Abstract
Despite evidence that mucin overproduction is critical in the pathogenesis of gallstones, the mechanisms triggering mucin production in gallstone disease are unknown. Here, we tested the potential implication of an inflammation-dependent epidermal growth factor receptor (EGF-R) pathway in the regulation of gallbladder mucin synthesis. In gallbladder tissue sections from subjects with cholesterol gallstones, mucus accumulation was associated with neutrophil infiltration and with increased expressions of EGF-R and of tumor necrosis factor-α (TNF-α). In primary cultures of human gallbladder epithelial cells, TNF-α induced EGF-R overexpression. In the presence of TNF-α, EGF-R ligands (either EGF or transforming growth factor-α) caused significant increases in MUC5AC mRNA and protein production, whereas expression of the other gallbladder mucins MUC1, MUC3, and MUC5B was unchanged. In addition, on gallbladder tissue sections from subjects with gallstones, increased MUC5AC immunoreactivity was detected in the epithelium and within mucus gel in the lumen. Studies in primary cultures demonstrated that MUC5AC up-regulation induced by the combination of TNF-α with EGF-R ligands was completely blunted by inhibitors of EGF-R tyrosine kinase and mitogen-activated protein/extracellular signal-related kinase kinase. In conclusion, an inflammation-dependent EGF-R cascade causes overproduction of the gel-forming mucin MUC5AC, which accumulates in cholesterol gallstone disease. The ability to interrupt this cascade is of potential interest in the prevention of cholesterol gallstones.
Gallstone disease is very common and has a major economic impact in developed countries.1,2 The vast majority of gallstones are of cholesterol or cholesterol-pre-dominant type. Previous observations suggest that gallbladder mucins are overproduced and act as pronucleating factors in gallstone disease. Mucins are found within the insoluble matrix of gallstones.3 In supersaturated model bile, they accelerate the nucleation of cholesterol monohydrate crystals.4,5 In animals fed a lithogenic diet, an increase in the synthesis and secretion of gallbladder mucins occurs before crystal formation, and, subsequently, crystals grow predominantly within a mucin gel that accumulates on the gallbladder wall.6–9 Likewise, in humans (eg, in morbidly obese subjects) who develop gallstones during rapid weight loss, the concentration of mucins in gallbladder bile increases before the appearance of crystals.10–12
Mucins comprise membrane-bound mucins that are primarily located at the cell surface and gel-forming mucins that are secreted and responsible for the rheologic properties of mucus.13 The gel-forming mucins have the ability to oligomerize14 and to form a mucus gel and thus are susceptible to promote gallstone formation. The possibility that this type of mucin contributes to cholelithiasis is further supported by the fact that overexpression of two gel-forming mucins, MUC5AC and, to a much lesser extent, MUC2, has been detected in stone-containing intrahepatic bile ducts of subjects with hepatolithiasis.15,16
The mechanisms leading to mucin overproduction in gallstone disease are unknown. However, there is evidence to indicate that inflammatory mechanisms might be involved. Thus, in animal models of cholelithiasis, mucin overproduction coincides with early inflammatory changes of the gallbladder mucosa,17,18 and anti-inflammatory drugs have been shown to prevent mucin gel accumulation in the gallbladder of these animals.7 Among pro-inflammatory cytokines, tumor necrosis factor-α (TNF-α) has been reported to affect gene expression of MUC5AC and of MUC2 in murine intrahepatic biliary epithelial cells.19 In airway epithelial cells, TNF-α acts on MUC5AC production by a mechanism implicating the epidermal growth factor receptor (EGF-R) signaling pathway.20,21
In the present study, we investigated potential links between gallbladder mucin overproduction, inflammation, and the EGF-R signaling pathway in gallstone disease. First, we examined mucin contents, the presence of inflammatory cell (neutrophil) infiltrate, and the expressions of EGF-R and the pro-inflammatory cytokine TNF-α in gallbladder tissue specimens from subjects with cholesterol gallstones. Then, in primary cultures of human gallbladder epithelial cells, we analyzed the influence of the EGF-R pathway on gene expression and production of gallbladder mucins.
Materials and Methods
Reagents
Dulbecco’s modified Eagle’s medium/Ham’s F12 and Moloney murine leukemia virus reverse transcriptase were purchased from Life Technologies (Cergy Pontoise, France). Ultroser G was from Biosepra (Villeneuve-la-Garenne, France), and human type IV collagen was from Tebu (Le Perray-en-Yvelines, France). Protease type XIV from Streptomyces griseus, recombinant human EGF, and recombinant human transforming growth factor-α (TGF-α) were provided by Sigma (Saint-Quentin Fallavier, France). Ribonuclease inhibitor RNazine was obtained from Promega (Charbonnières, France), and TaqDNA polymerase was from Perkin-Elmer (Les Ulis, France). Recombinant human TNF-α was purchased from Interchim (Montluçon, France). Tyrphostin AG 1478 and PD 98059 were purchased from Calbiochem (La Jolla, CA).
Tissue Specimens
Gallbladder tissue specimens excluding acute cholecystitis were obtained from 10 subjects with cholesterol gallstone disease undergoing elective cholecystectomy. These specimens displayed no or mild chronic cholecystitis at histological examination. Specimens used as controls and for primary cultures of gallbladder epithelial cells were obtained from subjects without gallstones who underwent cholecystectomy during liver surgery. Subjects were recruited using informed consent for a protocol approved by the Committee for Human Research at the University of California, San Francisco.
(Immuno)histochemical Analyses
Immediately after surgical ablation, samples were fixed in 10% formalin, embedded in paraffin, and cut into 5-μm sections. Tissue sections were deparaffinized, rehydrated, and treated with 0.3% H2O2 in methyl alcohol. For immunohistochemistry, phosphate-buffered saline (PBS) containing 0.05% Tween 20 and 1% bovine serum albumin was used as a diluent for monoclonal antibodies. We used monoclonal antibodies raised against MUC2 (clone M53, 1:100; Neomarkers, Fremont, CA), MUC5AC (clone 45M1, 1:500; Neomarkers), EGF-R (Ab-5, 1:100; Calbiochem), human neutrophil elastase (NP57, 1:100; DAKO Corp., Carpinteria, CA), and a polyclonal antibody raised against TNF-α (1:1000; Genzyme, Cambridge, MA). Tissue sections were incubated with 2% bovine serum albumin at room temperature for 1 hour and then incubated with primary antibodies at room temperature for 2 hours. After washing with PBS, sections were incubated with a biotinylated horse anti-mouse antibody (1:200; Vector Laboratories, Burlingame, CA) at room temperature for 1 hour. Bound antibody was visualized according to standard protocols for avidin-biotin-peroxidase complex method (Elite ABC kit; Vector Laboratories), and tissue sections were counterstained with hematoxylin. Alcian Blue/Periodic Acid Schiff (AB/PAS) staining was performed for the detection of mucous glycoconjugates, as previously described.22
Morphometric analyses of AB/PAS-stained and MUC5AC-immunoreactive areas in the gallbladder epithelium and lumen were performed using a semiautomatic imaging system that includes a microscope, a video camera, and a computer. We measured the values of AB/PAS- and MUC5AC-labeled areas and of corresponding total epithelial and luminal areas, from two randomly selected gallbladder sections in each subject. In each section, we first recorded 10 power-field images of epithelium and luminal spaces (between deep folds) at magnification ×400. Next, we measured epithelial and luminal areas on a computer screen using a grid composed of points: intersections of points with epithelium or lumen were counted by a single observer (unaware of the study group on two separate occasions in a coded random order) and were converted to areas (each point corresponding to a known area). Areas of epithelium or lumen occupied by AB/PAS and MUC5AC labeling were also measured by point counting using a grid. Results obtained in two sections were averaged and expressed as a percentage of total area.
Quantification of neutrophils in gallbladder tissue sections was performed by counting the number of human neutrophil elastase (HNE)-labeled cells in the subepithelium, ie, in the area immediately beneath the basement membrane. Ten high-power-field images (magnification, ×400) were selected randomly, and positively labeled cells were counted. Results are expressed as the number of cells per square millimeter in the subepithelial area. In these morphometric analyses, the within observer coefficient of variation for repeated counts of labeled cells or areas was <5%.
Cell Isolation and Cultures
Human gallbladder epithelial cells were isolated by incubation with 0.075% (w/v) protease type XIV at 4°C for 12 hours, as previously described.23 Isolated gallbladder epithelial cells were suspended in Dulbecco’s modified Eagle’s medium/Ham’s F12 containing 1 mmol/L Ca2+, supplemented with 5.35 g/L d-glucose, 14 mmol/L NaHCO3, 2% Ultroser G, and 200,000 IU of 200 mg/L penicillin G-streptomycin (pH 7.4) and plated in culture dishes coated with human type IV collagen. The cells were incubated with 95% air and 5% CO2 at 37°C. All experiments were performed at days 6 to 7 of primary culture when the gallbladder epithelial cells form a confluent monolayer and retain the ability to produce mucins.22 In cells pre-incubated in Ultroser G-free medium for 48 hours, EGF or TGF-α (both at 50 ng/ml) and TNF-α (20 or 40 ng/ml) were added alone or in combination and were maintained for 24 hours. In inhibition studies, cells were pretreated for 30 minutes with the selective EGF-R tyrosine kinase inhibitor AG 1478 (10 μmol/L) or with the mitogen-activating protein/extracellular signal-related kinase kinase (MEK) inhibitor PD 98059 (30 μmol/L).
Reverse Transcription and Real-Time Polymerase Chain Reaction
Total RNA was extracted from cultured cells, by RNA plus lysis solution (Quantum, Montreuil-sous-Bois, France), according to the method of Chomczynski and Sacchi.24 cDNA was synthesized using random hexamers and Moloney murine leukemia virus reverse transcriptase. Real-time polymerase chain reaction (PCR) was performed with the TaqMan system (Applied Biosystems, Foster City, CA). PCR was performed using the SYBR Green Master Mix (Applied Biosystems). The primers were designed according to published human cDNA sequences in GenBank database using the Primer Express software v1.5 (PE Applied Biosystems): EGF-R (GenBank accession no. BC094761) forward, 5′-TGACCGTTTGGGAGTTGATGA-3′, and reverse, 5′-TCGATGGTACATATGGGTGGC-3′; MUC1 (GenBank accession no. NM_182741) forward, 5′-CTGCTGGTGCTGGTCTGTGT-3′, and reverse, 5′-ATGTCCAGCTGCCCGTAGTT-3′; MUC2 (GenBank accession no. L21998) forward, 5′-GCCCTGGCTTCGAACTCAT-3′, and reverse, 5′-TCTTCGGGTCGCTCTTGAA-3′; MUC3 (GenBank accession no. AF143371) forward, 5′-CCACGGGCTATGAAGAGTTCTACT-3′, and reverse, 5′-GTGACAGTCGATG GCGTTGT-3′; MUC5AC (GenBank accession no. AJ298317) forward, 5′-TCAGCC CCGAGTTCAAGG-3′, and reverse, 5′-TTCCCAAACTCCAGCACGTC-3′; and MUC5B (GenBank accession no. U78551) forward, 5′-AGTCCATTTGCTGACCCCAC-3′, and reverse, 5′-GGATGGTCGTGTTGATGCG-3′. 18S rRNA TaqMan assay reagent was used for internal control. One-step reverse transcriptase-PCR (RT-PCR) was performed for both target gene and endogenous controls. Duplicate CT values were analyzed in Microsoft Excel using the comparative CT (ΔΔCT) method as described by the manufacturer (Applied Biosystems). The amount of target (2−ΔΔCT) was obtained as normalized to 18S rRNA.
EGF-R Immunoblotting
Freshly isolated and cultured cells were lysed on ice in PBS lysis buffer containing 25 mmol/L Tris-HCl, 300 mmol/L NaCl, 1 mmol/L CaCl2, 1% Triton X-100 (pH 7.4), and protease inhibitors (Complete Mini; Roche Diagnostics, Indianapolis, IN). Lysates were precleared by centrifugation at 14,000 rpm for 20 minutes at 4°C. Protein concentration was determined by the bicinchoninic acid-based BCA Protein Assay kit (Pierce, Rockford, IL). Proteins (50 μg) were subjected to 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Hercules, CA). Immunoblotting was performed using a polyclonal anti-EGF-R carboxy-terminal peptide antibody (1:500; Cell Signaling Technology Inc., Beverly, MA), as described previously.25 Immunoreactivity was examined by enhanced chemiluminescence using an ECL kit (Amersham, Les Ulis, France). The detected bands were quantified with the NIH Image software (National Institutes of Health, Bethesda, MD).
MUC5AC Immunoassay
Newly produced mucins are released from cultured gallbladder epithelial cells constitutively,22 so that the total production of mucins consists of both mucins in cell lysates and mucins secreted into the cell culture supernatants. Therefore, in the present studies, the total production of MUC5AC protein was calculated as the sum of MUC5AC in cell lysates and in cell culture supernatants. After 24 hours of incubation of gallbladder epithelial cells in various conditions, MUC5AC protein in cell lysate and cell culture supernatant was measured by enzyme-linked immunosorbent assay (ELISA), as described previously.26 Cells were collected in lysis buffer (1% Triton X-100, 1% deoxycholic acid, and proteinase inhibitors). Fifty microliters of cell lysate or cell culture supernatant was incubated with bicarbonate-carbonate buffer (50 μl) at 40°C in a 96-well plate (Nalge Nunc International, Rochester, NY) until dry. Plates were washed with PBS and blocked with 2% bovine serum albumin, fraction V (Sigma) for 1 hour at room temperature. Plates were again washed with PBS and then incubated with 100 μl of MUC5AC monoclonal antibody (clone 45M1, 1:500; NeoMarkers) in PBS containing 0.05% Tween 20. After 1 hour, the plates were washed, and 100 μl of horseradish peroxidase-conjugated goat anti-mouse IgG conjugate (1:10,000; Sigma) was dispensed into each well. After 1 hour, plates were washed with PBS. Color reaction was developed with 3,3′,5,5′-tetramethylbenzidine peroxidase solution (Sigma) and stopped with 1 mol/L H2SO4. Absorbance was read at 450 nm. The amount of MUC5AC was calculated with bovine submaxillary gland mucin (type I; Sigma) as a standard.
Statistical Analyses
All statistical analyses were performed with the StatView 5.01 software (SAS Institute, Inc., Cary, NC). The paired Wilcoxon signed rank test was used to analyze differences between groups. Pearson’s linear regression analysis and one-way analysis of variance were used to determine correlations between variables. Differences of P < 0.05 were considered statistically significant.
Results
Gallbladder Mucin Accumulation in Gallstone Disease
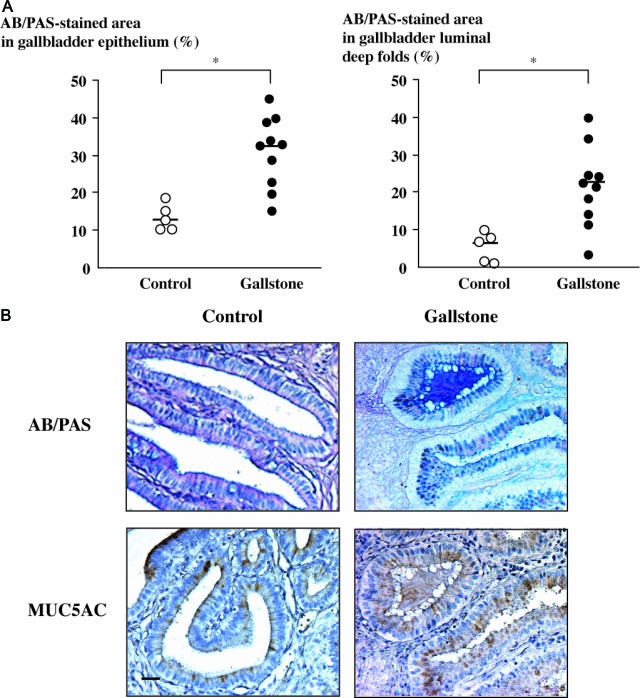
Mucous glycoconjugates were detected by AB/PAS staining on gallbladder tissue sections from both controls and subjects with cholesterol gallstone disease. Morphometric analyses of AB/PAS-stained areas on tissue sections showed that although variable among samples, mucous glycoconjugate contents were significantly higher in subjects with gallstones than in controls, both in the gallbladder epithelium (Figure 1A, left) and in the lumen, within spaces delimited by epithelial deep folds (Figure 1A, right) (P < 0.05). In subjects with gallstones, some of the luminal spaces between epithelial deep folds were completely filled with mucus, a feature that was not observed in controls (Figure 1B, top).
Figure 1.
Mucus content and MUC5AC expression in gallstone disease. A: Mucous glycoconjugate content was assessed by AB/PAS-stained areas in the epithelium (left) and in the luminal spaces delineated by epithelial deep folds (right) on gallbladder tissue sections from controls (n = 5, open circles) and from subjects with gallstones (n = 10, solid circles). AB/PAS-stained areas were measured by point counting and expressed as a percentage of total epithelial or luminal areas. Horizontal bars represent median values; *P < 0.05. B: Photomicrographs of AB/PAS-stained mucous glycoconjugates (top) and of MUC5AC immunoreactivity (bottom) in gallbladder tissue sections from a control (left) and from a subject with gallstones (right), with an example of deep fold luminal space filled with mucins. Bar = 50 μm; original magnification, ×100.
Because an altered expression of the gel-forming mucins MUC5AC and, to a lesser extent, MUC2 has been reported previously in the setting of intrahepatic lithiasis,15,16 we next performed immunohistochemical analyses of MUC5AC and MUC2 on gallbladder tissue sections to determine whether these mucins contributed to mucus accumulation in gallstone disease. In subjects with gallstones, MUC5AC immunoreactivity was more intense than in controls (Figure 1B, bottom), and its distribution coincided with that of AB/PAS staining both in the gallbladder epithelium and in the lumen (Figure 1B, right). No gastric or intestinal metaplasia that could account for MUC5AC overexpression was found at histological examination. MUC2 immunoreactivity was undetectable in the lumen and in the vast majority of gallbladder epithelial cells with the exception of a weak signal in very sparse cells, both in controls and in subjects with gallstones (data not shown).
From these findings, we concluded that in human gallstone disease, mucus accumulates both in the gallbladder epithelium and on the luminal surface of the epithelium and that MUC5AC makes a significant contribution to this mucus accumulation.
Subepithelial Neutrophil Infiltration in Gallstone Disease
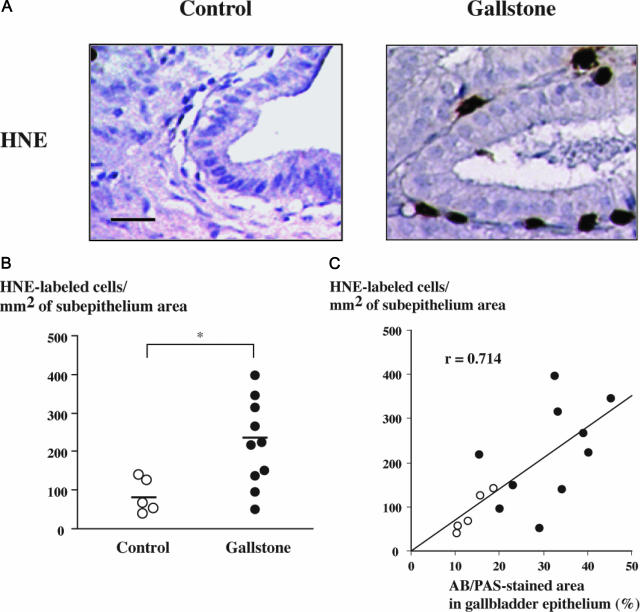
In animal models of lithogenesis, increased mucus content is associated with inflammatory changes including leukocyte infiltration of the gallbladder mucosa.17,18 In the airway epithelium, neutrophils contribute to the induction of mucin production.20,26,27 Here, we examined human gallbladder tissue specimens for the presence of neutrophils using HNE immunolabeling. Figure 2A shows HNE-labeled neutrophils in the subepithelium of a mucus-containing deep fold in a subject with gallstones, compared with a control subject. Morphometric analyses demonstrated that in subjects with gallstones, the number of neutrophils in the subepithelium was significantly increased compared with controls (P < 0.05) (Figure 2B). In addition, the number of neutrophils in the subepithelium was correlated with mucus content in the epithelium, as assessed by the proportion of AB/PAS-stained areas (r = 0.714, P < 0.05) (Figure 2C). In subjects with gallstones, there was also a correlation between the number of neutrophils in the subepithelium and MUC5AC content in the epithelium as assessed by the proportion of MUC5AC immunoreactive areas (r = 0.717, P < 0.05). These results provide evidence for a relationship between inflammation, mucus accumulation, and MUC5AC overproduction in the gallbladder.
Figure 2.
Neutrophil infiltration of the gallbladder subepithelium in gallstone disease. A: Photomicrographs of gallbladder tissue sections labeled for HNE showing no labeling in a control specimen (left) and neutrophil infiltration in the subepithelium of a gallbladder with gallstones (right). Bar = 50 μm; original magnification, ×200. B: HNE-labeled cells were counted in the subepithelium of gallbladder specimens from controls (n = 5, open circles) and from individuals with gallstones (n = 10, solid circles). Horizontal bars represent median values; *P < 0.05. C: Correlation between the number of HNE-labeled cells in the subepithelium and AB/PAS-stained areas in the epithelium of gallbladders from controls (open circles) and from individuals with gallstones (solid circles); r = 0.714; P < 0.05.
Increased Expression of EGF-R and TNF-α in Gallstone Disease
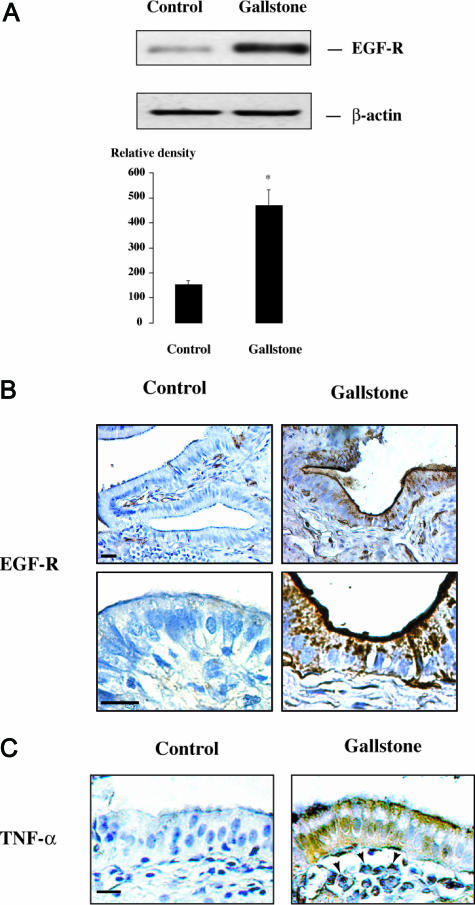
Because EGF-R regulates the synthesis of mucous glycoconjugates in other epithelia,21,28,29 we examined EGF-R expression in gallbladder specimens. Freshly isolated gallbladder epithelial cells were harvested from controls and from subjects with gallstones and were analyzed for EGF-R protein expression by Western blot. These analyses showed an overexpression of EGF-R in the gallbladder epithelial cells obtained from individuals with gallstones compared with controls (Figure 3A). Immunohistochemical analyses showed that EGF-R immunolabeling was faint in the gallbladder epithelium of control subjects (Figure 3B, left). By contrast, in subjects with gallstones, the majority of gallbladder epithelial cells displayed intense EGF-R immunoreactivity, which was detected predominantly in the apical region of the epithelium but also in the basal domain of the cells (Figure 3B, right).
Figure 3.
EGF-R and TNF-α expression in gallstone disease. A: Representative Western blot of EGF-R and β-actin in freshly isolated gallbladder epithelial cells from a control subject and from a subject with gallstones. In quantitative analysis, results are expressed as the ratio of EGF-R to β-actin signals. They represent the mean ± SEM of three preparations; *P < 0.05 versus control. B: Representative photomicrographs at low (top) and high (bottom) magnifications of EGF-R immunolabeling in gallbladder tissue sections from controls and subjects with gallstones. In the control specimen (left), EGF-R immunoreactivity is weak in the epithelium; in the specimen with gallstones (right), EGF-R immunoreactivity is intense and located predominantly in the apical region of epithelial cells but also in their basal region (bottom right). Bar = 50 μm; original magnification: top panels, ×100; bottom panels, ×400. C: Photomicrographs of gallbladder tissue sections labeled for TNF-α showing no labeling in a control specimen (left), whereas in a gallbladder with gallstones, TNF-α immunoreactivity is detected within both the epithelium and inflammatory cells in the subepithelium (right, arrowheads). Bar = 50 μm; original magnification, ×200.
Tissue specimens from subjects with gallstones also showed increased expression of the pro-inflammatory cytokine TNF-α, which was previously shown to stimulate EGF-R expression in other epithelia.29,30 In these subjects, TNF-α was detected both in the gallbladder epithelium and in inflammatory cells within the subepithelium (Figure 3C).
Up-Regulation of EGF-R Expression by TNF-α in Human Gallbladder Epithelial Cells
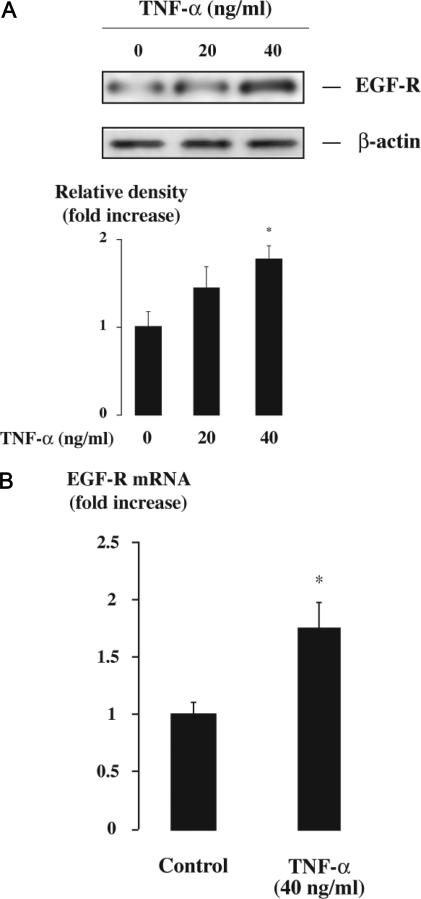
To dissect further the mechanisms underlying EGF-R overexpression and mucin production in the gallbladder, we investigated gallbladder epithelial cells in vitro. First, using primary cultures of human gallbladder epithelial cells, we tested the possibility that EGF-R expression is up-regulated by TNF-α in these cells. Incubation of gallbladder epithelial cells with TNF-α caused a dose-dependent increase in EGF-R protein, as ascertained by Western blot analysis (Figure 4A). We also found that TNF-α caused a significant increase in EGF-R transcripts in gallbladder epithelial cells (Figure 4B), consistent with a stimulation of gene transcription.
Figure 4.
Effect of TNF-α on EGF-R expression in gallbladder epithelial cells. Primary cultures of human gallbladder epithelial cells were incubated with or without TNF-α (20 or 40 ng/ml) for 24 hours and were then submitted to Western blot or real-time RT-PCR analyses. A: Western blot analysis of EGF-R examining the effect of TNF-α concentration on EGF-R expression. A representative Western blot of EGF-R and β-actin is shown. In quantitative analysis, results are expressed as the ratio of EGF-R/β-actin signals and relative to untreated cells. They represent the mean ± SEM of three preparations; *P < 0.05 versus untreated cells. B: Real-time RT-PCR analysis of EGF-R gene expression in response to stimulation with TNF-α (40 ng/ml). Results are expressed as mRNA levels normalized to 18S rRNA and relative to untreated cells. They represent the mean ± SEM of four experiments performed in duplicate; *P < 0.05 versus untreated cells.
Effect of EGF-R Cascade Activation on Mucin Production in Gallbladder Epithelial Cells
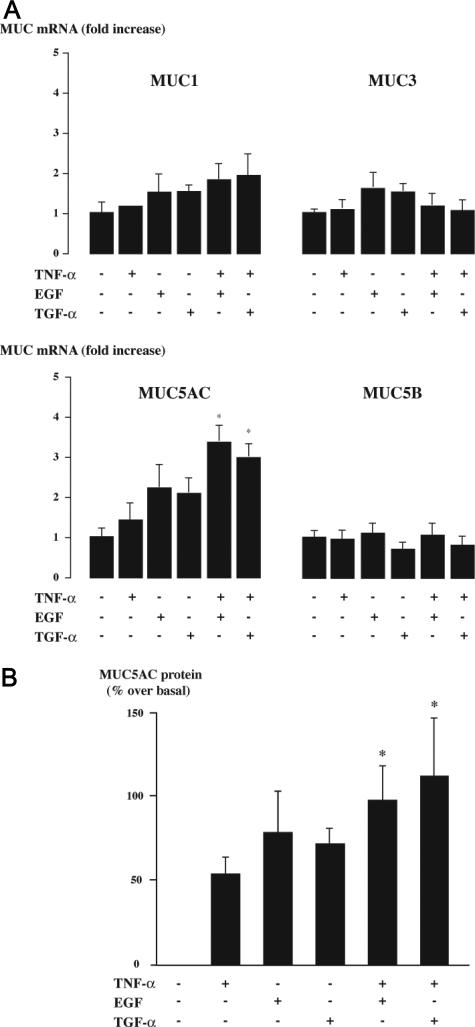
To examine the role of EGF-R pathway on mucin production, we first analyzed the effect of EGF-R cascade activation on the gene expression of the major gallbladder mucins, including membrane-bound (MUC1 and MUC3) and gel-forming mucins (MUC5AC and MUC5B).31–35 After treatment of gallbladder epithelial cells with EGF-R ligands (either TGF-α or EGF) and/or with TNF-α, which was used to up-regulate EGF-R expression, gene expression was assessed by real-time RT-PCR analysis. EGF-R ligands either alone or in combination with TNF-α induced no significant change in MUC1, MUC3, or MUC5B mRNA levels (Figure 5A). In cells exposed to TNF-α, EGF, or TGF-α alone, the levels of MUC5AC mRNA trended upward but were not significantly different from controls (Figure 5A). In contrast, when EGF-R ligands (EGF or TGF-α) were added in combination with TNF-α, MUC5AC mRNA levels increased further, and the difference with controls was significantly different (Figure 5A). Consistent with our immunohistochemical data, MUC2 expression, which was previously shown to be regulated by an EGF-R pathway in airway epithelial cells,21 was not found at the mRNA level in gallbladder epithelial cells (data not shown).
Figure 5.
Effects of TNF-α and EGF-R ligands on mucin synthesis in gallbladder epithelial cells. Primary cultures of human gallbladder epithelial cells were incubated with or without TNF-α (40 ng/ml), EGF (50 ng/ml), or TGF-α (50 ng/ml) for 24 hours and were then submitted to real-time RT-PCR or ELISA. A: Real-time RT-PCR analyses of gallbladder mucin genes (MUC1, MUC3, MUC5AC, and MUC5B) expressions. Results are expressed as mRNA levels normalized to 18S rRNA and relative to untreated cells. They represent the mean ± SEM of five experiments performed in duplicate; *P < 0.05 versus untreated cells. B: The analysis of MUC5AC protein production. MUC5AC was measured by ELISA in cell lysates and culture supernatants. The total production of MUC5AC was determined by the sum of MUC5AC in cell lysates and culture supernatants. Results are expressed as a percentage over basal. They represent the mean ± SEM of four experiments performed in duplicate; *P < 0.05 versus untreated cells.
To determine whether MUC5AC gene up-regulation thus identified was associated with increased mucin synthesis, we performed analyses of MUC5AC protein total production using combined measurements in cell lysates and supernatants. These analyses showed that the combination of TNF-α with EGF-R ligands also increased significantly MUC5AC protein production (Figure 5B).
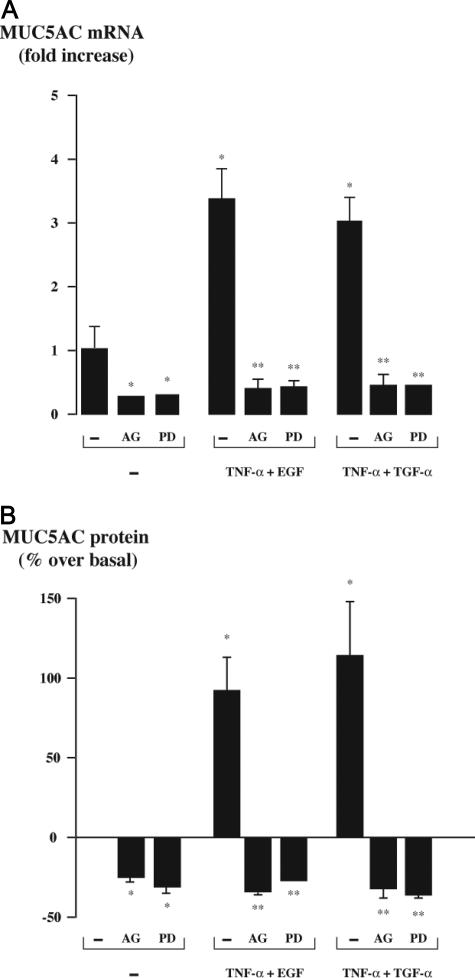
To ascertain the implication of EGF-R signaling pathway in the stimulation of MUC5AC synthesis by the combination of TNF-α with EGF-R ligands, we pre-incubated the cells with AG 1478, a selective inhibitor of EGF-R tyrosine kinase, or with PD 98059, an inhibitor of MEK. Both treatments completely blocked the stimulation of MUC5AC synthesis at mRNA (Figure 6A) and protein levels (Figure 6B). These treatments also decreased basal MUC5AC mRNA and protein levels significantly, indicating that EGF-R and MEK activation are required for the maintenance of baseline MUC5AC synthesis in gallbladder epithelial cells. Altogether, these data indicate that the EGF-R cascade is a key pathway in gallbladder MUC5AC production.
Figure 6.
Effect of inhibitors of the EGF-R signaling pathway on MUC5AC synthesis in gallbladder epithelial cells. Primary cultures of human gallbladder epithelial cells were pretreated with a selective EGF-R tyrosine kinase inhibitor (AG 1478, 10 μmol/L) for 30 minutes or with a MEK inhibitor (PD 98059, 30 μmol/L) for 1 hour and then incubated with the combination of TNF-α (40 ng/ml) with either TGF-α or EGF (both at 50 ng/ml) for 24 hours. Cultures were then submitted to real-time RT-PCR or ELISA. A: Real-time RT-PCR analysis of MUC5AC gene expression. Results are expressed as mRNA levels normalized to 18S rRNA and relative to untreated cells. They represent the mean ± SEM of five experiments performed in duplicate; *P < 0.05 versus untreated cells; **P < 0.05 versus the combination of TNF-α with either TGF-α or EGF without inhibitor. B: The analysis of MUC5AC protein production. MUC5AC was measured by ELISA in cell lysates and culture supernatants. The total production of MUC5AC was determined by the sum of MUC5AC in cell lysates and culture supernatants. Results are expressed as a percentage over basal. They represent the mean ± SEM of four experiments performed in duplicate. *P < 0.05 versus untreated cells; **P < 0.05 versus the combination of TNF-α with either TGF-α or EGF without inhibitor.
Discussion
Gallbladder mucin is regarded as a critical contributing factor in the pathogenesis of gallstone disease. Although previous studies, including those from our group, have identified regulatory pathways of mucin secretion in the gallbladder,22,36–38 the regulation of gallbladder mucin synthesis has been rarely investigated. Here, we demonstrate the pivotal role of an inflammation-dependent EGF-R pathway in the regulation of mucin synthesis in the setting of gallstone disease. First, we found that mucin accumulation is associated with inflammation and with increased EGF-R expression in the gallbladder from individuals with gallstones. Then, we demonstrated by in vitro studies that the pro-inflammatory cytokine TNF-α induces EGF-R expression and that activation of the EGF-R cascade increases the synthesis of a major gel-forming mucin, MUC5AC. Finally, we showed that inhibitors of the EGF-R signaling pathway completely block this overproduction.
Consistent with previous observations in animal models of gallstone disease6,8,39 and in obese subjects developing cholesterol crystals or gallstones during weight reduction,10–12 we observed an accumulation of mucins on the luminal surface of the gallbladder epithelium, along with mucin accumulation within the epithelium, in subjects with gallstones. This mucin accumulation was correlated with infiltration of the subepithelium by neutrophils. Inflammation and neutrophil infiltration of the gallbladder were previously shown to occur early in gallstone disease in response to cholesterol supersaturation of bile17,18 and have been attributed to a pro-inflammatory effect of absorbed sterols from saturated bile.40 However, the present study was not designed to determine the sequence of biliary events in vivo, and we cannot exclude other mechanisms of inflammation, including the possibility that cholesterol gallstones themselves, once they are formed, act as foreign bodies and stimulate the recruitment of neutrophils and the overproduction of mucins.41 Neutrophils can influence the EGF-R pathway and mucin production through several mechanisms. They can release elastase and reactive oxygen species, causing ligand-dependent EGF-R activation and mucin overproduction.20,26 Together with the biliary epithelial cells themselves,42,43 they may also contribute to the production of TNF-α,44 causing EGF-R overexpression. Implication of a TNF-α-dependent EGF-R pathway in the accumulation of mucins in gallstone disease was herein suggested by the fact that EGF-R together with TNF-α was overexpressed in subjects with gallstones. In a previous study, Lee et al45 described EGF-R immunoreactivity in chronic cholecystitis as weak to moderate, but this was in reference to a higher expression in gallbladder carcinoma, and the conditions of detection in this study compared with ours were likely less sensitive (eg, primary antibody used at a dilution of 1:1500 instead of 1:100). The fact that EGF-R immunoreactivity was detected in the apical domain of epithelial cells was also previously reported in the gastrointestinal tract46,47 and in the airways,48 other sites of mucin production. In the gallbladder epithelium, this finding was consistent with activation of the EGF-R pathway by luminal factors (ie, EGF and TGF-α that are present in bile).47,49 Altogether, the data obtained by the analysis of human gallbladder tissue led us to investigate the regulation of mucin synthesis by a TNF-α-dependent EGF-R pathway in primary cultures of human gallbladder epithelial cells.
In the human gallbladder epithelium, the membrane-bound mucins MUC1 and MUC3 and the gel-forming mucins MUC5AC and MUC5B have been identified as the predominant types of mucins.31–35 In primary cultures of human gallbladder epithelial cells, the combination of TNF-α with EGF-R ligands significantly increased MUC5AC expression, whereas expressions of MUC1, MUC3, and MUC5B were unchanged. The effect of TNF-α alone on mucin production is an issue of conflicting results. It has been reported on the basis of Northern blot analysis that TNF-α increased MUC5AC expression in murine intrahepatic biliary epithelial cells.19 Conversely, TNF-α was shown to have little or no effect on mucin secretion in dog gallbladder epithelial cells37 and on MUC5AC expression in intestinal cells.50 Likewise, we found here that TNF-α alone had only a minor effect on MUC5AC production in the gallbladder. Instead, TNF-α augmented the effect of EGF-R ligands on MUC5AC production. This synergistic effect may be attributed to the up-regulation of EGF-R expression induced by TNF-α in gallbladder epithelial cells. Although TNF-α was previously reported to induce transactivation of EGF-R in different cell lines,51–53 the exposure of gallbladder epithelial cells to TNF-α over a short time course showed no induction of EGF-R tyrosine phosphorylation (data not shown). This may be explained by a low constitutive expression of EGF-R in these cells. Direct evidence that the synergistic effect of TNF-α on MUC5AC production was mediated by EGF-R cascade was provided by the fact that this effect was completely blunted by inhibitors of the EGF-R signaling pathway.
We found that among gallbladder mucins, MUC5AC is selectively regulated by the EGF-R pathway. The expression of MUC2, previously shown to be up-regulated together with MUC5AC by an EGF-R pathway in lung cancer cells,21 was virtually absent in the gallbladder, as shown both by immunohistochemical and RT-PCR analyses. By contrast, immunohistochemical studies clearly indicated that an overproduction of MUC5AC occurs along with EGF-R overexpression in gallstone disease. Because a decrease in MUC5AC immunoreactivity in the epithelium was previously reported in acute cholecystitis,35 it should be kept in mind that part of the overproduced mucin is secreted in the lumen. If, as one would expect, severe inflammation increases secretion even more than total production, this could explain why intracellular MUC5AC may appear as decreased in acute/severe cholecystitis. Here, in the setting of no or only mild chronic cholecystitis, we found an increase of both MUC5AC immunoreactivity in the epithelium and of secreted MUC5AC at the surface of the epithelium, which may be of particular importance in the constitution of the backbone of gallstone by means of gel formation. Moreover, in individuals with gallstones, the distribution of MUC5AC mucin coincided with that of total mucous glycoconjugates. Together with the previous demonstration that MUC5AC is clearly overexpressed in the biliary tract of subjects with hepatolithiasis15 and that experimental reduction in MUC5AC expression results in decreased formation of cholesterol gallstones in mice fed a lithogenic diet,39 the present study indicates that selective regulation of MUC5AC production may be of particular importance in stone formation.
Identification of the EGF-R cascade as a major regulatory pathway in gallbladder mucin production provides new insights into the pathogenesis of gallstone disease. The ability shown here to block this cascade leading to gallbladder mucin overproduction is of potential interest in the prevention of cholesterol gallstones.
Acknowledgments
We are greatly indebted to Iris Ueki for excellent technical assistance and helpful discussion.
Footnotes
Address reprint requests to Chantal Housset, M.D., Ph.D., INSERM U680, Faculté de Médecine Pierre et Marie Curie (Université Pierre et Marie Curie-Paris 6), Site Saint-Antoine, 27 rue Chaligny, 75571 Paris cedex 12, France. E-mail: chantal.housset@st-antoine.inserm.fr.
Supported by Sanofi-Synthelabo (to L.F.), Association Française pour l’Etude du Foie, Association pour la Recherche sur le Cancer, and Institut National Du Cancer.
References
- Kim WR, Brown RS, Jr, Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–242. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- Shaffer EA. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep. 2005;7:132–140. doi: 10.1007/s11894-005-0051-8. [DOI] [PubMed] [Google Scholar]
- Smith BF, LaMont JT. Identification of gallbladder mucin-bilirubin complex in human cholesterol gallstone matrix: effects of reducing agents on in vitro dissolution of matrix and intact gallstones. J Clin Invest. 1985;76:439–445. doi: 10.1172/JCI111991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy PF, Smith BF, LaMont JT. Human gallbladder mucin accelerates nucleation of cholesterol in artificial bile. Gastroenterology. 1984;87:270–275. [PubMed] [Google Scholar]
- Smith BF. Human gallbladder mucin binds biliary lipids and promotes cholesterol crystal nucleation in model bile. J Lipid Res. 1987;28:1088–1097. [PubMed] [Google Scholar]
- Lee SP, LaMont JT, Carey MC. Role of gallbladder mucus hypersecretion in the evolution of cholesterol gallstones. J Clin Invest. 1981;67:1712–1723. doi: 10.1172/JCI110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SP, Carey MC, LaMont JT. Aspirin prevention of cholesterol gallstone formation in prairie dogs. Science. 1981;211:1429–1431. doi: 10.1126/science.7466399. [DOI] [PubMed] [Google Scholar]
- MacPherson BR, Pemsingh RS. Ground squirrel model for cholelithiasis: role of epithelial glycoproteins. Microsc Res Tech. 1997;39:39–55. doi: 10.1002/(SICI)1097-0029(19971001)39:1<39::AID-JEMT4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Wang DQ, Paigen B, Carey MC. Phenotypic characterization of Lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice: physical-chemistry of gallbladder bile. J Lipid Res. 1997;38:1395–1411. [PubMed] [Google Scholar]
- Marks JW, Bonorris GG, Albers G, Schoenfield LJ. The sequence of biliary events preceding the formation of gallstones in humans. Gastroenterology. 1992;103:566–570. doi: 10.1016/0016-5085(92)90848-s. [DOI] [PubMed] [Google Scholar]
- Shiffman ML, Shamburek RD, Schwartz CC, Sugerman HJ, Kellum JM, Moore EW. Gallbladder mucin, arachidonic acid, and bile lipids in patients who develop gallstones during weight reduction. Gastroenterology. 1993;105:1200–1208. doi: 10.1016/0016-5085(93)90968-i. [DOI] [PubMed] [Google Scholar]
- Gustafsson U, Benthin L, Granstrom L, Groen AK, Sahlin S, Einarsson C. Changes in gallbladder bile composition and crystal detection time in morbidly obese subjects after bariatric surgery. Hepatology. 2005;41:1322–1328. doi: 10.1002/hep.20686. [DOI] [PubMed] [Google Scholar]
- Thornton DJ, Sheehan JK. From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thorac Soc. 2004;1:54–61. doi: 10.1513/pats.2306016. [DOI] [PubMed] [Google Scholar]
- van Klinken BJ, Einerhand AW, Buller HA, Dekker J. The oligomerization of a family of four genetically clustered human gastrointestinal mucins. Glycobiology. 1998;8:67–75. doi: 10.1093/glycob/8.1.67. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Nakanuma Y, Kim YS. Expression of apomucins in the intrahepatic biliary tree in hepatolithiasis differs from that in normal liver and extrahepatic biliary obstruction. Hepatology. 1998;27:54–61. doi: 10.1002/hep.510270110. [DOI] [PubMed] [Google Scholar]
- Zen Y, Sasaki M, Fujii T, Chen TC, Chen MF, Yeh TS, Jan YY, Huang SF, Nimura Y, Nakanuma Y. Different expression patterns of mucin core proteins and cytokeratins during intrahepatic cholangiocarcinogenesis from biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile duct-an immunohistochemical study of 110 cases of hepatolithiasis. J Hepatol. 2006;44:350–358. doi: 10.1016/j.jhep.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Haley-Russell D, Husband KJ, Moody FG. Morphology of the prairie dog gallbladder: normal characteristics and changes during early lithogenesis. Am J Anat. 1989;186:133–143. doi: 10.1002/aja.1001860204. [DOI] [PubMed] [Google Scholar]
- Rege RV, Prystowsky JB. Inflammation and a thickened mucus layer in mice with cholesterol gallstones. J Surg Res. 1998;74:81–85. doi: 10.1006/jsre.1997.5213. [DOI] [PubMed] [Google Scholar]
- Zen Y, Harada K, Sasaki M, Tsuneyama K, Katayanagi K, Yamamoto Y, Nakanuma Y. Lipopolysaccharide induces overexpression of MUC2 and MUC5AC in cultured biliary epithelial cells: possible key phenomenon of hepatolithiasis. Am J Pathol. 2002;161:1475–1484. doi: 10.1016/S0002-9440(10)64423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol. 2000;164:1546–1552. doi: 10.4049/jimmunol.164.3.1546. [DOI] [PubMed] [Google Scholar]
- Perrais M, Pigny P, Copin MC, Aubert JP, Van Seuningen I. Induction of MUC2 and MUC5AC mucins by factors of the epidermal growth factor (EGF) family is mediated by EGF receptor/Ras/Raf/extracellular signal-regulated kinase cascade and Sp1. J Biol Chem. 2002;277:32258–32267. doi: 10.1074/jbc.M204862200. [DOI] [PubMed] [Google Scholar]
- Dray-Charier N, Paul A, Combettes L, Bouin M, Mergey M, Balladur P, Capeau J, Housset C. Regulation of mucin secretion in human gallbladder epithelial cells: predominant role of calcium and protein kinase C. Gastroenterology. 1997;112:978–990. doi: 10.1053/gast.1997.v112.pm9041261. [DOI] [PubMed] [Google Scholar]
- Housset C, Carayon A, Housset B, Legendre C, Hannoun L, Poupon R. Endothelin-1 secretion by human gallbladder epithelial cells in primary culture. Lab Invest. 1993;69:750–755. [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Schiffer E, Housset C, Cacheux W, Wendum D, Desbois-Mouthon C, Rey C, Clergue F, Poupon R, Barbu V, Rosmorduc O. Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology. 2005;41:307–314. doi: 10.1002/hep.20538. [DOI] [PubMed] [Google Scholar]
- Kohri K, Ueki IF, Nadel JA. Neutrophil elastase induces mucin production by ligand-dependent epidermal growth factor receptor activation. Am J Physiol. 2002;283:L531–L540. doi: 10.1152/ajplung.00455.2001. [DOI] [PubMed] [Google Scholar]
- Park JA, He F, Martin LD, Li Y, Chorley BN, Adler KB. Human neutrophil elastase induces hypersecretion of mucin from well-differentiated human bronchial epithelial cells in vitro via a protein kinase Cδ-mediated mechanism. Am J Pathol. 2005;167:651–661. doi: 10.1016/s0002-9440(10)62040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinter-Jensen L, Juhl CO, Djurhuus JC, Poulsen SS, Dajani EZ, Brown KD, Orntoft TF, Teglbjaerg PS, Nexo E. Chronic systemic treatment with epidermal growth factor in pigs causes pronounced urothelial growth with accumulation of glycoconjugates. Am J Pathol. 1995;147:1330–1338. [PMC free article] [PubMed] [Google Scholar]
- Takeyama K, Dabbagh K, Lee HM, Agusti C, Lausier JA, Ueki IF, Grattan KM, Nadel JA. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci USA. 1999;96:3081–3086. doi: 10.1073/pnas.96.6.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiegel W, Roeder C, Schmielau J, Rodeck U, Kalthoff H. Tumor necrosis factor alpha induces the expression of transforming growth factor alpha and the epidermal growth factor receptor in human pancreatic cancer cells. Proc Natl Acad Sci USA. 1993;90:863–867. doi: 10.1073/pnas.90.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SB, Niehans GA, Lyftogt C, Yan PS, Cherwitz DL, Gum ET, Dahiya R, Kim YS. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993;53:641–651. [PubMed] [Google Scholar]
- Baeckstrom D, Karlsson N, Hansson GC. Purification and characterization of sialyl-Le(a)-carrying mucins of human bile; evidence for the presence of MUC1 and MUC3 apoproteins. J Biol Chem. 1994;269:14430–14437. [PubMed] [Google Scholar]
- Campion JP, Porchet N, Aubert JP, L’Helgoualc’h A, Clement B. UW-preservation of cultured human gallbladder epithelial cells: phenotypic alterations and differential mucin gene expression in the presence of bile. Hepatology. 1995;21:223–231. [PubMed] [Google Scholar]
- van Klinken BJ, Dekker J, van Gool SA, van Marle J, Buller HA, Einerhand AW. MUC5B is the prominent mucin in human gallbladder and is also expressed in a subset of colonic goblet cells. Am J Physiol. 1998;274:G871–G878. doi: 10.1152/ajpgi.1998.274.5.G871. [DOI] [PubMed] [Google Scholar]
- Ho SB, Shekels LL, Toribara NW, Gipson IK, Kim YS, Purdum PP, 3rd, Cherwitz DL. Altered mucin core peptide expression in acute and chronic cholecystitis. Dig Dis Sci. 2000;45:1061–1071. doi: 10.1023/a:1005573213100. [DOI] [PubMed] [Google Scholar]
- O’Leary DP, Murray FE, Turner BS, LaMont JT. Bile salts stimulate glycoprotein release by guinea pig gallbladder in vitro. Hepatology. 1991;13:957–961. [PubMed] [Google Scholar]
- Choi J, Klinkspoor JH, Yoshida T, Lee SP. Lipopolysaccharide from Escherichia coli stimulates mucin secretion by cultured dog gallbladder epithelial cells. Hepatology. 1999;29:1352–1357. doi: 10.1002/hep.510290515. [DOI] [PubMed] [Google Scholar]
- Chignard N, Mergey M, Veissiere D, Parc R, Capeau J, Poupon R, Paul A, Housset C. Bile acid transport and regulating functions in the human biliary epithelium. Hepatology. 2001;33:496–503. doi: 10.1053/jhep.2001.22345. [DOI] [PubMed] [Google Scholar]
- Wang HH, Afdhal NH, Gendler SJ, Wang DQ. Targeted disruption of the murine mucin gene 1 decreases susceptibility to cholesterol gallstone formation. J Lipid Res. 2004;45:438–447. doi: 10.1194/jlr.M300468-JLR200. [DOI] [PubMed] [Google Scholar]
- Ginanni Corradini S, Ripani C, Della Guardia P, Giovannelli L, Elisei W, Cantafora A, Codacci Pisanelli M, Tebala GD, Nuzzo G, Corsi A, Attili AF, Capocaccia L, Ziparo V. The human gallbladder increases cholesterol solubility in bile by differential lipid absorption: a study using a new in vitro model of isolated intra-arterially perfused gallbladder. Hepatology. 1998;28:314–322. doi: 10.1002/hep.510280205. [DOI] [PubMed] [Google Scholar]
- Lee HM, Takeyama K, Dabbagh K, Lausier JA, Ueki IF, Nadel JA. Agarose plug instillation causes goblet cell metaplasia by activating EGF receptors in rat airways. Am J Physiol. 2000;278:L185–L192. doi: 10.1152/ajplung.2000.278.1.L185. [DOI] [PubMed] [Google Scholar]
- Yasoshima M, Kono N, Sugawara H, Katayanagi K, Harada K, Nakanuma Y. Increased expression of interleukin-6 and tumor necrosis factor-alpha in pathologic biliary epithelial cells: in situ and culture study. Lab Invest. 1998;78:89–100. [PubMed] [Google Scholar]
- Savard CE, Blinman TA, Choi HS, Lee SK, Pandol SJ, Lee SP. Expression of cytokine and chemokine mRNA and secretion of tumor necrosis factor-alpha by gallbladder epithelial cells: response to bacterial lipopolysaccharides. BMC Gastroenterol. 2002;2:23. doi: 10.1186/1471-230X-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JJ, Dabbagh K, Ueki IF, Dao-Pick T, Burgel PR, Takeyama K, Tam DC, Nadel JA. IL-13 induces mucin production by stimulating epidermal growth factor receptors and by activating neutrophils. Am J Physiol. 2001;280:L134–L140. doi: 10.1152/ajplung.2001.280.1.L134. [DOI] [PubMed] [Google Scholar]
- Lee CS, Pirdas A. Epidermal growth factor receptor immunoreactivity in gallbladder and extrahepatic biliary tract tumours. Pathol Res Pract. 1995;191:1087–1091. doi: 10.1016/S0344-0338(11)80652-7. [DOI] [PubMed] [Google Scholar]
- Hormi K, Lehy T. Developmental expression of transforming growth factor-alpha and epidermal growth factor receptor proteins in the human pancreas and digestive tract. Cell Tissue Res. 1994;278:439–450. doi: 10.1007/BF00331362. [DOI] [PubMed] [Google Scholar]
- Harada K, Terada T, Nakanuma Y. Detection of transforming growth factor-alpha protein and messenger RNA in hepatobiliary diseases by immunohistochemical and in situ hybridization techniques. Hum Pathol. 1996;27:787–792. doi: 10.1016/s0046-8177(96)90450-5. [DOI] [PubMed] [Google Scholar]
- Kim JH, Jung KH, Han JH, Shim JJ, In KH, Kang KH, Yoo SH. Relation of epidermal growth factor receptor expression to mucus hypersecretion in diffuse panbronchiolitis. Chest. 2004;126:888–895. doi: 10.1378/chest.126.3.888. [DOI] [PubMed] [Google Scholar]
- Kong WY, Koldovsky O, Rao RK. Appearance of exogenous epidermal growth factor in liver, bile, and intestinal lumen of suckling rats. Gastroenterology. 1992;102:661–667. doi: 10.1016/0016-5085(92)90117-h. [DOI] [PubMed] [Google Scholar]
- Enss ML, Cornberg M, Wagner S, Gebert A, Henrichs M, Eisenblatter R, Beil W, Kownatzki R, Hedrich HJ. Proinflammatory cytokines trigger MUC gene expression and mucin release in the intestinal cancer cell line LS180. Inflamm Res. 2000;49:162–169. doi: 10.1007/s000110050576. [DOI] [PubMed] [Google Scholar]
- Hirota K, Murata M, Itoh T, Yodoi J, Fukuda K. Redox-sensitive transactivation of epidermal growth factor receptor by tumor necrosis factor confers the NF-kappa B activation. J Biol Chem. 2001;276:25953–25958. doi: 10.1074/jbc.M011021200. [DOI] [PubMed] [Google Scholar]
- Chen WN, Woodbury RL, Kathmann LE, Opresko LK, Zangar RC, Wiley HS, Thrall BD. Induced autocrine signaling through the epidermal growth factor receptor contributes to the response of mammary epithelial cells to tumor necrosis factor alpha. J Biol Chem. 2004;279:18488–18496. doi: 10.1074/jbc.M310874200. [DOI] [PubMed] [Google Scholar]
- Argast GM, Campbell JS, Brooling JT, Fausto N. Epidermal growth factor receptor transactivation mediates tumor necrosis factor-induced hepatocyte replication. J Biol Chem. 2004;279:34530–34536. doi: 10.1074/jbc.M405703200. [DOI] [PubMed] [Google Scholar]