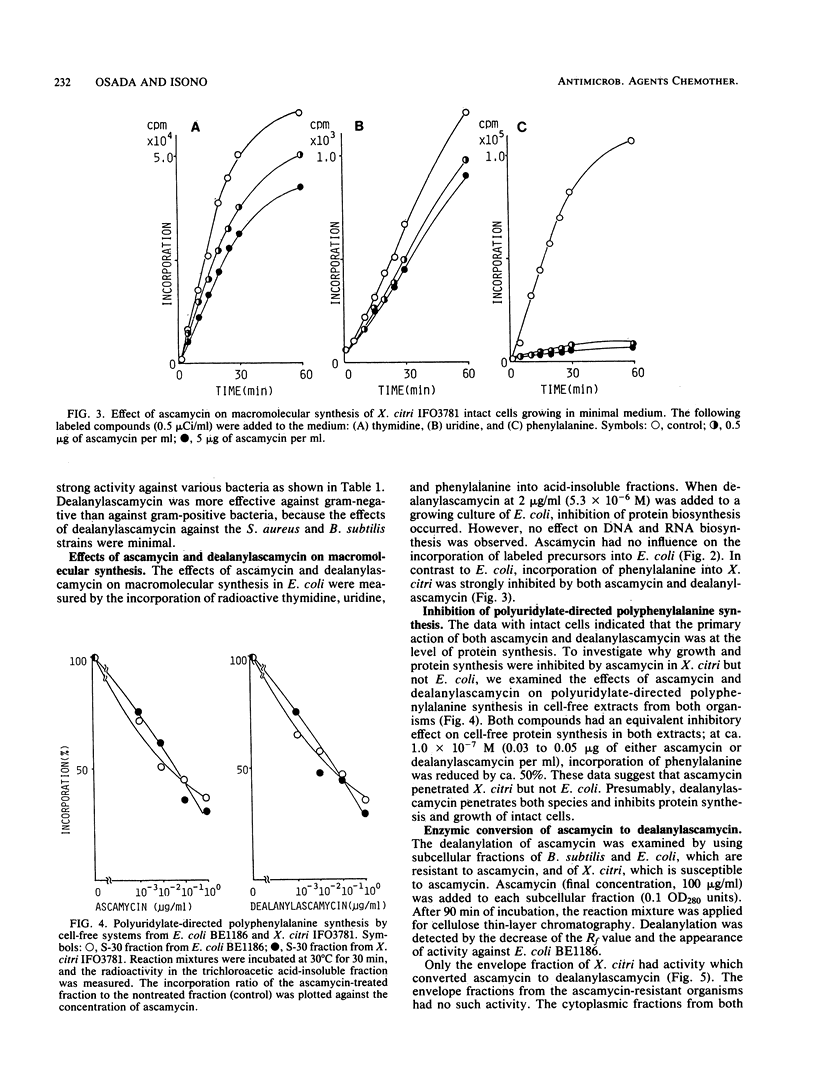
Abstract
An unidentified Streptomyces sp. produces two nucleoside antibiotics, ascamycin and its dealanyl derivative. In contrast to the broad antibacterial activity of dealanylascamycin against various gram-negative and gram-positive bacteria, ascamycin showed selective toxicity against Xanthomonas citri and X. oryzae. Both ascamycin and dealanylascamycin inhibited the protein synthesis of X. citri, but only dealanylascamycin inhibited that of Escherichia coli. In cell-free systems from E. coli and X. citri, both antibiotics, at ca. 0.04 micrograms/ml, inhibited the polyuridylate-directed synthesis of polyphenylalanine by ca. 50%. These data suggest that ascamycin cannot permeate the bacterial membrane. The dealanylating activity toward ascamycin was found only on the cell surface of bacteria susceptible to ascamycin. Dealanylascamycin must then have been transported into cytoplasm, where it inhibited protein synthesis.
Full text
PDF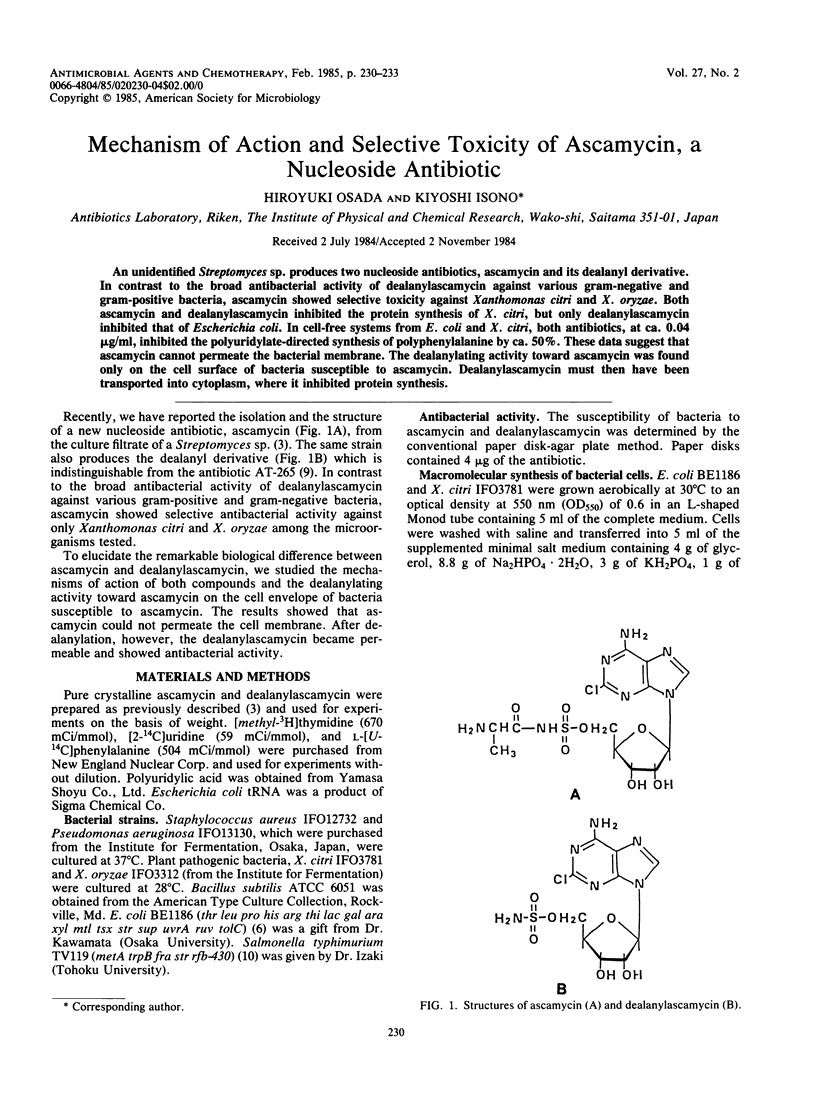


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton F. R., Hall M. J., Hassall C. H., Lambert R. W., Lloyd W. J., Ringrose P. S. Phosphonopeptides as antibacterial agents: mechanism of action of alaphosphin. Antimicrob Agents Chemother. 1979 May;15(5):696–705. doi: 10.1128/aac.15.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton F. R., Hall M. J., Hassall C. H., Lambert R. W., Ringrose P. S. Phosphonopeptides as antibacterial agents: rationale, chemistry, and structure-activity relationships. Antimicrob Agents Chemother. 1979 May;15(5):677–683. doi: 10.1128/aac.15.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isono K., Uramoto M., Kusakabe H., Miyata N., Koyama T., Ubukata M., Sethi S. K., McCloskey J. A. Ascamycin and dealanylascamycin, nucleoside antibiotics from Streptomyces sp. J Antibiot (Tokyo) 1984 Jun;37(6):670–672. doi: 10.7164/antibiotics.37.670. [DOI] [PubMed] [Google Scholar]
- Kenig M., Vandamme E., Abraham E. P. The mode of action of bacilysin and anticapsin and biochemical properties of bacilysin-resistant mutants. J Gen Microbiol. 1976 May;94(1):46–54. doi: 10.1099/00221287-94-1-46. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M. W., MATTHAEI J. H. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1588–1602. doi: 10.1073/pnas.47.10.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuji N., Horiuchi T., Nakata A., Kawamata J. Strains of Escherichia coli hypersensitive to representative carcinostatic and carcinogenic agents. J Antibiot (Tokyo) 1978 Aug;31(8):794–796. doi: 10.7164/antibiotics.31.794. [DOI] [PubMed] [Google Scholar]
- Rogers H. J., Newton G. G., Abraham E. P. Production and purification of bacilysin. Biochem J. 1965 Nov;97(2):573–578. doi: 10.1042/bj0970573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E., Beppu T. A new nucleosidic antibiotic AT-265. J Antibiot (Tokyo) 1982 Aug;35(8):939–947. doi: 10.7164/antibiotics.35.939. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Arai T., Hattori T. Effects of cell wall polysaccharide on the mating ability of Salmonella typhimurium. Nature. 1970 Jan 3;225(5227):70–71. doi: 10.1038/225070a0. [DOI] [PubMed] [Google Scholar]



