Abstract
Heterozygote advantage, or overdominance, remains a popular and persuasive explanation for the maintenance of genetic variation in natural populations in the face of selection. However, despite being first proposed more than 80 years ago, there remain few examples that fit the criteria for heterozygote advantage, all of which are associated with disease resistance and are maintained only in the presence of disease or other gene-by-environment interaction. Here we report five new examples of heterozygote advantage, based around polymorphisms in the BMP15 and GDF9 genes that affect female fecundity in domesticated sheep and are not reliant on disease for their maintenance. Five separate mutations in these members of the transforming growth factor β (TGFβ) superfamily give phenotypes with fitness differentials characteristic of heterozygous advantage. In each case, one copy of the mutant allele increases ovulation rate, and ultimately litter size per ewe lambing, relative to the wildtype. However, homozygous ewes inheriting mutant alleles from both parents have impaired oocyte development and maturation, which results in small undeveloped ovaries and infertility. Using data collected over many years on ovulation rates, litter size, and lambing rates, we have calculated the equilibrium solution for each of these polymorphisms using standard population genetic theory. The predicted equilibrium frequencies obtained for these mutant alleles range from 0.11 to 0.23, which are amongst the highest yet reported for a polymorphism maintained by heterozygote advantage. These are amongst the most frequent and compelling examples of heterozygote advantage yet described and the first documented examples of heterozygote advantage that are not reliant on a disease interaction for their maintenance.
Introduction
Heterozygote advantage, or overdominance, remains a popular and persuasive explanation for the maintenance of genetic variation in natural populations in the face of selection [1], [2]. However, despite being first proposed more than 80 years ago [3], [4] there remain only a small number of examples that fit the criteria for heterozygote advantage, all of which are associated with disease resistance and maintained only in the presence of disease or other gene-by-environment interaction (see supporting text and Table S1). In addition, it is hard to continue to portray heterozygote advantage as an important general concept in evolutionary biology when a single example is used repeatedly throughout the literature. Here we report a simple, but compelling new example of heterozygote advantage, based around polymorphisms in genes associated with fertility in sheep.
Several genes have recently been identified that affect female fecundity in domesticated sheep [5]–[7]. Specifically, mutations in the BMP15 [6] and GDF9 [7] genes have been shown to increase ovulation rate and ultimately litter size in multiple sheep breeds (Table 1 ). The BMP15 fecundity alleles show an X-linked overdominance inheritance pattern with infertility in homozygous females, while the GDF9 fecundity alleles have an autosomal overdominance inheritance pattern with infertility in homozygous females [5]–[7]. Surprisingly, BMP15 and GDF9 have never been explicitly referred to as characteristic of heterozygote advantage and genotype fitnesses have never been estimated at these genes.
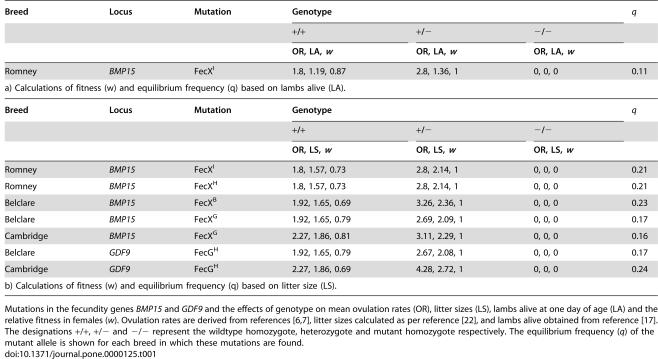
Table 1. Fecundity genes exhibiting heterozygote advantage in sheep.
| Breed | Locus | Mutation | Genotype | q | ||
| +/+ | +/− | −/− | ||||
| OR, LA, w | OR, LA, w | OR, LA, w | ||||
| Romney | BMP15 | FecXI | 1.8, 1.19, 0.87 | 2.8, 1.36, 1 | 0, 0, 0 | 0.11 |
| a) Calculations of fitness (w) and equilibrium frequency (q) based on lambs alive (LA). | ||||||
| Breed | Locus | Mutation | Genotype | q | ||
| +/+ | +/− | −/− | ||||
| OR, LS, w | OR, LS, w | OR, LS, w | ||||
| Romney | BMP15 | FecXI | 1.8, 1.57, 0.73 | 2.8, 2.14, 1 | 0, 0, 0 | 0.21 |
| Romney | BMP15 | FecXH | 1.8, 1.57, 0.73 | 2.8, 2.14, 1 | 0, 0, 0 | 0.21 |
| Belclare | BMP15 | FecXB | 1.92, 1.65, 0.69 | 3.26, 2.36, 1 | 0, 0, 0 | 0.23 |
| Belclare | BMP15 | FecXG | 1.92, 1.65, 0.79 | 2.69, 2.09, 1 | 0, 0, 0 | 0.17 |
| Cambridge | BMP15 | FecXG | 2.27, 1.86, 0.81 | 3.11, 2.29, 1 | 0, 0, 0 | 0.16 |
| Belclare | GDF9 | FecGH | 1.92, 1.65, 0.79 | 2.67, 2.08, 1 | 0, 0, 0 | 0.17 |
| Cambridge | GDF9 | FecGH | 2.27, 1.86, 0.69 | 4.28, 2.72, 1 | 0, 0, 0 | 0.24 |
| b) Calculations of fitness (w) and equilibrium frequency (q) based on litter size (LS). | ||||||
Mutations in the fecundity genes BMP15 and GDF9 and the effects of genotype on mean ovulation rates (OR), litter sizes (LS), lambs alive at one day of age (LA) and the relative fitness in females (w). Ovulation rates are derived from references [6], [7], litter sizes calculated as per reference [22], and lambs alive obtained from reference [17]. The designations +/+, +/− and −/− represent the wildtype homozygote, heterozygote and mutant homozygote respectively. The equilibrium frequency (q) of the mutant allele is shown for each breed in which these mutations are found.
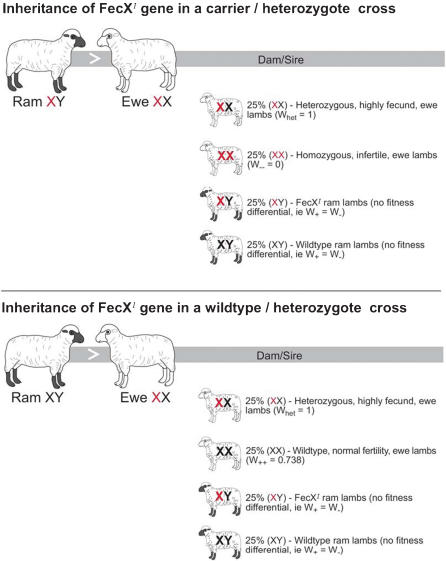
Four separate mutations (Inverdale, Hanna, Belclare and Galway) in the X-linked BMP15 gene (Table 1 ) have been described [5] that give rise to phenotypes with fitness differentials characteristic of heterozygous advantage (Figure 1). One copy of the Inverdale (FecXI), Hanna (FecXH), Belclare (FECXB) or Galway (FECXG) allele increases ovulation rate and ultimately litter size by about 0.6 lambs per ewe lambing compared to the wildtype [5]–[7]. However, homozygous ewes inheriting mutant alleles from both parents have small undeveloped ovaries and are infertile [6]–[8].
Figure 1. Genotypes and the fitness differentiations (w) for the Inverdale mutation (FecXI).
Mechanistically, the genetic basis of this heterozygote advantage is well understood. The BMP15 gene encodes bone morphogenic protein 15 (also known as growth differentiation factor 9B, GDF9B), which is a member of the transforming growth factor β (TGFβ) superfamily [6]. BMP15 acts through a cascade of other proteins (the SMAD pathway) that are responsible for a huge diversity of cellular behaviours, including oocyte development and maturation [6], [8], [9]. Without BMP15, oocytes continue to grow in the absence of granulosa cell proliferation until they are unable to be supported by the residual granulosa cells, whereupon they degenerate [6]. Curiously, although a lack of BMP15 blocks follicular growth in homozygotes, inactivation of only one copy of BMP15 increases ovulation rate [6], [8], conferring a fecundity advantage to the heterozygote (Table 1 , Figure 1).
A similar pattern of heterozygote advantage is observed in another closely related member of theTGFβ superfamily GDF9 [10]. Like BMP15, the autosomal GDF9 has an essential role in controlling follicular growth via its influence on granulosa cell function [7], [10]. An absence of GDF9 also blocks follicular growth in homozygotes, resulting in sterility, while inactivation of only one copy of GDF9 again increases ovulation rate [7], conferring a fecundity advantage to the heterozygote (Table 1 ).
Despite being very compelling examples of heterozygote advantage, the overdominant nature of these fertility polymorphisms is not widely recognised. One of the hurdles to these becoming a more widely known example of heterozygote advantage is the absence of data on the equilibrium solution for each of these polymorphisms and knowledge of their frequencies in a natural situation (see supporting text). However, using standard population genetic theory [11] the equilibrium solution can be readily calculated from data collected over many years on ovulation rates, litter size and lambing rates [5]–[7]. Here we report the predicted fitness and equilibrium solution for each of these fecundity polymorphisms and show that these are expected to be amongst the most frequent and compelling examples of heterozygote advantage yet described.
Results and Discussion
The equilibrium solution for each fecundity polymorphism was calculated from data collected on ovulation rates, litter size, and lambing rates as described below. For the Inverdale mutation (FecXI) the estimated relative fitness of ewes (w), based on lambs alive at one day of age, of the homozygote wildtype (++), heterozygote (+/−) and mutant homozygote (−/−) are 0.87, 1 and 0 respectively, leading to a predicted equilibrium frequency for the FecXI allele (q) of 0.11 (Table 1a). Other mutations in BMP15 and GDF9 show similar relative fitnesses and equilibrium frequencies for the more limited litter size data (Table 1b), which are collectively amongst the highest yet described for a polymorphism maintained by heterozygote advantage. For comparison, the equilibrium frequency of the recessive sickle cell allele (HbS) rarely exceeds 0.15 [12].
Heterozygote advantage as a concept holds considerable sway in evolutionary biology, but only a handful of examples have withstood close scrutiny (reviewed in Table S1). Of the 21 examples reported that invoke heterozygote advantage as an explanation for the maintenance of polymorphism, compelling evidence for heterozygote advantage is lacking in nearly every instance (Table S1). The following information is required to unequivocally demonstrate heterozygote advantage: (i) the gene and mutant alleles under selection must be known; (ii) the relative fitness of each genotype must be known (with heterozygotes exhibiting the greatest relative fitness); (iii) the mechanism of selection must be understood i.e. we need to known why heterozygotes are fitter than homozygotes.
Prior to the discovery of the sheep fecundity genes described here, perhaps only the classic example of heterozygote advantage, sickle cell anaemia, met these requirements. In African and Asian human populations where malaria is prevalent, fatalities in homozygotes for the sickle cell trait (HbSS) are offset by the survival advantage of heterozygotes bearing one copy of the sickle cell allele (HbS+) over the wildtype homozygotes (Hb++), when challenged with malaria [1], [12]. Sickle cell anaemia is an excellent example of heterozygote advantage, but it has two principal shortcomings. First, the advantage to heterozygotes is conditional, only operating where malaria is prevalent [1]. Second, the heterozygote advantage arises because of the super-position of two opposite directional selection pressures (malaria and sickle-cell anaemia), so it is not really a true case of heterozygote advantage arising from the heterozygote having superior fitness for a specific trait.
There are numerous other putative examples of heterozygote advantage that evoke contemporary and/or historical resistance to infectious disease to explain the relatively high frequency of otherwise deleterious alleles. Examples include the cystic fibrosis-causing alleles at the CFTR gene, the Gaucher Disease-causing allele at GBA, deafness-causing alleles at GJB2 and polymorphism at the major histocompatability locus MHC (Table S1). However, in the majority of these cases genotype fitnesses remain undetermined, and very often the infectious disease driving selection is unknown. This missing information makes it difficult to distinguish between heterozygote advantage and alternative explanations including other forms of balancing selection, such as negative frequency dependence or spatially/temporally variable directional selection [13].
The fertility polymorphisms documented in BMP15 and GDF9 represent one of the best examples of heterozygote advantage yet described, with the genetic basis, fitness differentials and the mechanism of selection well understood [5]–[7]. Crucially, in stark contrast to sickle cell anaemia, no external factor, such as disease [2], needs to be invoked to understand the fitness differentials among genotypes for these polymorphisms.
There are of course still substantial issues unresolved for these polymorphisms too. Among the most important of these issues is the frequency of the polymorphism in the wild. At equilibrium our theoretical expectation, based on data from domesticated sheep, is that fertility alleles such as FecXI will occur at a frequency of 0.11–0.23 (Table 1 ). The obvious extension of this work then is to screen wild populations of sheep for these mutations to determine if they are present and if the frequencies match expectations. To date such an investigation has not been undertaken widely. One study of a small population of Soay sheep on the remote Islands of St Kilda found no evidence for any of the BMP15 fertility polymorphisms described in Table 1 (Gratten pers. comm.). However since this is an isolated population, subject to regular population crashes [14], the absence of polymorphism in BMP15 in this population may not be overly surprising and further studies in other wild sheep populations are warranted.
Heterozygote advantage is commonly assumed to slow the rate of loss of genetic diversity by genetic drift. However, Robertson [15] described scenarios where it can accelerate loss of diversity by drift, and provided solutions to the relative rate that heterozygote advantage accelerates or retards loss of diversity for populations of finite size. Extrapolating from Table 1 of Robertson [15], and assuming that s1 = 0.125, s2 = 0.5 because selection is only in one sex, and q = 0.20, then heterozygote advantage at the loci described here would retard the effects of genetic drift 100-fold in a population with effective population size (Ne) 176 (retardation of loss would be greater in larger populations). In other words, the polymorphism is likely to be maintained in moderate or large sized populations.
It may well transpire that the fertility polymorphisms described here are only relatively common in domesticated sheep as a consequence of selective breeding and that they do not actually confer an appreciable fitness advantage to heterozygotes in the wild, due perhaps to the existence of other genes under stronger selection that are in linkage or have epistatic effects on these fertility polymorphisms. One prominent example of a mutation maintained at high frequency by human mediated selection for the heterozygote is the Halothane mutation in pigs [16]. The Halothane mutation occurs as a result of a missense mutation in a calcium release gene, the ryanodine receptor gene (RYR1), expressed in muscle. Heterozygous carriers show a higher lean content than homozygous wild type individuals, whereas the mutant homozygotes are highly susceptible to malignant hyperthermia [16]. Selection for leanness in domesticated pigs created an overdominant situation maintaining the mutation at high frequency until a DNA based diagnostic became available that enabled the targeted culling of individuals bearing the Halothane mutation [16].
It remains possible that the fertility polymorphisms in BMP15 and GDF9 are also maintained through selective breeding, but these mutations differ significantly from the Halothane mutation in that the heterozygotes clearly have a natural advantage in terms of fecundity over the wild type, with little demonstrable decrease in other fitness parameters for the heterozygote reported to date [5]–[7], [17]. However, an obvious possibility is that there is a trade off between birthing many lambs and their survival to weaning, particularly when environmental conditions are unfavourable. To explore this further we have examined data on the mean frequency of single and multiple births for carriers of the Inverdale mutation (FecXI) and for wildtype Romney sheep [18]. We have then estimated the relative fitness of each genotype using data on average survival rates to weaning, for lambs for each birth category, across environmental conditions that range from intensive farming to harsh hill country (Table 2 ) [18]. Due to an absence of data on the frequency of different litter sizes under different environmental conditions, we have made the conservative assumption that this is unaffected by environmental conditions.
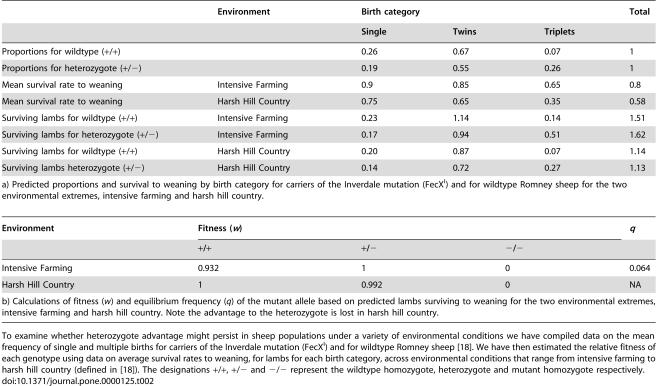
Table 2. Table 2. Heterozygote advantage for fecundity in sheep under two different environment conditions.
| Environment | Birth category | Total | |||
| Single | Twins | Triplets | |||
| Proportions for wildtype (+/+) | 0.26 | 0.67 | 0.07 | 1 | |
| Proportions for heterozygote (+/−) | 0.19 | 0.55 | 0.26 | 1 | |
| Mean survival rate to weaning | Intensive Farming | 0.9 | 0.85 | 0.65 | 0.8 |
| Mean survival rate to weaning | Harsh Hill Country | 0.75 | 0.65 | 0.35 | 0.58 |
| Surviving lambs for wildtype (+/+) | Intensive Farming | 0.23 | 1.14 | 0.14 | 1.51 |
| Surviving lambs for heterozygote (+/−) | Intensive Farming | 0.17 | 0.94 | 0.51 | 1.62 |
| Surviving lambs for wildtype (+/+) | Harsh Hill Country | 0.20 | 0.87 | 0.07 | 1.14 |
| Surviving lambs heterozygote (+/−) | Harsh Hill Country | 0.14 | 0.72 | 0.27 | 1.13 |
| a) Predicted proportions and survival to weaning by birth category for carriers of the Inverdale mutation (FecXI) and for wildtype Romney sheep for the two environmental extremes, intensive farming and harsh hill country. | |||||
| Environment | Fitness (w) | q | ||
| +/+ | +/− | −/− | ||
| Intensive Farming | 0.932 | 1 | 0 | 0.064 |
| Harsh Hill Country | 1 | 0.992 | 0 | NA |
| b) Calculations of fitness (w) and equilibrium frequency (q) of the mutant allele based on predicted lambs surviving to weaning for the two environmental extremes, intensive farming and harsh hill country. Note the advantage to the heterozygote is lost in harsh hill country. | ||||
To examine whether heterozygote advantage might persist in sheep populations under a variety of environmental conditions we have compiled data on the mean frequency of single and multiple births for carriers of the Inverdale mutation (FecXI) and for wildtype Romney sheep [18]. We have then estimated the relative fitness of each genotype using data on average survival rates to weaning, for lambs for each birth category, across environmental conditions that range from intensive farming to harsh hill country (defined in [18]). The designations +/+, +/− and −/− represent the wildtype homozygote, heterozygote and mutant homozygote respectively.
We found that lamb survival to weaning does decrease with increasing litter size [17], [18], but that the advantage to heterozygotes seems to hold across a wide variety of environmental conditions (Table 2 ), even with our rather simplistic assumption that the frequency of births by category is unaffected by environment. Under intensive farming the selection coefficient (s) in favour of the heterozygote is 0.068, whilst in harsh conditions the wildtype is favoured slightly with s of 0.008 (Table 2 ). These calculations suggest that the heterozygote advantage for fecundity conferred by BMP15 has some dependence on environmental conditions. Under benign conditions (e.g. intensive farming, easy and hard hill country), the heterozygous advantage would be promoted and equilibrium might be reached, but under harsh conditions (e.g. harsh hill country), lamb survival for multiple births drops [18] and the advantage to heterozygotes is apparently lost (Table 2 ).
Recalculating the equilibrium solution for the mutant allele under each of these environmental scenarios results in an expected equilibrium frequency, q, of no greater than 0.064 predicted for intensive farming, the most benign situation (Table 2 ). This is an important result because based on the calculations of Robertson [15] a value of q below 0.2 will usually result in heterozygous advantage actually accelerating the effects of drift unless Ne is very large, making a stable equilibrium increasingly unlikely as q becomes smaller.
Overall our calculations suggest that a stable equilibrium for these fecundity alleles might only be expected in populations of large size (Ne>400) [15] under favourable or predominantly favourable environmental conditions. However, it remains possible that the heterozygous advantage could be maintained in natural populations even under harsh conditions if females modify the number of lambs they produce in response to environmental conditions, which a range of studies suggest they will [19]–[21]. This is a factor that we have been unable to incorporate in our calculations due to an absence of condition specific rates of single, twin and other multiple births. However, if heterozygous carriers and wildtype ewes responded in the same way to the harsher environments, producing fewer multiple births, it may transpire that the heterozygote may still have superior fitness across all environmental conditions.
The possibility remains that the alleles that confer this heterozygous advantage may be maintained in wild populations and further research is warranted. Investigations of the patterns of genomic diversity adjacent to each of these loci would provide insights into any obvious pattern of selection around these genes. In addition, such a study might enable the estimation of the age of these polymorphisms, which we would predict would be old, if indeed they are maintained by overdominance in the wild. While there is still much to be determined about the maintenance of these new examples of heterozygote advantage in the wild, the overdominant effect of these mutations under domestication is incontrovertible, and provides a useful complement to the perennial example of heterozygote advantage based upon the sickle cell [1].
Materials and Methods
The relative genotype fitnesses in females (w) and the equilibrium frequency (q) of the mutant allele were calculated for each of the BMP15 and GDF9 polymorphisms found in each breed of sheep (Table 1 ). For these calculations we assume that litter size (LS), is an accurate measure of female reproductive fitness. To calculate litter size (LS), we used reports of ovulation rate (OR) [6], [7], the standard measure of fecundity in this system, which we converted using the quadratic equation:
Subsequently, where data were available, we have adjusted for neo-natal mortality, to give an estimate of the lambs alive at one day of age per ewe lambing (LA), which incorporates barrenness, litter size and lamb survival into a single measure [17].
BMP15 (GDF9B) is X-linked therefore males have only one copy of the gene and there is no evidence of a fitness difference between alleles in males [5]. Therefore, assuming that wild type females have relative fitness 1-s, heterozygous females have relative fitness 1, and female mutant homozygotes have relative fitness 1-t, and that male carriers have fitness equal to male wild types, the equilibrium frequency of the mutant allele is given by q = s/(s+t) [11]. Note that since FecXB and FecXG both segregate in Belclare sheep a stable equilibrium cannot be attained when both mutants are present [6], [7]. Therefore, for simplicity, the reported equilibrium frequency is based on the assumption that only the focal mutant and the wild type segregate.
GDF9 maps to sheep chromosome 5 and there is no evidence of a fitness difference between genotypes in males [5]. Again, we assumed that the three genotypes have fitness 1-s, 1 and 1-t in females as above, but in this case the three male genotypes have relative fitness 1, halving the selection coefficient against the two homozygous genotypes. The equilibrium frequency of the mutant allele is given by q = 0.5s/(0.5s+0.5t) = s/(s+t) [11].
Supporting Information
A review of genes proposed to exhibit heterozygote advantage.
(0.12 MB DOC)
Acknowledgments
We thank Roger Butlin, Brian Charlesworth, Brent Emerson, Greg Hurst, Laurence Hurst, and Hamish Spencer for helpful comments and/or discussion on an earlier draft of this manuscript. We also thank Bill Sherwin and an anonymous reviewer for constructive critic of this manuscript prior to publication. We acknowledge the expert assistance of Matt Walters in developing Figure 1.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This collaboration was facilitated by a University of Canterbury Erskine Grant to J.S.
References
- 1.Allison AC. Protection afforded by sickle-cell trait against subtertian malarial infection. Br Med J. 1954;1:290–294. doi: 10.1136/bmj.1.4857.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haldane JBS. Disease and evolution. Ricarea Scientifica Supplement A. 1949;19:68–76. [Google Scholar]
- 3.Fisher RA. On the dominance ratio. Proc Royal Soc Edin. 1922;42:321–341. [Google Scholar]
- 4.Fisher RA. The Genetical Theory of Natural Selection. Oxford: Clarendon Press; 1930. [Google Scholar]
- 5.Davis GH. Major genes affecting ovulation rate in sheep. Genet Sel Evol. 2005;37:S11–S23. doi: 10.1186/1297-9686-37-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galloway SM, McNatty KP, Cambridge LM, Laitinen MPE, Juengel JL, et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25:279–283. doi: 10.1038/77033. [DOI] [PubMed] [Google Scholar]
- 7.Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, et al. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol Reprod. 2004;70:900–909. doi: 10.1095/biolreprod.103.023093. [DOI] [PubMed] [Google Scholar]
- 8.Galloway SM, Gregan SM, Wilson T, McNatty KP, Juengel JL, et al. BMP15 mutations and ovarian function. Mol Cell Endo. 2002;191:15–18. doi: 10.1016/s0303-7207(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 9.Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25:72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- 10.Dong JW, Albertini DF, Nishimori K, Kumar TR, Lu NF, et al. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 11.Hedrick P. Genetics of Populations. Sudbury, Massachusetts: Jones and Bartlett; 2005. p. 737. [Google Scholar]
- 12.Williams TN, Mwangi TW, Wambua S, Peto TEA, Weatherall DJ, et al. Negative epistasis between the malaria-protective effects of α+-thalassemia and the sickle cell trait. Nat Genet. 2005;37:1253–1257. doi: 10.1038/ng1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreitman M, Di Rienzo A. Balancing claims for balancing selection. Trends Genet. 2004;20:300–304. doi: 10.1016/j.tig.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Clutton-Brock TH, Pemberton JM, editors. Soay Sheep: Dynamics and Selection in an Island Population. Cambridge, U.K.: Cambridge University Press; 2004. p. 396. [Google Scholar]
- 15.Robertson A. Selection for heterozygotes in small populations. Genetics. 1962;47:1291–1300. doi: 10.1093/genetics/47.9.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii J, Otsu K, Zorzato F, de Leon S, Khanna VK, et al. Identification of a mutation in porcine ryanodine receptor associated with malignant hyperthermia. Science. 1991;253:448–451. doi: 10.1126/science.1862346. [DOI] [PubMed] [Google Scholar]
- 17.Davis GH, Dodds KG, McEwan JC, Fennessy PF. Liveweight, fleece weight and prolificacy of Romney ewes carrying the Inverdale prolificacy gene (FecX I) located on the X-chromosome. Livestock Production Science. 1992;34:83–91. [Google Scholar]
- 18.Amer PR, McEwan JC, Dodds KG, Davis GH. Economic values for ewe prolificacy and lamb survival in New Zealand sheep. Livestock Production Science. 1999;58:75–90. [Google Scholar]
- 19.Lassoued N, Rekik M, Mahouachi M, Ben Hamouda M. The effect of nutrition prior to and during mating on ovulation rate, reproductive wastage, and lambing rate in three sheep breeds. Small Ruminant Research. 2004;52:117–125. [Google Scholar]
- 20.O'Callaghan D, Boland MP. Nutritional effects on ovulation, embryo development and the establishment of pregnancy in ruminants. Animal Science. 1999;68:299–314. [Google Scholar]
- 21.Robinson JJ. Nutrition and reproduction. Animal Reproduction Science. 1996;42:25–34. [Google Scholar]
- 22.Hanrahan JP. Selection for increased ovulation rate, litter size and embryo survival. 2nd World Congress on Genetics Applied to Livestock Production. 1982;V:294. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A review of genes proposed to exhibit heterozygote advantage.
(0.12 MB DOC)