Abstract
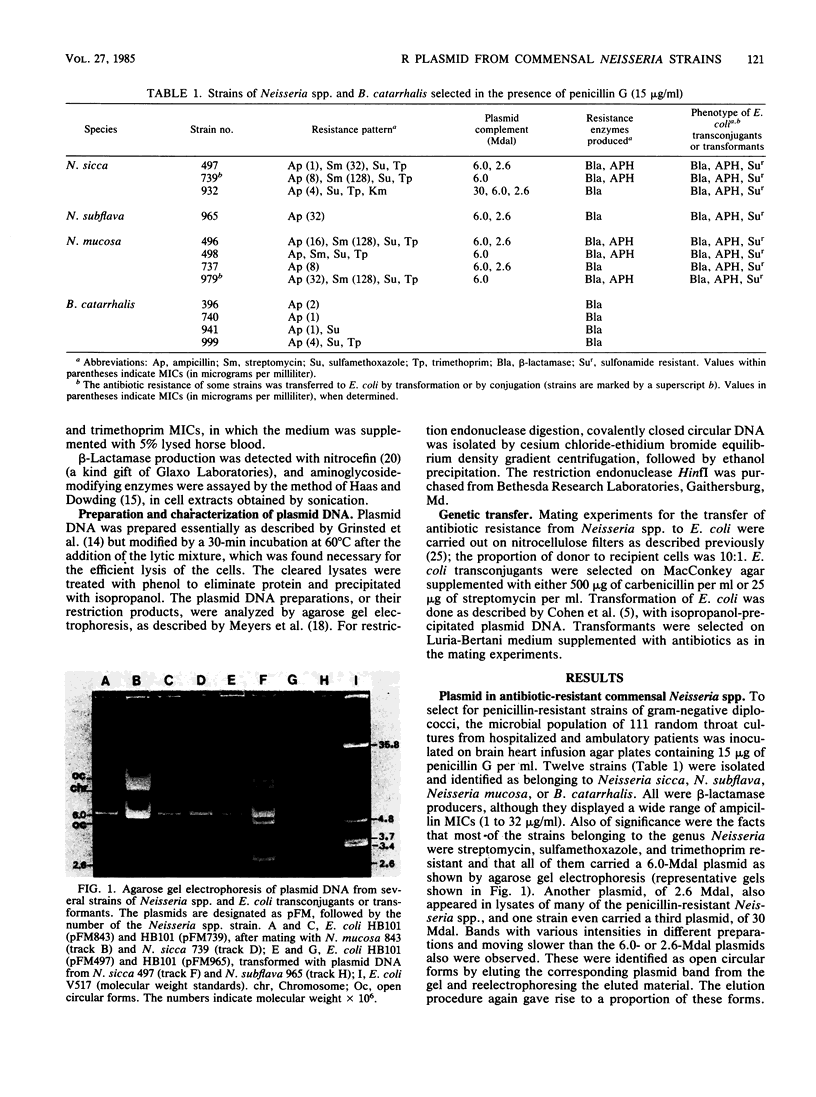
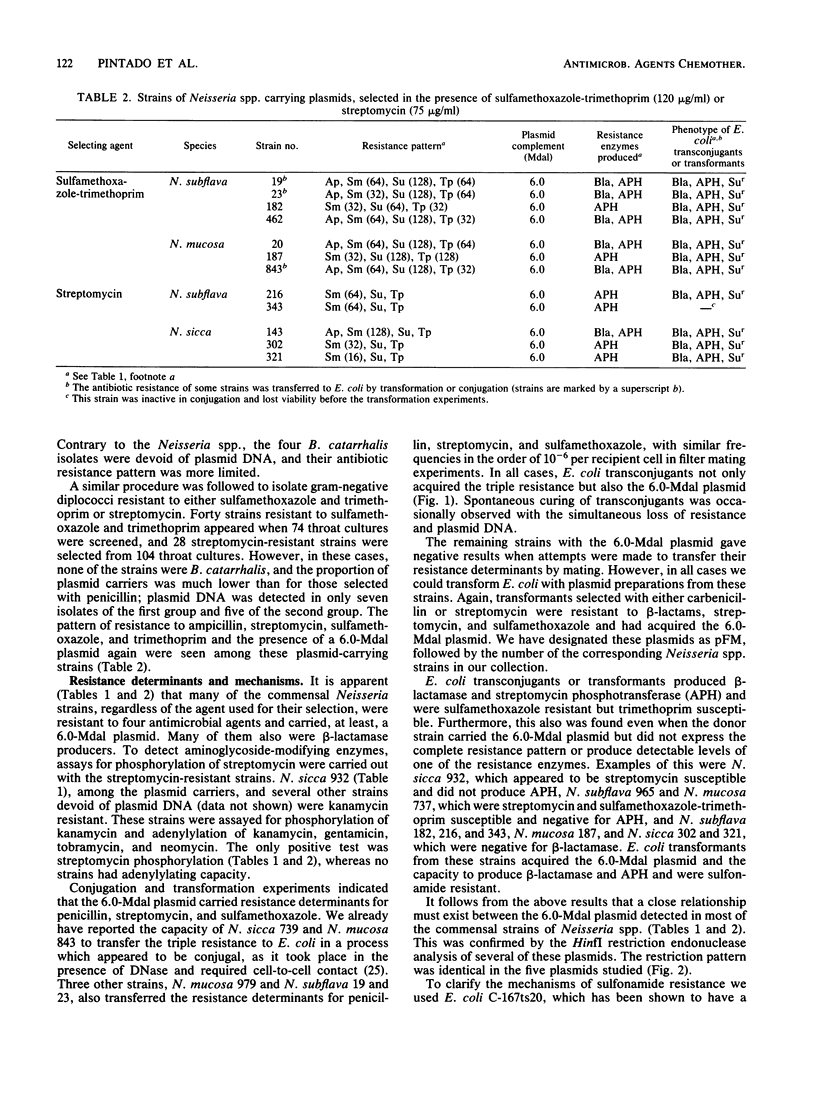
Antibiotic-resistant commensal strains of Neisseria spp. and Branhamella catarrhalis were isolated from throat cultures, on the basis of their capacity to grow in the presence of penicillin, streptomycin, or sulfamethoxazole-trimethoprim. Several strains, which belonged to different species of Neisseria, were resistant to beta-lactams, streptomycin, sulfamethoxazole, and trimethoprim, harbored a 6.0-megadalton plasmid with identical HinfI restriction patterns, and produced beta-lactamase and streptomycin phosphotransferase. The resistance determinants for beta-lactams, streptomycin, and sulfamethoxazole, but not for trimethoprim, were transferred from all these strains to Escherichia coli by conjugation or transformation. The resulting transconjugants or transformants acquired the plasmid and the capacity to produce beta-lactamase and streptomycin phosphotransferase. The 6.0-megadalton plasmid complemented a mutation which determines production of thermosensitive dihydropteroate synthetase in E. coli. We conclude that an R plasmid coding for beta-lactamase, streptomycin phosphotransferase, and a sulfonamide-resistant dihydropteroate synthetase is common to these strains.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B., Albritton W. L., Biddle J., Johnson S. R. Common beta-lactamase-specifying plasmid in Haemophilus ducreyi and Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1984 Feb;25(2):296–297. doi: 10.1128/aac.25.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron E. S., Saz A. K., Kopecko D. J., Wohlhieter J. A. Transfer of plasmid-borne beta-lactamase in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1977 Aug;12(2):270–280. doi: 10.1128/aac.12.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Dillon J. R., Pauzé M., Yeung K. H. Spread of penicillinase-producing and transfer plasmids from the gonococcus to Neisseria meningitidis. Lancet. 1983 Apr 9;1(8328):779–781. doi: 10.1016/s0140-6736(83)91846-9. [DOI] [PubMed] [Google Scholar]
- Doern G. V., Siebers K. G., Hallick L. M., Morse S. A. Antibiotic susceptibility of beta-lactamase-producing strains of Branhamella (Neisseria) catarrhalis. Antimicrob Agents Chemother. 1980 Jan;17(1):24–29. doi: 10.1128/aac.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Sox T., Biswas G., Blackman E., Sparling P. F. Conjugal transfer of the gonococcal penicillinase plasmid. Science. 1977 Mar 11;195(4282):998–1000. doi: 10.1126/science.402693. [DOI] [PubMed] [Google Scholar]
- Elwell L. P., Roberts M., Mayer L. W., Falkow S. Plasmid-mediated beta-lactamase production in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1977 Mar;11(3):528–533. doi: 10.1128/aac.11.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer T., Reading C. beta-Lactamases of Branhamella catarrhalis and their inhibition by clavulanic acid. Antimicrob Agents Chemother. 1982 Mar;21(3):506–508. doi: 10.1128/aac.21.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted J., Bennett P. M., Higginson S., Richmond M. H. Regional preference of insertion of Tn501 and Tn802 into RP1 and its derivatives. Mol Gen Genet. 1978 Nov 9;166(3):313–320. doi: 10.1007/BF00267624. [DOI] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Translocation of a plasmid DNA sequence which mediates ampicillin resistance: molecular nature and specificity of insertion. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3623–3627. doi: 10.1073/pnas.72.9.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piziak M. V., Woodbury C., Berliner D., Takafuji E., Kirkpatrick J., Opal S., Tramont E. Resistance trends of Neisseria gonorrhoeae in the Republic of Korea. Antimicrob Agents Chemother. 1984 Jan;25(1):7–9. doi: 10.1128/aac.25.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Smith A. L. Molecular characterization of "plasmid-free" antibiotic-resistant Haemophilus influenzae. J Bacteriol. 1980 Oct;144(1):476–479. doi: 10.1128/jb.144.1.476-479.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Falkow S. Conjugal transfer of R plasmids in Neisseria gonorrhoeae. Nature. 1977 Apr 14;266(5603):630–631. doi: 10.1038/266630a0. [DOI] [PubMed] [Google Scholar]
- Rotger R., Nombela C. Characterization of penicillin-resistant beta-lactamase producing-strains of Neisseria gonorrhoeae isolated in Spain (1978-81). Microbiol Esp. 1983 Jul-Dec;36(3-4):115–122. [PubMed] [Google Scholar]
- Sköld O. R-factor-mediated resistance to sulfonamides by a plasmid-borne, drug-resistant dihydropteroate synthase. Antimicrob Agents Chemother. 1976 Jan;9(1):49–54. doi: 10.1128/aac.9.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Chromosomally integrated conjugative plasmids are common in antibiotic-resistant Haemophilus influenzae. J Bacteriol. 1980 Jun;142(3):925–930. doi: 10.1128/jb.142.3.925-930.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Plasmid transfer in Haemophilus influenzae. J Bacteriol. 1979 Aug;139(2):520–529. doi: 10.1128/jb.139.2.520-529.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]