Caveolin-1 (Cav-1) was first identified as a marker protein for the purification of caveolae organelles.1–3 Subsequently, it was later determined that Cav-1 expression is essential for caveolae formation.4,5 Thus, Cav-1(−/−)-deficient mice morphologically lack caveolae organelles. Surprisingly, these mice are viable and fertile.4,5 In striking contrast, zebrafish (Danio rerio) lacking Cav-1 display important developmental abnormalities and embryonic lethality. These novel findings by Fang et al are described and highlighted in this issue of The American Journal of Pathology.6
Caveolin-1 Isoforms: Structure and Tissue-Specific Expression Patterns
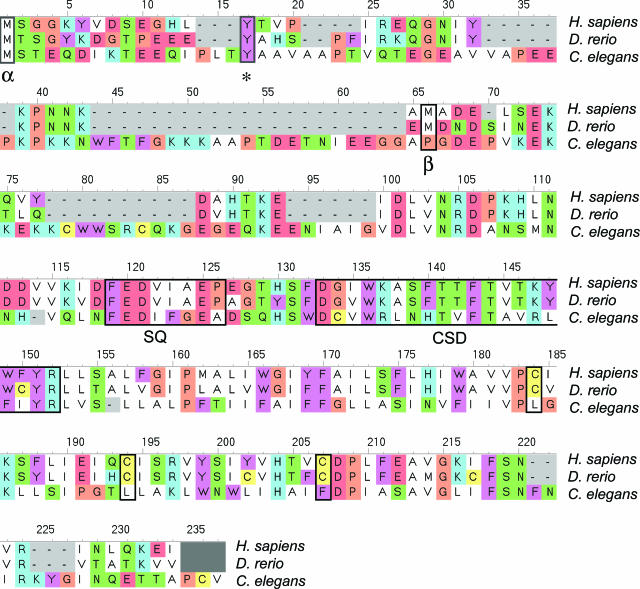
The gene encoding Cav-1 has the same organization, with three exons and two introns, in human,7 mouse,8 and zebrafish.6 This suggests an important and conserved role for Cav-1 in whole-organismal biology. In fact, sequence alignment reveals that the Cav-1 protein is highly evolutionarily conserved, from Caenorhabditis elegans to humans (Figure 1).
Figure 1.
Evolutionary conservation of the caveolin-1 (Cav-1) protein. Alignment of the Cav-1 protein sequences from C. elegans (accession no. Q94051), D. rerio (accession no. Q6YLH9), and Homo sapiens (accession no. Q2TNI1) was produced using CINEMA.25 Note that the β-isoform uses an internal methionine (M32 in humans; M34 in zebrafish) as an initiation codon, as indicated (α versus β). As such, only the α-isoform undergoes tyrosine phosphorylation [Y14 in humans and zebrafish; see asterisk], since the β-isoform lacks tyrosine 14. The positions of the caveolin-signature-sequence (SQ) and the caveolin-scaffolding-domain (CSD) are highlighted. Palmitoylated cysteine residues have been boxed, in the case of the human and zebrafish sequences. Color coding is as follows: white, hydrophobic residues; blue, positively charged residues; red, negatively charged residues; green, small hydrogen bonding residues; brown, glycine/proline residues; yellow, cysteine residues; and cyan, aromatic residues.
Interestingly, a single Cav-1 gene encodes two protein isoforms that differ slightly, only by their N-terminal sequence.9 More specifically, Cav-1α is a 178-amino acid protein, whereas Cav-1β is 147 amino acids and lacks the first 31 N-terminal residues of Cav-1α. These two Cav-1 isoforms have been shown to be translated from distinct mRNA species.10 Until now, the specific functional role of each Cav-1 isoform had not been clearly defined. Nonetheless, Cav-1α and -1β have different subcellular distributions, as demonstrated by recent studies.9,11,12 Moreover, Cav-1α has been shown to form caveolae more readily than Cav-1β.13
In zebrafish, Cav-1 mRNAs are detected during the very early stages of development. Late in development, the Cav-1α mRNA is the only isoform detectable in intestinal epithelium, whereas both Cav-1α and -1β mRNAs are produced in the heart, pharyngeal vasculature, notochord, somites, skin, and neuromast tissues. Interestingly, these data are similar to those obtained in Xenopus laevis.14
In the mouse, Cav-1α protein expression is detected early in the embryo (E15).12 Maximal expression is observed in the vasculature, the lungs, the kidneys, and the gut. Interestingly, in the lungs, Cav-1α expression first appears in endothelial cells. The importance of Cav-1 in the vasculature has also been highlighted by Bullejos et al, who observed high levels of Cav-1 mRNA in the developing ovaries but not testes.15 This difference is due to the formation of a more dense and more complex vascular network in the ovaries.15
Roles of Caveolin-1 during Development
In mice, Cav-1 expression does not appear to be as essential as in zebrafish, since its elimination is not lethal.16,17 However, its role in the vasculature and other tissues is clearly important, since its absence has been associated with many disease-related phenotypes, most notably in the lung, vasculature, heart, adipose tissue, and the mammary gland. However, the detailed developmental progression of the Cav-1-deficient mouse embryo has yet to be determined. For example, alterations observed in Cav-1-deficient murine lungs could result from developmental abnormalities.
In zebrafish, Cav-1 down-regulation, in the case of both isoforms (Cav-1α and Cav-1β), is associated with important defects occurring by 12 hours after fertilization. This time point is normally associated with a remarkable increase in Cav-1 mRNA levels. As expected, reductions in the Cav-1 protein are also associated with a major reduction in the number of caveolae.
One of the first proteins shown to associate with caveolae is actin.18,19 This “anchoring” interaction appears to be responsible, at least in part, for the extremely reduced mobility of caveolae at the cell surface.20 In addition, during cellular migration, Cav-1 has been shown to assume a polarized distribution in migrating endothelial cells.21,22 Moreover, it was also shown that this specific polarization during trans-migration requires the presence of the Tyr14 residue within Cav-1 for phosphorylation, since the distribution of other forms of Cav-1 (Cav-1α (Y14A) and Cav-1β) are not polarized.22
It is important to note that phosphorylation of Cav-1α at Tyr14 has been associated with its subcellular localization in close proximity to focal adhesions,23 as well as caveolae-mediated endocytosis.24 Interestingly, in zebrafish, a deficiency in Cav-1α cannot be rescued by a mutant form of Cav-1α (Y14F) that cannot undergo phosphorylation. In addition, Cav-1 deficiency is associated with severe disruption of the actin cytoskeleton. These findings suggest that Cav-1 plays a critical role in cell migration and/or endocytosis in zebrafish. Likewise, overexpression of the full-length Cav-1α isoform could not rescue the phenotype induced by the absence of the Cav-1β isoform, and visa versa. Taken together, these data suggest for the first time that Cav-1α and Cav-1β have nonoverlapping functions and that these differences may be related to the ability of the Cav-1α isoform to undergo tyrosine phosphorylation at residue 14.
Replacement of zebrafish Cav-1 by the corresponding human Cav-1 isoform could complement the phenotypes associated with the absence of each isoform. This finding further suggests that the function of the Cav-1 protein is highly conserved throughout evolution. In mammals, however, the absence of Cav-1 may not be as lethal as in zebrafish because of the existence of redundant compensatory mechanisms.
Conclusions
Clearly, the mouse and human systems are more complicated than zebrafish. However, the zebrafish model of development will provide, for the first time, a genetically tractable system to perform rapid and detailed mutagenesis of both Cav-1α and Cav-1β isoforms. As such, the zebrafish system is a new experimental tool for investigators to directly dissect the relationship between the primary structure of Cav-1 and its essential developmental and whole organismal functions.
Footnotes
Address reprint requests to Philippe Frank and Michael Lisanti, Department of Cancer Biology, Kimmel Cancer Center, Thomas Jefferson University, 233 S. 10th Street, BLSB 933, Philadelphia, PA 19107. E-mail: Philippe.Frank@jefferson.edu or Michael.Lisanti@jefferson.edu.
Related article on page 2209
M.P.L. was supported by grants from the National Cancer Institute (R01CA98779 and R01CA80250), the Charlotte Geyer Foundation, the American Heart Association, and the Muscular Dystrophy Association. P.G.F. was supported by a grant from the Elsa U. Pardee Foundation. The Lisanti and Frank laboratories are funded, in part, under a grant with the Pennsylvania Department of Health. The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
This commentary relates to Fang et al, Am J Pathol 2006, 169:2209–2222, published in this issue.
References
- Glenney JRJ, Soppet D. Sequence and expression of caveolin, a protein component of caveolae plasma membrane domains phosphorylated on tyrosine in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci USA. 1992;89:10517–10521. doi: 10.1073/pnas.89.21.10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia T, Dupree P, Parton RG, Kellner R, Virta H, Lehnert M, Simons K. VIP 21, a 21-kD membrane protein is an integral component of trans-Golgi-network-derived transport vesicles. J Cell Biol. 1992;118:1003–1014. doi: 10.1083/jcb.118.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Fang P-K, Solomon KR, Zhuang L, Qi M, McKee M, Freeman MR, Yelick PC. Caveolin-1α and 1β perform non-redundant roles in early vertebrate development. Am J Pathol. 2006;169:2209–2222. doi: 10.2353/ajpath.2006.060562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Zhang XL, Lisanti MP. Sequence and detailed organization of the human caveolin-1 and -2 genes located near the D7S522 locus (7q31.1). Methylation of a CpG island in the 5′ promoter region of the caveolin-1 gene in human breast cancer cell lines. FEBS Lett. 1999;448:221–230. doi: 10.1016/s0014-5793(99)00365-8. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Zhang XL, Galbiati F, Lisanti MP. Chromosomal localization, genomic organization, and developmental expression of the murine caveolin gene family (Cav-1, -2, and -3). Cav-1 and Cav-2 genes map to a known tumor suppressor locus (6-A2/7q31). FEBS Lett. 1998;429:330–336. doi: 10.1016/s0014-5793(98)00619-x. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Tang Z, Chun M, Sargiacomo M, Lodish HF, Lisanti MP. Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution. Identification and epitope mapping of an isoform-specific monoclonal antibody probe. J Biol Chem. 1995;270:16395–16401. doi: 10.1074/jbc.270.27.16395. [DOI] [PubMed] [Google Scholar]
- Kogo H, Fujimoto T. Caveolin-1 isoforms are encoded by distinct mRNAs. Identification of mouse caveolin-1 mRNA variants caused by alternative transcription initiation and splicing. FEBS Lett. 2000;465:119–123. doi: 10.1016/s0014-5793(99)01730-5. [DOI] [PubMed] [Google Scholar]
- Kogo H, Aiba T, Fujimoto T. Cell type-specific occurrence of caveolin-1alpha and -1beta in the lung caused by expression of distinct mRNAs. J Biol Chem. 2004;279:25574–25581. doi: 10.1074/jbc.M310807200. [DOI] [PubMed] [Google Scholar]
- Ramirez MI, Pollack L, Millien G, Cao YX, Hinds A, Williams MC. The alpha-isoform of caveolin-1 is a marker of vasculogenesis in early lung development. J Histochem Cytochem. 2002;50:33–42. doi: 10.1177/002215540205000104. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Kogo H, Nomura R, Une T. Isoforms of caveolin-1 and caveolar structure. J Cell Sci. 2000;113(Pt 19):3509–3517. doi: 10.1242/jcs.113.19.3509. [DOI] [PubMed] [Google Scholar]
- Razani B, Park DS, Miyanaga Y, Ghatpande A, Cohen J, Wang XB, Scherer PE, Evans T, Lisanti MP. Molecular cloning and developmental expression of the caveolin gene family in the amphibian Xenopus laevis. Biochemistry. 2002;41:7914–7924. doi: 10.1021/bi020043n. [DOI] [PubMed] [Google Scholar]
- Bullejos M, Bowles J, Koopman P. Extensive vascularization of developing mouse ovaries revealed by caveolin-1 expression. Dev Dyn. 2002;225:95–99. doi: 10.1002/dvdy.10128. [DOI] [PubMed] [Google Scholar]
- Williams TM, Lisanti MP. The caveolin genes: from cell biology to medicine. Ann Med. 2004;36:584–595. doi: 10.1080/07853890410018899. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–79. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Jacobson BS, Dvorak AM. Caveolae from luminal plasmalemma of rat lung endothelium: microdomains enriched in caveolin, Ca(2+)-ATPase, and inositol trisphosphate receptor. Proc Natl Acad Sci USA. 1995;92:1759–1763. doi: 10.1073/pnas.92.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen P, Roepstorff K, Stahlhut M, van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol Biol Cell. 2002;13:238–250. doi: 10.1091/mbc.01-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isshiki M, Ando J, Yamamoto K, Fujita T, Ying Y, Anderson RG. Sites of Ca(2+) wave initiation move with caveolae to the trailing edge of migrating cells. J Cell Sci. 2002;115:475–484. doi: 10.1242/jcs.115.3.475. [DOI] [PubMed] [Google Scholar]
- Parat MO, Anand-Apte B, Fox PL. Differential caveolin-1 polarization in endothelial cells during migration in two and three dimensions. Mol Biol Cell. 2003;14:3156–3168. doi: 10.1091/mbc.E02-11-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Volonte D, Galbiati F, Iyengar P, Lublin DM, Bregman DB, Wilson MT, Campos-Gonzalez R, Bouzahzah B, Pestell RG, Scherer PE, Lisanti MP. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol. 2000;14:1750–1775. doi: 10.1210/mend.14.11.0553. [DOI] [PubMed] [Google Scholar]
- Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am J Physiol. 2003;285:L1179–L1183. doi: 10.1152/ajplung.00242.2003. [DOI] [PubMed] [Google Scholar]
- Parry-Smith DJ, Payne AW, Michie AD, Attwood TK. CINEMA—a novel colour interactive editor for multiple alignments. Gene. 1998;221:GC57–GC63. doi: 10.1016/s0378-1119(97)00650-1. [DOI] [PubMed] [Google Scholar]