 during disease does not negate the possibility that Hsp70 is getting into the circulation via a necrotic cell death pathway. However, three lines of evidence seem to point to an active release mechanism. First, data from the Febbraio laboratory demonstrate that physical exercise results in the appearance of Hsp70 in the circulation prior to any increase in gene or protein expression in contracting skeletal muscle (68). Additional studies have confirmed these findings and recently it was demonstrated that both intensity and duration of exercise influences the concentration of released eHsp72 in the plasma (23). Second, psychological stress induced by exposure of Sprague Dawley rats to cats results in the release of Hsp72 into the circulation (24). Third, the human brain is able to release Hsp72 into the circulation in response to exercise (35).
In an in vitro study, Guzhova and colleagues demonstrated that Hsp70 is released by glia cells in the absence of necrotic cell death (31). More recently, Hsp70 has been shown to be released by B cells (19) and peripheral blood mononuclear cells (33) and also under non necrotic conditions. Using conditions will not induce significant cell death, we showed that IFN-γ and IL-10 induce the active release of constitutive seventy-kilo-Dalton heat shock protein, Hsc70 from tumors (7). However, these initial studies did not address the mechanism of release. Recently, our group (9, 28) and others (34) have begun to elucidate the mechanism of active release of Hsp70 from viable cells.
In our study, we showed that certain cytokines normally found in high concentrations within inflammatory foci including IFN-γ, IL-10 but not TGF-β1, mediate the active release of Hsp72. We further showed that whereas some Hsp72 could be found as free Hsp72, a proportion of Hsp72 was released within exosomes (9). Exosomes are internal vesicles of multi-vesicular bodies (MVB) that are released into the extracellular milieu upon fusion of multivesicular bodies MVB with the cell surface (53, 71, 72). In addition to containing Hsp72 (9, 28), exosomes are highly packed with immunostimulatory mediators including MHC class I and II (53, 71, 72) and costimulatory molecules (22). This seems to help explain the potent effect observed by HSP-based immunotherapy against certain cancer (59, 61). Our group recently demonstrated that Hsp72 was released by a non classical protein transport pathway and that intact surface membrane lipid rafts were required for efficient stress-induced Hsp72 release (9, 28). Similar findings were demonstrated in B cells (19). Studies by Lancaster & Febbraio recently demonstrate that exosomes provide the major pathway for secretory vesicular release of Hsp72 (34). However, using methyl-β-cyclodextrin (the cholesterol depleting agent) to disrupt lipid raft function, these authors were unable to confirm a role for lipid rafts in stress-induced Hsp72 release from human peripheral blood mononuclear cells PBMC (34). In order to address the cellular location of Hsp72 after stress, a recent study demonstrated that newly synthesized Hsp72 protein localizes within the Golgi region of HELA cells and also concentrates on the surface of the plasma membrane and in the ruffled zone of migrating cells (56).
The binding step: Hsp70 receptor
For Hsp70 to be an effective danger signal, it must bind to specific cell receptors. Hsp70 has been shown to bind selectively and with high specificity and affinity to a number of cells including natural killer (NK) cells (30, 45, 46), dendritic cells (DC) (6, 54), macrophages, peripheral blood monocytes (4-6), and B cells (2). In contrast, T lymphocytes do not bind HSP70 (2). Our group was the first to demonstrate that the Toll-like receptors 2 and 4 with their cofactor CD14 is a receptor for Hsp70 (6). These results were independently corroborated by others (66). In addition, to date the list of putative Hsp70 receptors have grown and now includes the scavenger receptor, CD36 (20, 47), the co-stimulatory molecule, CD40 (10), the low-density lipoprotein receptor-related protein CD91 (14-17), Lox-1 (20, 64) and SR-A, another member of the scavenger superfamily (11, 32).
The signaling step
Immediately following binding, extracellular Hsp70 (eHsp70) stimulates signal transduction cascade dependent on the receptor to which it is bound (for review see (3)). Binding of eHsp70 to the surface of human monocytes stimulates rapid intracellular Ca2+ flux (10 seconds), activate NF-κB (30 minutes) and upregulate the expression of pro-inflammatory cytokines in human monocytes (2 h post challenge) (5). We reported that eHSP70-induced pro-inflammatory cytokine production is mediated via the myeloid differentiation factor 88/IL-1R-associated kinase/NF-κB (MyD88/IRAK/NF-κB) signal transduction pathway and that eHSP70 utilizes both TLR2 (receptor for Gram positive bacteria) and TLR4 (receptor for Gram negative bacteria) to transduce its pro-inflammatory signal in a CD14-dependant fashion (6). Human monocytic cells, THP1 transfected with the dominant negative MyD88 plasmid or a combination of both dominant negative TLR2 and TLR4 inhibited HSP70-induced IL-1β expression (3). However, only a combination of dominant negative MyD88/TLR2/TLR4 completely inhibited eHSP70-induced IL-1β expression (3). These results were independently corroborated by the work of Vabulus and co-workers (66). In addition, these authors demonstrated that Hsp70 directly stimulated IKK and JNK kinase signaling by APCs and that this effect could be partially eliminated by knocking out either MyD88 or TRAF6 (66).
Signaling of eHsp70 via the scavenger superfamily including CD91, Lox-1 and SR-A has been shown to activate NF-κB signaling (17, 64). Further upstream signaling remains to be elucidated. On the other hand, binding of eHsp70 to the costimulatory molecule, CD40 has not been shown to directly activate the MyD88 adaptor protein but to instead activate TNF-associated factor-6 (TRAF6) (10) before bifurcating to either the NF-κB or the mitogen activated protein kinase (MAPK) signal transduction pathway.
Initiation of immune responses: the chaperokine activity of Hsp70
Chaperokine, is a recent term coined to better describe the unique function of extracellular Hsp72 (eHsp72) as both chaperone and cytokine (for review, see Ref. (3)). After admixing eHsp70 to APCs, specific signal transduction pathways are activated that result in the stimulation of an immune response. eHsp72 induces a plethora of immune responses and the list continues to grow. Our group demonstrated that as early as 2-4 hours post exposure of APC to exogenous eHsp70, there is significant release of cytokines including TNF-α, IL-1β, IL-6 and IL-12 (5, 6) and GM-CSF (58); nitric oxide, a potent apotogenic mediator (49); chemokines including MIP-1, MCP-1 and RANTES (36, 49) (Fig. 1). Earlier, we had demonstrated that both peptide-bearing and non peptide-bearing eHsp70 is capable of inducing pro-inflammatory cytokine production by APCs (4). eHsp72 induces the DC maturation by augmenting the surface expression of CD40, CD83, CD86 and MHC class II molecules on DC (6, 8, 48, 57) and migration of DC (13) and NK cells (28)(Fig. 1).
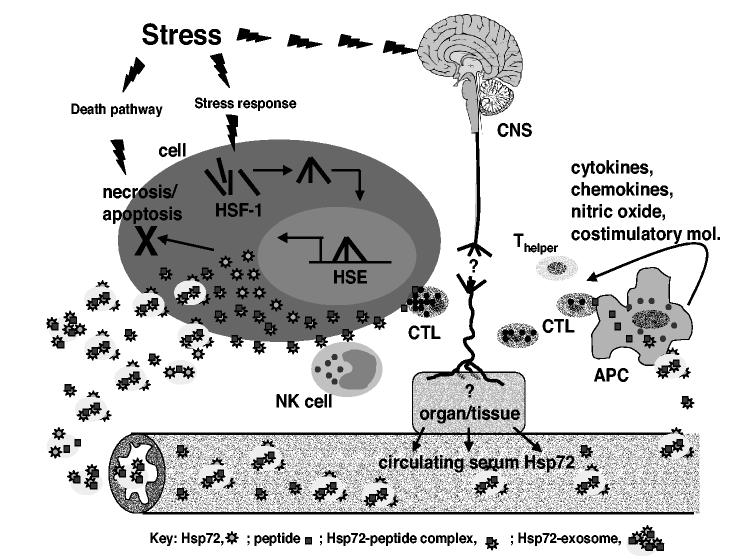
Figure 1.
Schematic representation of stress-induced release of eHsp72. Stress (lightning blot) activates at least three pathways that result in the release of Hsp72 into the circulation. First, if trauma to the cell is overwhelming stress will activates the death pathway either by necrosis or apoptosis and Hsp72 is released from the cell. Second, stress can activate the stress response by triggering the trimerization and nuclear translocation of cytoplasmic HSF-1 (brown rods) to the heat shock element (HSE) and subsequent transcription of Hsp72 (stars). The increased intracellular Hsp72 chaperones peptides (Hsp72-peptide complex) and protects the cell from cell death under certain conditions. The Hsp72-peptide complex is expressed on the cell surface and released into the extracellular milieu within exosomes; Hsp72-exosomes (yellow circle). Hsp72-peptide complexes (Hsp72-pc) and Hsp72-exosomes (Hsp72-ex) make their way into the circulation and can be measured by classical sandwich ELISA. Antigen presenting cells bind and internalize Hsp72-pc and Hsp72-ex. Binding to specific receptors mediated a signal transduction cascade that results in the initiation of an immune response characterized by the upregulation of pro-inflammatory cytokines, chemokines, nitric oxide and costimulatory molecules. Internalization of Hsp72-pc and Hsp72-ex allows the peptides to be processed and presented in the context of MHC class I to cytotoxic T lymphocytes (CTL). CTL's become activated and will recognize and destroy cells presenting the specific peptide. Circulating Hsp72-ex induces NK cells migration and the expression of Hsp72-pc on the surface of stressed cells activates NK lytic functions. Thirdly, stress in form of physical of psychological stress will stimulate the release of Hsp72-pc and Hsp72-ex into the circulation by a hitherto unknown mechanism and by a yet to be discovered tissue/organ.
The Hsp70-endotoxin debate
It is imperative to note that important questions still remain unanswered about the putative Hsp70 receptor and clearly much work remains, especially pertaining the structural data on interaction of Hsp70 with nearly all of the receptor candidates (for review see (17)). In addition, the debate that endotoxin contamination of certain recombinant Hsp72 preparations mediates the chaperokine activity of Hsp70 rather than pure Hsp70 itself (26, 27, 65), has now been addressed by our group (9) and independently corroborated by others (37, 40, 42, 69). Briefly, when special care is taken to control for endotoxin contamination, clear effects of Hsp70 can be demonstrated. Some precautions including physical assays like addition of an additional microdialysis step to remove unbound peptide and other contaminants, passing all Hsp70 protein preparations through polymyxin B column and only using preparations with less than <1.0 endotoxin units per 20 μg of protein must be performed. In addition, functional assays include boiling Hsp70 protein preparations at 100°C, 60 minute, which denatures Hsp70 but not LPS. Pre-treatment of Hsp70 preparations with protinase K, which inhibit Hsp70-induced, but not LPS induced-cytokine release by APC. Addition of soluble CD14 to macrophages enhances LPS-induced, but not Hsp70-induced cytokine release. Pre-treatment of macrophages with BAPTA-AM, an intracellular calcium chelator, inhibits efficient Hsp70-induced, but not LPS induced-cytokine release, and measurement of intracellular calcium flux, which is only induced by Hsp70, not LPS (5, 40). When similar measures were used to control for LPS contamination of rHsp70 preparations, Hsp70 augments dendritic cell (DC) effector functions and when admixed with specific antigens, triggers autoimmune diseases in vivo (42), and DC pulsed with peptide loaded rHsp70 generated potent antigen-specific CTL responses (40). However, mutation of the peptide binding domain of Hsp70, rendered the mutants incapable of generating antigen-specific CTL responses (40). Since the results were based on recombinant technology and yet both preparations elicit disparate effects, this seems to exclude the possibility that endotoxin contamination of Hsp70 preparations is responsible for their observed effects. Finally, it must be noted that although the most up-to-date techniques to eliminate endotoxin contamination may be used, it is virtually impossible to completely eliminate this possibility. This is a problem for all researchers using recombinant proteins and not just Hsp70. However, all these studies strongly suggest that when special care is taken to control for LPS contamination, clear effects of Hsp70 can be demonstrated.
Interestingly, the immune effects of circulating Hsp72 in the host are not encumbered by these questions. It is quite plausible that in the circulation, Hsp70 might chaperone endotoxin. Due to eHsp72s chaperone ability, it is highly unlikely that one will find peptide-free eHsp72. We predict that in vivo, circulating Hsp72 will chaperone not only peptides from the parent cell, but quite possibly bacterial fragments/products it finds in the circulation. To underline this point, a recent study demonstrated that psychological stress induced the release of bacteria from the gut (67). Since psychological stress induces the release of eHsp70 into the circulation, it is quite probable that when isolated, purified, and enriched, samples of circulating eHsp70 will be found chaperoning endotoxin. However, this hypothesis needs to be tested.
Conclusion
This review has draw attention to recent advances in our understanding the mechanism by which eHsp70 initiates hosts immune response. eHsp72 is released into the extracellular milieu within exosomes by a non classical protein transport mechanism, requiring intact lipid rafts. In addition, eHsp72 can also be released via necrotic as would be expected during non-physiological conditions including trauma. eHsp72 is found in the circulation of healthy individuals, however elevated levels can be found in various disease conditions and in response to moderate exercise and acute psychological stress. eHsp72 binds with high affinity and avidity to specific cells of the immune system and activates specific signal transduction cascades depending on which receptor it bind. Finally, eHsp72 stimulates the hosts immune response in an attempt to rid the host of infection or tumors.
Exciting work now remains to be performed that will decipher the source and exact target of circulating serum Hsp72 in psychological stress and exercise. Since this information will allow researchers to develop highly effective pharmacological or molecular tools for patients with “burnout” syndrome (44) and the super athletes (29) both of whom are exquisitely prone to infections.
Acknowledgements
This work was supported in part by the National Institute of Health grant RO1CA91889, Scott & White Clinic, the Central Texas Veterans Health Administration and an Endowment from the Cain Foundation.
Abbreviations used in this paper
- AChE
acetylcholinesterase
- eHsp72
extracellular Hsp72
- ER
endoplasmic reticulum
- Hsp
heat shock proteins
- Hsc70
constitutively expressed seventy-kilo Dalton heat shock protein
- Hsp72
stress inducible seventy-kilo Dalton heat shock protein
- HSF-1
heat shock factor-1
- IFN-γ
interferon-gamma
- IL
interleukin
- LDH
lactate dehydrogenase
- MßD
methyl ß-cyclodextrin
- TGF-β1
transforming growth factor-beta1.
References
- 1.Adewoye AH, Klings ES, Farber HW, Palaima E, Bausero MA, McMahon L, Odhiambo A, Surinder S, Yoder M, Steinberg MH, Asea A. Sickle cell vaso-occlusive crisis induces the release of circulating serum heat shock protein-70. Am J Hematol. 2005;78:240–242. doi: 10.1002/ajh.20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee H-G, de la Salle H, Schild H. Receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162:3757–3760. [PubMed] [Google Scholar]
- 3.Asea A. Chaperokine-induced signal transduction pathways. Exerc Immunol Rev. 2003;9:25–33. [PMC free article] [PubMed] [Google Scholar]
- 4.Asea A, Kabingu E, Stevenson MA, Calderwood SK. HSP70 peptide-bearing and peptide-negative preparations act as chaperokines. Cell Stress Chaperones. 2000;5:425–431. doi: 10.1379/1466-1268(2000)005<0425:hpbapn>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 6.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 7.Barreto A, Gonzalez JM, Kabingu E, Asea A, Fiorentino S. Stress-induced release of HSC70 from human tumors. Cell Immunol. 2003;222:97–104. doi: 10.1016/s0008-8749(03)00115-1. [DOI] [PubMed] [Google Scholar]
- 8.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 9.Bausero MA, Gastpar R, Multhoff G, Asea A. Alternative Mechanism by which IFN-{gamma} Enhances Tumor Recognition: Active Release of Heat Shock Protein 72. J Immunol. 2005;175:2900–2912. doi: 10.4049/jimmunol.175.5.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker T, Hartl FU, Wieland F. CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J Cell Biol. 2002;158:1277–1285. doi: 10.1083/jcb.200208083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berwin B, Hart JP, Rice S, Gass C, Pizzo SV, Post SR, Nicchitta CV. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. EMBO J. 2003;22:6127–6136. doi: 10.1093/emboj/cdg572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berwin B, Reed RC, Nicchitta CV. Virally induced lytic cell death elicits the release of immunogenic GRP94/gp96. J Biol Chem. 2001;276:21083–21088. doi: 10.1074/jbc.M101836200. [DOI] [PubMed] [Google Scholar]
- 13.Binder RJ, Anderson KM, Basu S, Srivastava PK. Cutting edge: heat shock protein gp96 induces maturation and migration of CD11c+ cells in vivo. J Immunol. 2000;165:6029–6035. doi: 10.4049/jimmunol.165.11.6029. [DOI] [PubMed] [Google Scholar]
- 14.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 15.Binder RJ, Karimeddini D, Srivastava PK. Adjuvanticity of alpha 2-macro-globulin, an independent ligand for the heat shock protein receptor CD91. J Immunol. 2001;166:4968–4972. doi: 10.4049/jimmunol.166.8.4968. [DOI] [PubMed] [Google Scholar]
- 16.Binder RJ, Srivastava PK. Essential role of CD91 in re-presentation of gp96-chaperoned peptides. Proc Natl Acad Sci U S A. 2004;101:6128–6133. doi: 10.1073/pnas.0308180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binder RJ, Vatner R, Srivastava P. The heat-shock protein receptors: some answers and more questions. Tissue Antigens. 2004;64:442–451. doi: 10.1111/j.1399-0039.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 18.Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 19.Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci. 2005;118:3631–3638. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- 20.Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, Kawakami-Honda N, Goetsch L, Sawamura T, Bonnefoy J, Jeannin P. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 21.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 22.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 23.Fehrenbach E, Niess AM, Voelker K, Northoff H, Mooren FC. Exercise intensity and duration affect blood soluble HSP72. Int J Sports Med. 2005;26:552–557. doi: 10.1055/s-2004-830334. [DOI] [PubMed] [Google Scholar]
- 24.Fleshner M, Campisi J, Amiri L, Diamond DM. Cat exposure induces both intraand extracellular Hsp72: the role of adrenal hormones. Psychoneuroendocrinology. 2004;29:1142–1152. doi: 10.1016/j.psyneuen.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 26.Gao B, Tsan MF. Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor alpha release by murine macrophages. J Biol Chem. 2003;278:174–179. doi: 10.1074/jbc.M208742200. [DOI] [PubMed] [Google Scholar]
- 27.Gao B, Tsan MF. Recombinant human heat shock protein 60 does not induce the release of tumor necrosis factor alpha from murine macrophages. J Biol Chem. 2003;278:22523–22529. doi: 10.1074/jbc.M303161200. [DOI] [PubMed] [Google Scholar]
- 28.Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65:5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gleeson M, Nieman DC, Pedersen BK. Exercise, nutrition and immune function. J Sports Sci. 2004;22:115–125. doi: 10.1080/0264041031000140590. [DOI] [PubMed] [Google Scholar]
- 30.Gross C, Hansch D, Gastpar R, Multhoff G. Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biol Chem. 2003;384:267–279. doi: 10.1515/BC.2003.030. [DOI] [PubMed] [Google Scholar]
- 31.Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73. doi: 10.1016/s0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- 32.Haworth R, Platt N, Keshav S, Hughes D, Darley E, Suzuki H, Kurihara Y, Kodama T, Gordon S. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J Exp Med. 1997;186:1431–1439. doi: 10.1084/jem.186.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunter-Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM, Williams JH. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–517. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 34.Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280:23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- 35.Lancaster GI, Moller K, Nielsen B, Secher NH, Febbraio MA, Nybo L. Exercise induces the release of heat shock protein 72 from the human brain in vivo. Cell Stress Chaperones. 2004;9:276–280. doi: 10.1379/CSC-18R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehner T, Bergmeier LA, Wang Y, Tao L, Sing M, Spallek R, van der Zee R. Heat shock proteins generate beta-chemokines which function as innate adjuvants enhancing adaptive immunity. Eur J Immunol. 2000;30:594–603. doi: 10.1002/1521-4141(200002)30:2<594::AID-IMMU594>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 37.Lehner T, Wang Y, Whittall T, McGowan E, Kelly CG, Singh M. Functional domains of HSP70 stimulate generation of cytokines and chemokines, maturation of dendritic cells and adjuvanticity. Biochem Soc Trans. 2004;32:629–632. doi: 10.1042/BST0320629. [DOI] [PubMed] [Google Scholar]
- 38.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 39.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 40.MacAry PA, Javid B, Floto RA, Smith KG, Oehlmann W, Singh M, Lehner PJ. HSP70 peptide binding mutants separate antigen delivery from dendritic cell stimulation. Immunity. 2004;20:95–106. doi: 10.1016/s1074-7613(03)00357-1. [DOI] [PubMed] [Google Scholar]
- 41.Mayer MP, Bukau B. Hsp70 chaperone systems: diversity of cellular functions and mechanism of action. Biol Chem. 1998;379:261–268. [PubMed] [Google Scholar]
- 42.Millar DG, Garza KM, Odermatt B, Elford AR, Ono N, Li Z, Ohashi PS. Hsp70 promotes antigen-presenting cell function and converts T-cell tolerance to autoimmunity in vivo. Nat Med. 2003;9:1469–1476. doi: 10.1038/nm962. [DOI] [PubMed] [Google Scholar]
- 43.Minota S, Cameron B, Welch WJ, Winfield JB. Autoantibodies to the constitutive 73-kD member of the hsp70 family of heat shock proteins in systemic lupus erythematosus. J Exp Med. 1988;168:1475–1480. doi: 10.1084/jem.168.4.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mommersteeg PM, Heijnen CJ, Verbraak MJ, van Doornen LJ. Clinical burnout is not reflected in the cortisol awakening response, the day-curve or the response to a low-dose dexamethasone suppression test. Psychoneuroendocrinology. 2005 doi: 10.1016/j.psyneuen.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Multhoff G, Botzler C, Jennen L, Schmidt J, Ellwart J, Issels R. Heat shock protein 72 on tumor cells: a recognition structure for natural killer cells. J Immunol. 1997;158:4341–4350. [PubMed] [Google Scholar]
- 46.Multhoff G, Botzler C, Wiesnet M, Eissner G, Issels R. CD3- large granular lymphocytes recognize a heat-inducible immunogenic determinant associated with the 72-kD heat shock protein on human sarcoma cells. Blood. 1995;86:1374–1382. [PubMed] [Google Scholar]
- 47.Nakamura T, Hinagata J, Tanaka T, Imanishi T, Wada Y, Kodama T, Doi T. HSP90, HSP70, and GAPDH directly interact with the cytoplasmic domain of macrophage scavenger receptors. Biochem Biophys Res Commun. 2002;290:858–864. doi: 10.1006/bbrc.2001.6271. [DOI] [PubMed] [Google Scholar]
- 48.Noessner E, Gastpar R, Milani V, Brandl A, Hutzler PJ, Kuppner MC, Roos M, Kremmer E, Asea A, Calderwood SK, Issels RD. Tumor-derived heat shock protein 70 peptide complexes are cross- presented by human dendritic cells. J Immunol. 2002;169:5424–5432. doi: 10.4049/jimmunol.169.10.5424. [DOI] [PubMed] [Google Scholar]
- 49.Panjwani NN, Popova L, Srivastava PK. Heat shock proteins gp96 and hsp70 activate the release of nitric oxide by APCs. J Immunol. 2002;168:2997–3003. doi: 10.4049/jimmunol.168.6.2997. [DOI] [PubMed] [Google Scholar]
- 50.Pockley AG, De Faire U, Kiessling R, Lemne C, Thulin T, Frostegard J. Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens. 2002;20:1815–1820. doi: 10.1097/00004872-200209000-00027. [DOI] [PubMed] [Google Scholar]
- 51.Pockley AG, Georgiades A, Thulin T, de Faire U, Frostegard J. Serum heat shock protein 70 levels predict the development of atherosclerosis in subjects with established hypertension. Hypertension. 2003;42:235–238. doi: 10.1161/01.HYP.0000086522.13672.23. [DOI] [PubMed] [Google Scholar]
- 52.Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–377. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- 53.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reed RC, Nicchitta CV. Chaperone-mediated cross-priming: a hitchhiker's guide to vesicle transport (review) Int J Mol Med. 2000;6:259–264. doi: 10.3892/ijmm.6.3.259. [DOI] [PubMed] [Google Scholar]
- 55.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schneider EM, Niess AM, Lorenz I, Northoff H, Fehrenbach E. Inducible hsp70 expression analysis after heat and physical exercise: transcriptional, protein expression, and subcellular localization. Ann N Y Acad Sci. 2002;973:8–12. doi: 10.1111/j.1749-6632.2002.tb04598.x. [DOI] [PubMed] [Google Scholar]
- 57.Singh-Jasuja H, Scherer HU, Hilf N, Arnold-Schild D, Rammensee HG, Toes RE, Schild H. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol. 2000;30:2211–2215. doi: 10.1002/1521-4141(2000)30:8<2211::AID-IMMU2211>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 58.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 59.Srivastava PK. Heat shock protein-based novel immunotherapies. Drug News Perspect. 2000;13:517–522. doi: 10.1358/dnp.2000.13.9.858479. [DOI] [PubMed] [Google Scholar]
- 60.Srivastava PK. Hypothesis: controlled necrosis as a tool for immunotherapy of human cancer. Cancer Immun. 2003;3:4. [PubMed] [Google Scholar]
- 61.Srivastava PK. Immunotherapy for human cancer using heat shock protein-Peptide complexes. Curr Oncol Rep. 2005;7:104–108. doi: 10.1007/s11912-005-0035-8. [DOI] [PubMed] [Google Scholar]
- 62.Srivastava PK, Amato RJ. Heat shock proteins: the ‘Swiss Army Knife’ vaccines against cancers and infectious agents. Vaccine. 2001;19:2590–2597. doi: 10.1016/s0264-410x(00)00492-8. [DOI] [PubMed] [Google Scholar]
- 63.Terry DF, McCormick M, Andersen S, Pennington J, Schoenhofen E, Palaima E, Bausero M, Ogawa K, Perls TT, Asea A. Cardiovascular disease delay in centenarian offspring: role of heat shock proteins. Ann N Y Acad Sci. 2004;1019:502–505. doi: 10.1196/annals.l297.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Theriault JR, Mambula SS, Sawamura T, Stevenson MA, Calderwood SK. Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS Lett. 2005;579:1951–1960. doi: 10.1016/j.febslet.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 65.Tsan MF, Gao B. Cytokine function of heat shock proteins. Am J Physiol Cell Physiol. 2004;286:C739–744. doi: 10.1152/ajpcell.00364.2003. [DOI] [PubMed] [Google Scholar]
- 66.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 67.Velin AK, Ericson AC, Braaf Y, Wallon C, Soderholm JD. Increased antigen and bacterial uptake in follicle associated epithelium induced by chronic psychological stress in rats. Gut. 2004;53:494–500. doi: 10.1136/gut.2003.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6:386–393. doi: 10.1379/1466-1268(2001)006<0386:eishih>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y, Whittall T, McGowan E, Younson J, Kelly C, Bergmeier LA, Singh M, Lehner T. Identification of stimulating and inhibitory epitopes within the heat shock protein 70 molecule that modulate cytokine production and maturation of dendritic cells. J Immunol. 2005;174:3306–3316. doi: 10.4049/jimmunol.174.6.3306. [DOI] [PubMed] [Google Scholar]
- 70.Wright BH, Corton JM, El-Nahas AM, Wood RF, Pockley AG. Elevated levels of circulating heat shock protein 70 (Hsp70) in peripheral and renal vascular disease. Heart Vessels. 2000;15:18–22. doi: 10.1007/s003800070043. [DOI] [PubMed] [Google Scholar]
- 71.Zitvogel L, Fernandez N, Lozier A, Wolfers J, Regnault A, Raposo G, Amigorena S. Dendritic cells or their exosomes are effective biotherapies of cancer. Eur J Cancer. 1999;35(Suppl 3):S36–38. doi: 10.1016/s0959-8049(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 72.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]