Abstract
Shigatoxin (Stx) is the offending agent of post-diarrheal hemolytic uremic syndrome, characterized by glomerular ischemic changes preceding microvascular thrombosis. Because podocytes are highly sensitive to Stx cytotoxicity and represent a source of vasoactive molecules, we studied whether Stx-2 modulated the production of endothelin-1 (ET-1), taken as candidate mediator of podocyte dysfunction. Stx-2 enhanced ET-1 mRNA and protein expression via activation of nuclear factor κB (NF-κB) and activator protein-1 (Ap-1) to the extent that transfection with the dominant-negative mutant of IκB-kinase 2 or with Ap-1 decoy oligodeoxynucleotides reduced ET-1 mRNA levels. We propose a role for p38 and p42/44 mitogen-activated protein kinases (MAPKs) in mediating NF-κB-dependent gene transcription induced by Stx-2, based on data that Stx-2 phosphorylated p38 and p42/44 MAPKs and that MAPK inhibitors reduced transcription of NF-κB promoter/luciferase reporter gene construct induced by Stx-2. Stx-2 caused F-actin redistribution and intercellular gaps via production of ET-1 acting on ETA receptor, because cytoskeleton changes were prevented by ETA receptor blockade. Exogenous ET-1 induced cytoskeleton rearrangement and intercellular gaps via phosphatidylinositol-3 kinase and Rho-kinase pathway and increased protein permeability across the podocyte monolayer. These data suggest that the podocyte is a target of Stx, a novel stimulus for the synthesis of ET-1, which may control cytoskeleton remodeling and glomerular permeability in an autocrine fashion.
Shigatoxin (Stx)-producing Escherichia coli has been strongly indicated as the offending agent of typical postdiarrheal hemolytic uremic syndrome (D+HUS), a disorder of thrombocytopenia, microangiopathic hemolytic anemia, and acute renal failure that mainly affects infants and small children.1–3 The kidney is the privileged organ of Stx toxicity because it expresses high levels of the specific receptor glycosphingolipid globotriaosyl ceramide (Gb3).4,5 The characteristic lesion consists of swelling and detachment of glomerular endothelial cells that have been extensively recognized as the main target of Stx.6 Retraction and collapse of the capillary tuft in the glomerulus are prominent and typically occur in association with fusion of foot processes and swelling of podocytes.6,7 Although ischemic lesion in the glomerular microcirculation can significantly contribute to renal dysfunction, the precise role of podocyte injury in the toxic response to Stx and the underlying cellular and molecular mechanisms have not been established yet.
Recent studies have indicated that glomerular visceral epithelial cells (podocytes) are sensitive to the toxic effects of Stx-1 and -2 isoforms because they express Gb3 and bind Stx, as documented either in cultured cells8 or in human renal biopsies.9 In vitro, Stx-1 activates podocytes to release inflammatory cytokines like interleukin (IL)-1 and tumor necrosis factor (TNF), which remarkably increased the cellular content of Gb3 receptor, thereby enhancing renal toxin responsiveness.8,10 In an experimental model of HUS in baboons, swelling of podocytes with osmophilic inclusions was found in association with the typical glomerular endothelial lesions after intravenous infusion of Stx-1.11
Podocytes represent a crucial component of the glomerular filter barrier. They are highly specialized cells endowed with foot processes that, through a contractile structure composed of actin and associated proteins connected to the glomerular basement membrane, stabilize glomerular architecture by counteracting the distension of the basement membrane.12,13 The contractile apparatus of the foot processes responds to vasoactive hormones, thus controlling the glomerular capillary surface area and the ultrafiltration coefficient. Podocytes are an important source of the vasoconstrictor peptide endothelin-1 (ET-1),14–16 which is recognized to play a key role in the control of glomerular hemodynamics. They constitutively express pre-proET-1 mRNA and synthesize the mature peptide, the generation of which is markedly up-regulated by transforming growth factor-β, membrane attack complex, and thrombin.14 Studies have shown that rat podocytes are targets of ET-1 because they express ETA and ETB receptors.17,18
In an attempt to identify possible mechanisms evoked by Stx that could contribute to podocyte dysfunction, we tested in cultured murine podocytes whether Stx-2 induced the expression and synthesis of ET-1, instrumental for cytoskeletal remodeling and cellular retraction. Intracellular signals involved in ET-1 gene regulation were also investigated.
Materials and Methods
Cell Culture and Incubation
Immortalized mouse podocytes (obtained from Dr. Peter Mundel, Department of Medicine, Mount Sinai School of Medicine, New York, NY) were grown as previously described.19 Briefly, cells were cultured under growth-permissive conditions on rat tail collagen type I-coated plastic dishes (BD Bioscience, Bedford, MA) at 33°C in RPMI 1640 (Invitrogen, Gaithersburg, MD) supplemented with 10% fetal bovine serum (Invitrogen), 10 U/ml mouse recombinant interferon-γ (Sigma Chemical Co., St. Louis, MO), and 100 U/ml penicillin plus 0.1 mg/ml streptomycin (Sigma). To induce differentiation, podocytes were maintained in nonpermissive conditions at 37°C without interferon-γ for 14 days and used for the experiments. In this culture condition, cells stopped proliferating and were identified as differentiated podocytes by their arborized morphology and the presence of high levels of synaptopodin, determined using indirect immunofluorescence microscopy. Cells were routinely maintained for 24 hours in serum-free medium before all of the experiments.
To investigate the effect of Stx-2 on the expression of the ET-1 gene, differentiated podocytes were exposed for 3, 6, and 24 hours to medium alone or to Stx-2, 50 pmol/L and 1 nmol/L (Toxin Technology Inc., Sarasota, FL). Preliminary experiments showed that these concentrations did not affect podocyte viability until 48 hours of incubation, as evaluated by viable cell count using trypan blue dye exclusion (Sigma). ET-1 mRNA transcript levels were measured by Northern blot analysis and real-time PCR. To exclude any possible effect of lipopolysaccharide (LPS) contamination of Stx-2 preparation on ET-1 mRNA expression, additional experiments were performed in podocytes incubated with Stx-2 in the presence of the LPS inhibitor polymyxin B, 10 μg/ml (Sigma). This concentration was chosen on the basis of previous experiments20 showing that 20 μg of polymyxin B was capable of neutralizing the effect of 1 ng of purified E. coli LPS. Because the Limulus test assay (Cambrex, Walkersville, MD) revealed the presence of 117 pg LPS/μg Stx-2 protein preparation, the concentration of polymyxin B used here far exceeded that needed to inhibit the detected LPS traces. The time course of ET-1 protein synthesis was assessed by radioimmunoassay (RIA) in supernatants of podocytes exposed to both concentrations of Stx-2.
To study intracellular signaling pathways that regulate ET-1 gene transcription in Stx-2-loaded podocytes, we first assessed the potential role of nuclear factor κB (NF-κB) and activator protein-1 (Ap-1) by determining the activity of both transcription factors in nuclear extracts from podocytes exposed for 30 minutes to Stx-2 (50 pmol/L and 1 nmol/L) and by evaluating the effect of NF-κB and Ap-1 inhibition on ET-1 gene expression. Podocytes were transfected for 3 hours with a dominant-negative mutant of the IκB kinase 2 (IKK2),21 a kinase that acts as an upstream activator of NF-κB, and then exposed to Stx-2 (50 pmol/L) for 24 hours. In other experiments, podocytes were transfected for 2 hours with double-stranded oligodeoxynucleotide (ODN)22 that scavenge Ap-1 activity by competitive reaction or with mutated control ODNs and then exposed to the toxin for 6 hours. Then, we studied whether Stx-2 induced activation/phosphorylation of p38 mitogen-activated protein kinase (MAPK) and p42/44 MAPK, known activators of NF-κB and Ap-1, in podocytes treated with 50 pmol/L Stx-2 for 15, 30, 60, and 180 minutes. To elucidate whether MAPKs were involved in NF-κB regulation, podocytes were transfected with NF-κB luciferase reporter gene and incubated with Stx-2 (50 pmol/L, 6 hours) in the presence or absence of the p38 inhibitor SB-202190 (20 μmol/L)23 or the p42/44 inhibitor PD-98059 (10 μmol/L).24
The effect of Stx-2 on F-actin cytoskeletal rearrangement and gap formation was assessed in differentiated podocytes exposed for 15 hours to Stx-2 (50 pmol/L). To investigate the role of ET-1 in Stx-2-induced cytoskeleton rearrangement, we first studied whether murine podocytes expressed ETA and ETB receptor mRNA and protein by real-time PCR and immunofluorescence studies. The effect of Stx-2 on ETA and ETB receptor expression was also evaluated. Then, podocytes were treated with the ETA receptor antagonist LU-302146 (1 μmol/L; Knoll AG, Ludwighafen, Germany),25 added 1 hour before and during 15 hours of Stx-2 incubation, and F-actin changes and gap formation were assessed.
Finally, the effect of exogenous ET-126 on cytoskeletal rearrangement and permeability to protein was evaluated. The involvement of phosphatidylinositol-3 kinase (PI3K) and Rho kinase in ET-1-induced F-actin redistribution was studied by incubating cells with ET-1 for 6 hours in the presence of the specific inhibitors wortmannin (100 nmol/L; Sigma)27 and Y27632 (1 μmol/L; Sigma),28 respectively, added 30 minutes before and during ET-1 stimulation.
Flow Cytometry Analysis
The surface expression of Stx receptor Gb3 (CD77) was evaluated by flow cytometry analysis (FACSort; Becton, Dickinson and Company, San Jose, CA) in differentiated podocytes treated with medium alone, α-galactosidase (7.5 U/ml), or IL-1 β (100 U/ml, 24 hours). Unstimulated human umbilical vein endothelial cells were used as positive control. At the end of incubations, cells in suspension were exposed for 45 minutes at 4°C to fluorescein isothiocyanate (FITC)-labeled mouse anti-human CD77 monoclonal antibody (25 μg/ml; BD Biosciences Pharmingen, Milan, Italy). Unstimulated cells incubated with FITC-conjugated mouse IgM (BD Biosciences Pharmingen) were used as negative control. Then cells were washed and fixed with 2% paraformaldehyde plus 4% sucrose in phosphate-buffered saline (PBS; 10 minutes, 4°C) and assayed within 1 hour.
Northern Blot Analysis
Total RNA was isolated from podocytes by the guanidium isothiocyanate/cesium chloride procedure. Fifteen micrograms of total RNA was then fractionated on 1.2% agarose gel and blotted onto synthetic membranes (Zeta-probe; Bio-Rad, Richmond, CA). ET-1 mRNA was detected by using a 319-bp fragment of rat ET-1 cDNA. The probe was labeled with [α-32P]dCTP by the random-primed method. Hybridization was performed overnight at 60°C in 0.25 mol/L Na2HPO4, pH 7.2, and 7% sodium dodecyl sulfate (SDS). Filters were washed twice for 30 minutes with 20 mmol/L Na2HPO4, pH 7.2, and 5% SDS and twice for 10 minutes with 20 mmol/L Na2HPO4, pH 7.2, and 1% SDS at 60°C. Membranes were subsequently probed with β-actin cDNA, taken as internal standard of equal loading of the samples on the membrane. Expression of ET-1 mRNA was corrected for β-actin expression and quantitated densitometrically.
Quantitative Real-Time PCR
Total RNA was extracted from podocytes by the guanidium isothiocyanate/cesium chloride procedure. Contaminating genomic DNA was removed by RNase-free DNase (Promega, Ingelheim, Germany) for 1 hour at 37°C. The purified RNA (1 μg) was reverse transcribed using random examers (50 ng) and 200 U of SuperScript II RT (Life Technologies, San Giuliano Milanese, Italy) for 1 hour at 42°C. No enzyme was added for reverse transcriptase-negative controls.
Real-time PCR was performed with ABI PRISM 5700 Sequence Detection System (PE Biosystems, Warrington, UK) using heat-activated TaqDNA polymerase (Amplitaq Gold; PE Biosystems).16 The SYBR Green I PCR Reagents kit was used according to the manufacturer’s protocol. After an initial hold of 2 minutes at 50°C and 10 minutes at 95°C, the samples were cycled 40 times at 95°C for 15 seconds and 60°C for 60 seconds. Fluorescence detection, defined as threshold cycle (Ct), was chosen in the exponential phase of the PCR and used for relative quantification of the target gene. The comparative Ct method normalizes the number of target gene copies to the housekeeping gene as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (ΔCt). Gene expression was then evaluated by the quantification of cDNA corresponding with the target gene relative to a calibrator sample serving as a physiological reference (eg, untreated cells, ΔΔCt). On the basis of exponential amplification of target gene as well as calibrator, the amount of amplified molecules at the Ct is given by 2−ΔΔCt.
The following specific primers (300 nmol/L) were used. Mouse ET-1: sense 5′-AACTACGAAGGTTGGAGGCCA-3′, antisense 5′-CACGAAAAGATGCCTTGATGC-3′; mouse ETA receptor: sense 5′-CTTGCGGATCGCCCTTAGT-3′, antisense 5′-TTTGCCACTTCTCGACGCT-3′; mouse ETB receptor: sense 5′-CCTACAAGTTGCTCGCAGAGG-3′, antisense 5′-GCTTACACATCTCAGCTCCAAATG-3′; and GAPDH: sense 5′-TCATCCCTGCATCCACTGGT-3′, antisense 5′-CTGGGATGACCTTGCCCAC-3′. All primers were obtained from Sigma Genosys (Cambridgeshire, UK). True identity of the amplification products was ensured by primer specificity for mouse sequences, the presence of a single dissociation curve at a constant T melting, and the lack of genomic DNA contamination or primer dimers in the reverse transcriptase-negative control sample.
Radioimmunoassay
ET-1 production was assayed in podocyte supernatants by RIA. Either standard compounds or unknown samples (100 μl) were mixed with 100 μl of antiserum (Peninsula Laboratories Inc., Belmont, CA) diluted in phosphate buffer, pH 7.2 (RIA buffer) at a final dilution of 1:72,000. The reaction mixture was incubated for 24 hours at 4°C, then 15,000 cpm of 125I-labeled ET-1 in 100 μl was added, and the incubation was prolonged for 24 hours at 4°C. Separation of free ET-1 from antibody-bound 125I-labeled ET-1 was achieved by the addition of a second antibody (500 μl of immunoprecipitating mixture consisting of a sheep anti-rabbit IgG and polyethylene glycol) for 30 minutes at room temperature. Finally, 500 μl of RIA buffer was added to stop the reaction, and the immunoprecipitates were centrifuged at 5000 × g for 30 minutes. Supernatants were discarded, and pellet radioactivity was detected by gamma counter (Beckman, Irvine, CA). Results were expressed as picograms per 106 cells. The minimum detectable concentration was 0.4 pg/tube. Nonspecific binding did not exceed 2% of total radioactivity.
The cross-reactivity of the antibody with other endothelins is as follows: endothelin-2, 46.9%; endothelin-3, 17%; and big endothelin-1, 9.4%. Intra-assay and interassay variability averaged 10 and 12%, respectively, over a range between 0.4 and 100 pg/tube.
Preparation of Nuclear Extracts and Electrophoretic Mobility Shift Assay
Nuclear extracts were prepared from podocytes with the NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Pierce/Celbio, Pero, Italy) according to the manufacturer’s instructions. To minimize proteolysis, all buffers contained protease inhibitor cocktail (Boehringer Mannheim, Mannheim, Germany). The protein concentration was determined by the Bradford assay using the Bio-Rad protein assay reagent.
Electrophoretic mobility shift assays were performed as previously described23 with the κB DNA sequence of the immunoglobulin gene (5′-CCGGTCAGAGGGGACTTTCCGAGACT-3′) and consensus binding site for Ap-1 (5′-CGCTTGATGACTCAGCCGGAA-3′). Nuclear extracts (3 μg) were incubated with 50,000 cpm of 32P-labeled oligonucleotide in a binding reaction mixture [10 mmol/L Tris-HCl, pH 7.5, 80 mmol/L NaCl, 1 mmol/L ethylenediamine tetraacetic acid, 1 mmol/L dithiothreitol, 5% glycerol, and 1.5 μg of poly(dI-dC)] for 30 minutes on ice. In competition studies, a 100-fold molar excess of unlabeled oligonucleotide was added to the binding reaction mixture before the addition of the labeled κB or Ap-1 probes. For densitometric analysis, the volume density for each band was determined in arbitrary units. The sum of the volume density of bands for a single sample was used as an indirect measure of NF-κB or Ap-1 activation and expressed as a fold increase of the mean densitometry of respective control (represented as 1).
Adenoviral Vector-Mediated Gene Transfer in Podocytes
Replication-deficient adenovirus encoding for kinase-defective dominant-negative form of human IKK2 (AdV-dnIKK2) was a kind gift of Dr. R. de Martin (University of Vienna, Vienna, Austria).21 Replication-deficient adenoviral vector having no insert (AdV-0) was from Novartis Pharma (Basel, Switzerland). All viruses used belong to Ad5 serotype.
For transfection experiments, podocytes were incubated with adenoviruses at a multiplicity of infection of 50 in RPMI 1640 without serum for 3 hours at 37°C. The adenovirus was washed off, and cells were maintained in serum-free medium for 24 hours. Then cells were exposed to 50 pmol/L Stx-2 for an additional 24 hours and processed for endothelin-1 mRNA expression (real-time PCR analysis). Transfection did not affect cell viability.
Ap-1 Decoy ODN Technique
The sequences of the phosphorothioate double-stranded ODN against the Ap-1 binding site and the mutated control ODN were as follows: Ap-1 decoy ODN (consensus sequences are underlined), 5′-CGCTTGATGACTCAGCCGGAA-3′ and 3′-GCGAACTACTGAGTCGGCCTT-5′ and mutated control ODN, 5′-CGCTTGATGACTTGGCCGGAA-3′ and 3′-TTCCGGCCAAGTCATCAAGCG-5′.
Double-stranded ODNs22 were prepared from complementary single-stranded phosphorothioate oligonucleotides by melting at 80°C for 5 minutes followed by a 3- to 4-hour reconstitution period at room temperature. To study the effect of Ap-1 decoy, podocytes were transfected with 200 nmol/L Ap-1 decoy ODN or mutated control ODN in serum-free medium, using Oligofectamine Reagent according to the manufacturer’s instructions (Invitrogen, San Giuliano Milanese, Italy). Two hours after transfection, Stx-2 at the final concentration of 50 pmol/L was added to the cells for 6 hours without removing the transfection mixture. Cells were then processed for ET-1 mRNA expression (real-time PCR analysis).
Western Blot Analyses
Podocytes were lysed with lysis buffer (20 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 2 mmol/L ethylenediamine tetraacetic acid, 1% Triton X-100, 2.5 mmol/L sodium pyrophosphate, and 1 mmol/L β-glycerophosphate) plus phosphatase inhibitors (1 mmol/L Na3VO4 and 50 mmol/L NaF) and protease inhibitors (1 mmol/L phenylmethylsulfonyl fluoride and 1 μg/ml leupeptin). Protein concentration was determined by protein assay based on bicinchoninic acid color formation (Pierce, Milan, Italy). Proteins (30 μg) were separated on 10% polyacrylamide gels by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Membranes were blocked for 1 hour at room temperature with PBS containing 0.1% Tween 20 and 5% bovine serum albumin (BSA; for phosphorylated protein detection) or 5% nonfat dry milk (for unphosphorylated protein detection) and then incubated overnight at 4°C with the following primary antibodies: mouse monoclonal IgM anti-phospho-p38 (1:300) or mouse monoclonal IgG phospho-p42/44 [Thr202/Tyr204] (1:1000) in PBS plus 1% BSA; mouse monoclonal IgG anti-p38 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) or mouse monoclonal IgG anti-p42/44 (1:2000; Cell Signaling Technology Inc., Beverly, MA) in PBS plus 1% nonfat dry milk. After incubation with the secondary antibodies, horseradish peroxidase-conjugated rabbit anti-IgG mouse or goat anti-IgM mouse (Sigma) for 1 hour at room temperature in PBS with 0.1% Tween 20 and 1% BSA or 1% nonfat dry milk, protein bands were detected by Supersignal chemiluminescent substrate (Pierce).
Reporter Luciferase Gene Assay
Podocytes were transfected with 1 μg of NF-κB luciferase reporter gene (Stratagene; M-Medical, Florence, Italy) by the Superfect transfection reagent following the manufacturer’s protocol (Qiagen, Milan, Italy). Three hours after transfection, the reporter gene was washed off, and cells were maintained overnight in fresh medium without serum.23 Then cells were exposed to Stx-2 (50 pmol/L) for an additional 6 hours. The p38 inhibitor SB-202190 (20 μmol/L) or the p42/44 inhibitor PD-98059 (10 μmol/L) was added 1 hour before and during stimulation with Stx-2. Podocytes were subsequently lysed in 1× reporter lysis buffer for 15 minutes at room temperature. The lysates were cleaned by centrifugation. The luciferase activity was measured according to standard protocols (Stratagene; M-Medical) with a Lumat LB9507 luminometer (EG&G Berthold, Bad Wildbad, Germany). Induced luciferase activities were normalized on the basis of protein content and expressed as fold stimulation compared with unstimulated controls.
Immunofluorescence
Podocytes plated on type I collagen-coated glass coverslips were maintained in nonpermissive conditions for 14 days and incubated with medium alone or Stx-2 at 50 pmol/L for 15 hours or in additional experiments with ET-1 (0.1 to 100 nmol/L)26 for 6 hours in the presence of the specific inhibitors wortmannin (100 nmol/L) and Y27632 (1 μmol/L). At the end of incubation, cells were fixed in 2% paraformaldehyde plus 4% sucrose in PBS, pH 7.4, for 10 minutes at 37°C and then permeabilized with 0.3% Triton X-100 (Sigma) in PBS for 4 minutes at room temperature. After three washings with PBS, nonspecific binding sites were saturated in blocking solution (2% fetal bovine serum, 2% bovine serum albumin, and 0.2% bovine gelatin in PBS) for 30 minutes at room temperature. Then podocytes were incubated with 20 U/ml rhodamine-phalloidin for 45 minutes (Molecular Probes Inc., Eugene, OR); negative control experiments without rhodamine-phalloidin were also performed. Coverslips were washed and mounted in 1% N-propyl-gallate in 50% glycerol and 0.1 mol/L Tris-HCl, pH 8, and examined using fluorescence microscopy Olympus IX70 (Olympus Italia srl, Segrate-Milano, Italy). To evaluate gap formation, we counted the number of gaps of 15 fields taken randomly for each sample as previously described.29
To quantify the number of cells with peripheral F-actin distribution, we performed a triple staining for ZO-1, taken as a cell membrane marker defining podocyte edge, F-actin, and nuclei. Fixed and permeabilized podocytes were incubated overnight at 4°C with polyclonal rabbit anti-ZO-1 antibody (10 μg/ml; Zymed Laboratories, San Francisco, CA) and then for 1 hour with FITC-conjugated goat anti-rabbit (13.6 μg/ml; Jackson Immunoresearch, West Grove, PA) followed by rhodamine phalloidin staining as indicated above. Finally, cells were treated with the nuclear cell marker 4,6-diamidine-2′-phenylindole dihydrochloride (0.25 μg/ml; Sigma) for 15 minutes at 37°C. Triple fluorescence labeling was analyzed by an inverted confocal laser scanning microscope (LS 510 Meta; Zeiss, Jena, Germany), and 10 random images for each sample were acquired. Percentage of cells with F-actin rearrangement with respect to total cells for each field was then evaluated.
Surface expression of ETA receptor was also assessed in podocytes treated for 15 hours with control medium or Stx-2 (50 pmol/L). Fixed cells were incubated with 10% goat serum in PBS for 6 hours to block nonspecific binding sites, and then a rabbit polyclonal anti-ETA receptor antibody (12 μg/ml; Abcam Cambridge Science Park, Cambridge, UK), diluted in PBS containing 1.5% goat serum, was added for 1 hour at room temperature. The incubation of the secondary antibody was performed using Cy3-conjugated goat anti-rabbit IgG (12.5 μg/ml; Jackson Immunoresearch) for 1 hour at room temperature, and representative images were acquired with an inverted confocal laser scanning microscope.
Permeability Studies
Permeability was determined by measuring the transepithelial passage of FITC-BSA from apical to basolateral compartment of transwell bicameral chambers (0.4-μm pore; Corning Costar Corporation, Cambridge, MA).30 Podocytes were exposed to test medium or ET-1 (100 nmol/L; Sigma)26 for 6 hours, and then 100 μg/ml FITC-BSA (Sigma) was loaded into the apical compartment for 1 hour at 37°C. At the end of the incubation, fluorescence in the basolateral compartment was measured using fluorescence spectroscopy (excitation, 490; emission, 525). To quantify the trans-membrane flux of FITC-BSA in micrograms per hour, the BSA concentration in the lower chambers was calculated using arbitrary fluorescent units of the albumin solution added to the apical compartment, taking into account the volume of the basolateral compartment.
Statistical Analysis
Results are expressed as means ± SEM. Statistical analysis was performed using Student’s t-test, analysis of variance followed by Tukey’s test for multiple comparisons, and Kruskal-Wallis test, as appropriate. Statistical significance was defined as P < 0.05.
Results
Gb3 Receptor Expression in Differentiated Murine Podocytes
In line with previous studies in human podocytes,8 differentiated murine podocytes expressed the Stx receptor Gb3 (CD77). Flow cytometry analysis revealed 8% of fluorescent podocytes for Gb3. Human umbilical vein endothelial cells used as positive control displayed 5.9% of fluorescent cells. Staining specificity was established by treating podocytes with α-galactosidase that removes terminal α-galactosyl residues.5 α-Galactosidase reduced the percentage of fluorescent cells for Gb3 down to 2.8%, a value comparable with the negative control. When podocytes were stimulated with IL-1β for 24 hours, up-regulation of Gb3 expression was observed (13.8% of fluorescent cells).
Stx-2 Increases ET-1 Expression and Protein in Cultured Podocytes
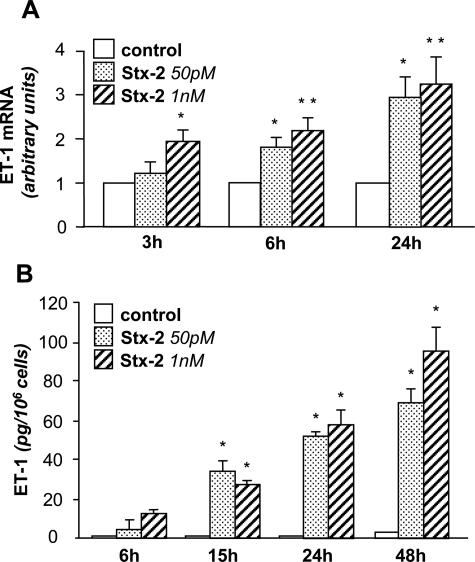
We assessed the capability of Stx-2 to modulate ET-1 mRNA expression and protein synthesis in differentiated mouse podocytes. By Northern blot, a 1.3-kb mRNA transcript specific for ET-1 was observed in unstimulated control podocytes. Stx-2 at the subtoxic concentrations of 50 pmol/L and 1 nmol/L induced a significant increase of ET-1 transcript levels over control at 3 and 6 hours, which was further enhanced after 24 hours of incubation (2.9- and 3.2-fold increase) (Figure 1A). The up-regulation of ET-1 gene was also confirmed by real-time PCR studies. The stimulatory effect of Stx-2 on ET-1 mRNA expression (24 hours) was not due to LPS contamination, as indicated by experiments using the LPS inhibitor polymyxin B (Stx-2, 3.1-fold increase of ET-1 mRNA over control; Stx-2+polymyxin B, 3.6-fold increase of ET-1 mRNA over control).
Figure 1.
ET-1 mRNA expression and protein synthesis in podocytes exposed to Stx-2. A: Northern blot experiments were performed using total RNA isolated from podocytes exposed to medium alone (control) or Stx-2 (50 pmol/L, 1 nmol/L) for 3, 6, and 24 hours. The results are representative of five independent experiments. The optical density of the autoradiographic signals was quantified and calculated as the ratio of ET-1 to β-actin mRNA. Results (means ± SEM) are expressed as fold increase over control (considered as 1) in densitometric arbitrary units. *P < 0.05, **P < 0.01 versus control. B: Stx-2 stimulates ET-1 production by podocytes. Podocytes were incubated with medium alone or Stx-2 (50 pmol/L, 1 nmol/L) for 6, 15, 24, and 48 hours. ET-1 production was measured in cell supernatants by RIA. The results are representative of three independent experiments. Data are expressed as means ± SEM. *P < 0.01 versus control.
Overexpression of ET-1 gene was paralleled by a significant time-dependent increase of the native peptide released into the supernatants of Stx-treated podocytes. ET-1 production, already detected at 6 hours, was more pronounced starting from 15-hour exposure to both Stx-2 concentrations (Figure 1B).
Up-Regulation of ET-1 mRNA in Response to Stx-2 Is Dependent on NF-κB and Ap-1
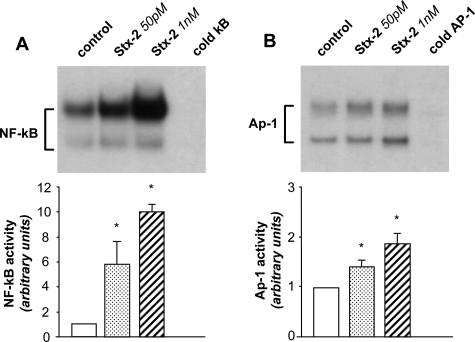
Because the promoter region of ET-1 has consensus sequences for NF-κB and Ap-1,31,32 we studied the DNA binding activity of these transcription factors in podocytes challenged with Stx-2. As shown in Figure 2A, nuclear extracts from unstimulated cells displayed two constitutive bands of NF-κB: an upper complex and a faster migrating lower complex. Thirty-minute incubation of podocytes with Stx-2 (50 pmol/L and 1 nmol/L) elicited a substantial rise in NF-κB binding activity of the two complexes. Densitometric analysis of three independent experiments revealed a 6- and 10-fold increase of NF-κB activity induced by Stx-2 over control (P < 0.05). Similarly, Ap-1 activation was detected in podocytes exposed to both concentrations of Stx-2 (P < 0.05 versus control) (Figure 2B). The specificity of binding reaction was confirmed by the ability of excess unlabeled NF-κB and Ap-1 oligonucleotides to inhibit binding.
Figure 2.
Activation of NF-κB and Ap-1 in podocytes exposed to Stx-2. Top: Electrophoretic mobility shift assay for NF-κB (A) and Ap-1 (B) was performed in nuclear extracts of podocytes exposed for 30 minutes to medium alone or Stx-2 (50 pmol/L, 1 nmol/L). To confirm the specificity of the binding reaction, a 100-fold molar excess of unlabeled (cold) nucleotide was used to compete with the labeled NF-κB or AP-1 probes for binding to nuclear proteins. The results are representative of three independent experiments using different nuclear extracts. Bottom: Densitometric analysis of autoradiographic signals of NF-κB (A) and AP-1 (B). Results are means ± SEM. *P < 0.05 versus control.
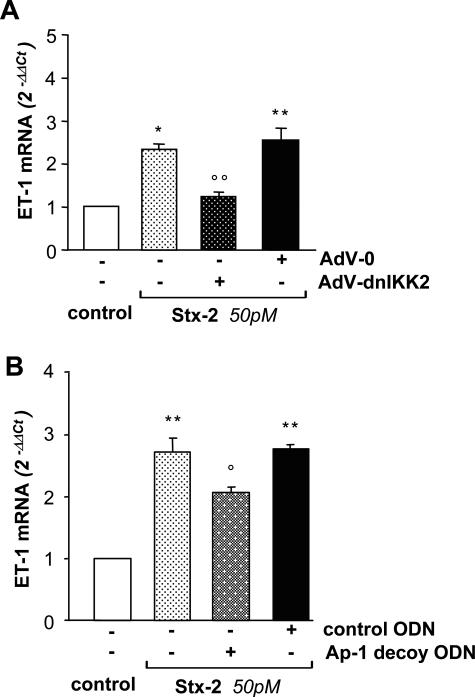
To establish whether up-regulation of ET-1 mRNA in response to Stx-2 was dependent on NF-κB, podocytes were infected with a recombinant adenovirus encoding the dominant-negative mutant of the IκB kinase 2 (AdV-dnIKK2), which fails in promoting the dissociation of IκBα from NF-κB, and then treated for 24 hours with Stx-2 (50 pmol/L). Real-time PCR experiments indicated that overexpression of dnIKK2 resulted in a significant (P < 0.01) reduction of ET-1 mRNA with respect to Stx-treated cells infected with the control adenovirus (AdV-0) (Figure 3A). Notably, dnIKK2 almost normalized ET-1 mRNA levels. No difference in ET-1 mRNA levels was observed between Stx-treated podocytes infected or not infected with AdV-0.
Figure 3.
ET-1 mRNA up-regulation induced by Stx-2 is inhibited by adenovirus-mediated dominant-negative mutant of IKK2 or by Ap-1 decoy ODN. A: Cells were left untreated or infected for 3 hours with a recombinant adenovirus coding for a dominant kinase-negative mutant of IKK2 (AdV-dnIKK2) or with a control adenovirus (AdV-0) and were then maintained in serum-free medium for 24 hours before incubation with medium alone or Stx-2 (50 pmol/L) for 24 hours. Cells were processed for ET-1 mRNA expression by real-time PCR. The results shown are means ± SEM of three independent experiments. *P < 0.05, **P < 0.01 versus control; °°P < 0.01 versus AdV-0+ Stx-2. B: Ap-1 decoy ODN or mutated control ODN was added to podocytes 2 hours before incubation with medium alone or Stx-2 (50 pmol/L) for 6 hours. ET-1 mRNA was assessed by real-time PCR. The results shown are means ± SEM of three independent experiments. **P < 0.01 versus control; °P < 0.05 versus control ODN+Stx-2.
The role of Ap-1 was assessed by transfection of podocytes with double-stranded (decoy) ODN that scavenge active Ap-1, thereby blocking its binding to the promoter region of target genes. A partial, although significant, reduction (26%) of ET-1 expression was found in cells transfected with decoy ODN against Ap-1 with respect to mutated control ODN podocytes treated with Stx-2 (Figure 3B).
Stx-2 Activates p38 and p42/44 MAPKs, Instrumental for NF-κB Transcriptional Activity
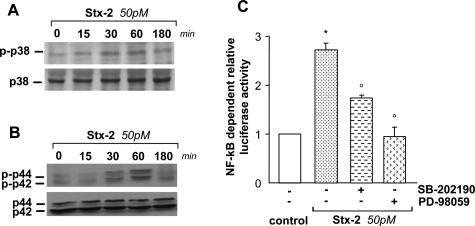
Given that in other cellular systems, p38 and p42/44 MAPKs modulate the transcriptional activity of NF-κB and Ap-1,23,33–37 we investigated whether Stx-2 induced activation/phosphorylation of both MAPKs. By Western blot analysis, podocytes challenged with Stx-2 (50 pmol/L) exhibited a rapid p38 MAPK phosphorylation within 15 minutes that further increased at 30 and 60 minutes with respect to control (Figure 4A). As shown in Figure 4B, Stx-2 induced a marked increase in the level of p42/44 MAPK phosphorylation starting at 30 minutes and then declining to the basal level by 180 minutes.
Figure 4.
Stx-2 activates p38 and p42/44 MAPKs, instrumental for NF-κB activation. Phosphorylation of p38 (A) and p42/44 (B) MAPKs in podocytes exposed to Stx-2 (50 pmol/L) for 15, 30, 60, and 180 minutes. Cell lysates were analyzed by Western blot using antibodies against the phosphorylated form of each MAPK. The blots were stripped and reprobed with anti-nonphosphorylated p38 or p42/44 antibodies to confirm equal loading of the proteins on the gel. C: Effect of pharmacological inhibition of p38 and p42/44 MAPKs on Stx-2-induced NF-κB-dependent promoter activity. Podocytes were transfected for 3 hours with NF-κB luciferase reporter gene. Then cells were maintained in serum-free medium for 15 hours before exposure to Stx-2 (50 pmol/L) for an additional 6 hours. The p38 inhibitor SB-202190 (20 μmol/L) and the p42/44 inhibitor PD-98059 (10 μmol/L) were added 1 hour before and during stimulation with Stx-2. Relative luciferase activity is expressed as fold stimulation by assuming control as 1. Data are means ± SEM (n = 3 experiments). *P < 0.01 versus control; °P < 0.01 versus Stx-2.
Next, we assessed whether p38 and p42/44 MAPKs activated by Stx-2 were involved in NF-κB transcriptional activity responsible for ET-1 gene regulation. The effect of p38 and p42/44 MAPK inhibitors SB-202190 and PD-98059 was assessed in Stx-2-treated podocytes transfected with a vector encoding the luciferase reporter gene driven by a promoter containing consensus sequence for NF-κB. Results showed that Stx-2 increased luciferase activity by 2.7-fold with respect to control, which was reduced by 37% by the p38 MAPK inhibitor and almost abrogated after p42/44 MAPK functional blockade (Figure 4C).
Stx-2 Promotes Cytoskeleton Rearrangement and Gap Formation via ET-1
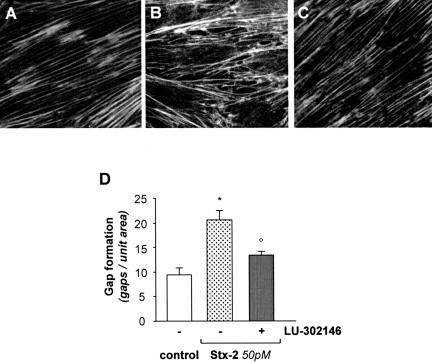
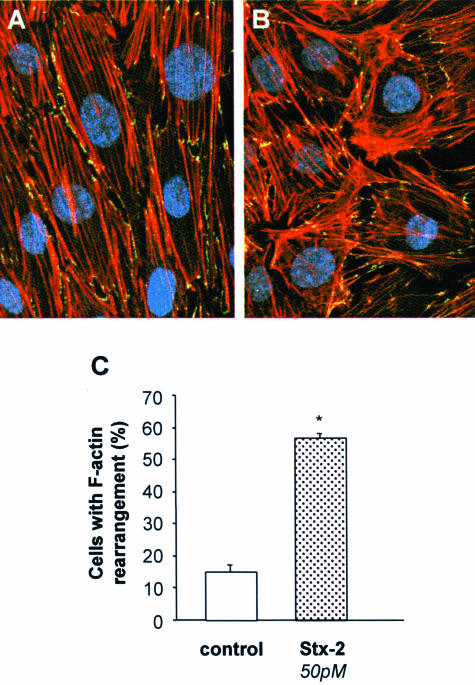
Differentiated podocytes possess a contractile structure composed of F-actin fibers extended across the entire cell body (Figure 5A) that, after Stx-2 (50 pmol/L), were greatly redistributed to the cell periphery (Figure 5B). Alteration in actin-based cytoskeleton was associated with the formation of intercellular gaps (Figure 5B). Higher frequency of gaps in podocytes exposed to Stx-2 was observed compared with unstimulated cells (Figure 5D). Quantitative assessment of podocytes undergoing cytoskeletal rearrangement in response to Stx-2 was performed by triple immunostaining of cells for ZO-1, a membrane marker that defines podocyte outline, F-actin, and nuclei. As shown in Figure 6, Stx-2 induced F-actin rearrangement at the cell periphery in 56.7 ± 1.7% of podocytes compared with 15.2 ± 2.1% of unstimulated cells that exhibited mild cytoskeletal changes.
Figure 5.
Stx-2 induces cytoskeletal F-actin redistribution and gap formation via ETA receptor. Immunofluorescence staining of F-actin fibers in podocytes stimulated for 15 hours with medium alone (A), Stx-2 (50 pmol/L) (B), and Stx-2 in the presence of the ETA receptor antagonist LU-302146 (1 μmol/L) (C). The ET-1 receptor antagonist was added 1 hour before and during the incubation with Stx-2. In unstimulated cells, F-actin microfilaments are arranged in parallel, whereas Stx-2 leads to F-actin redistribution at the cell periphery in association with intercellular gap formation. LU-302146 prevents cytoskeletal redistribution and gaps. Magnification, ×600. D: The number of gaps was counted in 15 random fields for each sample, and the results are expressed as means ± SEM (n = 7 experiments). *P < 0.01 versus control; °P < 0.01 versus Stx-2.
Figure 6.
Quantification of podocytes with F-actin rearrangement in response to Stx-2. Representative images of immunofluorescence staining for F-actin fibers (red), ZO-1 (green), and nuclei (blue) in podocytes stimulated for 15 hours with medium alone (A) or Stx-2 50 pmol/L (B). Magnification, ×630. C: Percentage of cells with F-actin rearrangement with respect to total cells was quantified (n = 10 fields for each sample). Results are expressed as means ± SEM (n = 3 experiments). *P < 0.01 versus control.
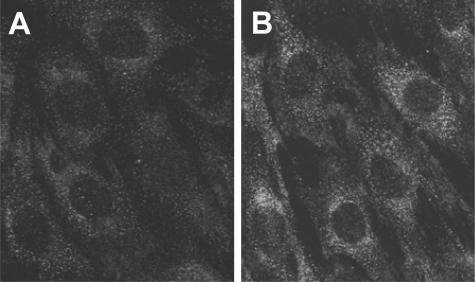
To document that ET-1 is one of the major mediators of Stx-induced podocyte structural changes, we first studied whether differentiated murine podocytes expressed ET-1 receptors. Murine podocytes constitutively expressed ETA but not ETB receptor as assessed by real-time PCR. Stx-2 up-regulated ETA receptor mRNA expression at 6 and 15 hours of incubation (2.17 ± 0.76- and 1.96 ± 0.18-fold increase over control). At variance, no effect on ETB receptor expression was observed in response to the toxin. ETA receptor protein was visualized in control cells (Figure 7A) by immunofluorescence staining and was found to be enhanced in response to Stx-2 (Figure 7B).
Figure 7.
ETA receptor expression in differentiated podocytes. Immunofluorescence staining for ETA receptor in podocytes exposed for 15 hours to medium alone (A) or Stx-2 50 pmol/L (B). ETA receptor is expressed constitutively by podocytes and up-regulated after Stx-2 incubation. Magnification, ×600.
We then tested the effect of the ETA receptor antagonist LU-302146 on cytoskeleton rearrangement and intercellular gap formation and found that it prevented F-actin redistribution induced by Stx-2 (Figure 5C) and reduced intercellular gaps (Figure 5, C and D), thus suggesting a role of ET-1 induced by Stx-2 via ETA receptor.
ET-1 Induces F-Actin Redistribution and Alters Protein Permeability in Podocytes
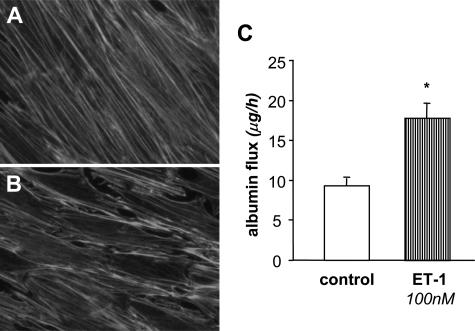
That ET-1 modifies podocyte cytoskeleton was further confirmed by another series of experiments in which the peptide was exogenously added to cultured podocytes. After a 6-hour exposure to ET-1 (0.1 to 100 nmol/L), the percentage of podocytes that underwent cytoskeleton alterations increased in a concentration-dependent manner, reaching values significantly different at 100 nmol/L ET-1 (Table 1). As shown in Figure 8B, challenge of podocytes with 100 nmol/L ET-1 induced a marked redistribution of F-actin fibers to the cell periphery and intercellular gap formation with respect to unstimulated cells (Figure 8A). Evaluation of transepithelial passage of fluorescent albumin to the basolateral compartment of bicameral chambers showed unequivocal increase in albumin permeability across podocyte monolayer on ET-1 challenge (Figure 8C).
Table 1.
ET-1 Induced a Redistribution of F-Actin Fibers
| Cells with F-actin rearrangement (%) | |
|---|---|
| Control | 15.75 ± 2.38 |
| ET-1 | |
| 0.1 nmol/L | 24.11 ± 3.54 |
| 1 nmol/L | 24.36 ± 2.74 |
| 10 nmol/L | 26.91 ± 2.55 |
| 100 nmol/L | 44.48 ± 1.99* |
Percentage of cells with F-actin rearrangement to total cells was quantified (n = 10 fields for each sample). Results are expressed as means ± SEM.
P < 0.01 versus control.
Figure 8.
Effect of exogenous ET-1 on cytoskeletal F-actin distribution and albumin permeability. Immunofluorescence staining of F-actin fibers in podocytes exposed for 6 hours to control medium (A) or ET-1 (100 nmol/L) (B). ET-1 promotes changes in cytoskeleton distribution leading to F-actin rearrangement at the cell periphery and intercellular gap formation. Magnification, ×600. C: Transepithelial albumin permeability is increased in podocytes exposed to ET-1 (100 nmol/L, 6 hours) versus unstimulated cells (n = 3 experiments). *P < 0.01 versus control.
Next, we investigated whether structural alterations induced by ET-1 could be mediated by PI3K and Rho kinase, known to regulate stress fiber formation.38,39 Treatment with wortmannin or Y27632, selective inhibitors of PI3K and Rho kinase, respectively, resulted in a significant reduction of cells with F-actin rearrangement induced by ET-1 (wortmannin+ET-1, 26.2 ± 1.6; Y27631+ ET-1, 34.0 ± 1.9; versus ET-1, 48.7 ± 0.8% of F-actin rearranged cells; P < 0.01 versus ET-1).
Discussion
The kidney is the privileged target of Shigatoxin, the causative agent of D+HUS. The role of podocyte in the toxic response to Stx and the underlying cellular and molecular mechanisms have been explored in the present study. We focused on the vasoconstrictor peptide ET-1 found to be elevated in plasma and urine of children during the acute phase of HUS.40,41 In the kidney, ET-1 is produced by all glomerular cell types and by tubules,42 and when bound to the ETA receptor, it elicits different biological activities, including contraction, proliferation, and inflammatory cell recruitment.43 The present results indicate that Stx-2, at subtoxic concentrations, enhanced gene expression of ET-1 in cultured murine podocytes, followed by increased synthesis of the mature peptide. Considering the podocyte location in the glomerulus at close proximity with the endothelium, the increased production of vasoactive ET-1 triggered by Stx in podocytes might have an impact on glomerular microcirculation.
A pivotal aspect of this study was the identification of the intracellular signals involved in Stx-induced ET-1 overexpression in podocytes. Regulation of the pre-pro ET-1 gene is complex and has been attributed to multiple regulatory elements. Evidence is available showing that the pre-proET-1 gene possesses in the promoter region specific consensus sequences for the transcription factors NF-κB and Ap-1.31,32 Podocytes exposed to Stx-2 exhibited a rapid and massive activation of NF-κB. Ap-1 was also significantly activated by the toxin, although to a lesser extent. In other cellular systems, such as monocytes44 and endothelial cells,45 previous reports showed that Stx increased either NF-κB or AP-1 binding activity, responsible for cytokine and chemokine gene expression. The direct demonstration that NF-κB modulates ET-1 mRNA expression derives from experiments in which transfected podocytes that overexpressed a dominant-negative mutant of the IKK221,23—a specific kinase that acts as upstream activator of NF-κB46—failed to increase ET-1 gene expression in response to Stx-2. In addition, the finding that Stx-treated podocytes transfected with the Ap-1 decoy ODN showed a significant decrease of ET-1 mRNA indicates that ET-1 gene expression is, at least in part, dependent on Ap-1 activation.
It has been consistently documented that p38 and p42/44 MAPK pathways are fundamental for initiating the transcriptional activity of NF-κB and Ap-1.23,33–37 The involvement of p38 and p42/44 MAPKs in mediating NF-κB-dependent gene transcription rests on data that Stx-2 phosphorylated p38 and p42/44 MAPKs and that inhibitors of both MAPKs decreased the transcription of NF-κB promoter/luciferase reporter gene construct induced by the toxin. Consistently with our results, other studies have implicated MAPK activation in Stx-induced gene transcription in various target cells. In a human adenocarcinoma-derived renal tubular cell line (ACHN), Stx-2 induced p38 and p42/44 MAPK activation instrumental for NF-κB-induced TNF-α transcription.33 In addition, Stx-1-induced TNF-α gene expression in a mononuclear cell line was decreased by blockade of the p38 pathway.47
Another major finding that arises from this study is that Stx-2 caused a marked rearrangement of the contractile F-actin apparatus of podocytes associated with the formation of intercellular gaps, reflecting effective podocyte process retraction. In vivo, podocyte cytoskeletal derangement, consisting of marked disaggregation and redistribution of actin filaments, results in foot process effacement and cell retraction, structural alterations common to both human and experimental glomerulopathies associated with proteinuria and renal function impairment.48–50 Foot process effacement, which possibly develops in association with increased mechanical stress, can be considered as an adaptive change in the podocyte phenotype to counteract glomerular capillary expansion at the price of reducing the actual filtration area.48 As for possible mechanisms by which Stx may influence cytoskeleton remodeling, a study51 performed in ACHN cells derived from renal tubular epithelial carcinoma showed that binding of Stx-1 B subunit to Gb3 receptor caused the phosphorylation of ezrin, a linker protein that connects the plasma membrane with actin filaments. Ezrin phosphorylation occurred via activation of Src protein-tyrosine kinase, a crucial determinant of cell contraction and cytoskeleton remodeling,52 and was followed by redistribution of cytoskeletal organizing proteins including actin, vimentin, cytokeratin, paxillin, and focal adhesion kinase.51 In our experimental setting, we tested the hypothesis that cytoskeletal changes induced by Stx-2 in podocytes could be related to the production of ET-1, because of its ability to induce stress-fiber formation via Rho/ROCK system53 and to activate Src PTK family.54 The finding that podocytes expressed ETA receptor and that treatment with the ETA receptor antagonist prevented F-actin redistribution and decreased intercellular gap formation induced by Stx-2 does suggest an autocrine role for ET-1, via ETA receptor, in the dysfunction of the contractile apparatus eventuating to cell retraction.
Notably, morphological alterations similar to those caused by Stx-2 were observed when exogenous ET-1 was added to cultured podocytes, causing F-actin redistribution and gap formation. Changes were already evident using ET-1 concentrations similar to those measured in the supernatant of podocytes exposed to Stx-2, although a significant effect was achieved only with the highest concentration (100 nmol/L ET-1). Cytoskeletal rearrangement was associated with a significant increase in transepithelial passage of fluorescent albumin to the basolateral compartment of podocytes grown on a bicameral chamber, which reflected podocyte-podocyte contact alteration in response to ET-1. Inhibition of Rho kinases, which is crucial for the formation of stress fibers,39 and of PI3K, a known upstream activator of Rho pathway,38 resulted in a significant decrease of the number of cells with F-actin rearrangement in response to ET-1, thus suggesting a possible molecular mechanism through which ET-1 induced podocyte structural changes. In conclusion, our results document that the podocyte is a functionally relevant target of Stx-2 that, via up-regulation of ET-1 gene and protein, may amplify its noxious effects, thus contributing to glomerular dysfunction.
Acknowledgments
We thank Dr. Peter Mundel (Department of Medicine, Mount Sinai School of Medicine, New York, NY) for providing the conditionally immortalized murine cell line and Dr. Rainer de Martin (Department of Vascular Biology and Thrombosis Research, University of Vienna, Vienna, Austria) for providing recombinant adenovirus expressing dnIKK2. We are indebted to Dr. Mauro Abbate and Dr. Susanna Tomasoni for their precious contribution. Manuela Passera helped preparing the manuscript.
Footnotes
Address reprint requests to Marina Morigi, Ph.D., ‘Mario Negri’ Institute for Pharmacological Research, Via Gavazzeni 11, 24125 Bergamo, Italy. E-mail: morigi@marionegri.it.
S.B. is the recipient of a Helsinn Healthcare SA fellowship from Fondazione Aiuti per la Ricerca sulle Malattie Rare.
Part of this work was presented at the annual meeting of the American Society of Nephrology (San Diego, CA, November 12–17, 2003).
References
- Ruggenenti P, Noris M, Remuzzi G. Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. Kidney Int. 2001;60:831–846. doi: 10.1046/j.1523-1755.2001.060003831.x. [DOI] [PubMed] [Google Scholar]
- Andreoli SP. The pathophysiology of the hemolytic uremic syndrome. Curr Opin Nephrol Hypertens. 1999;8:459–464. doi: 10.1097/00041552-199907000-00010. [DOI] [PubMed] [Google Scholar]
- Karmali MA. Infection by Shiga toxin-producing Escherichia coli: an overview. Mol Biotechnol. 2004;26:117–122. doi: 10.1385/MB:26:2:117. [DOI] [PubMed] [Google Scholar]
- Lingwood CA. Role of verotoxin receptors in pathogenesis. Trends Microbiol. 1996;4:147–153. doi: 10.1016/0966-842x(96)10017-2. [DOI] [PubMed] [Google Scholar]
- Lingwood CA. Verotoxin-binding in human renal sections. Nephron. 1994;66:21–28. doi: 10.1159/000187761. [DOI] [PubMed] [Google Scholar]
- Habib R. Pathology of the hemolytic uremic syndrome. Kaplan BS, Trompeter RS, Moake JL, editors. New York: M. Dekker, Inc.,; Hemolytic Uremic Syndrome and Thrombotic Thrombocytopenic Purpura. 1992:pp 315–353. [Google Scholar]
- Striker GE, Striker LJ, D’Agati V. Renal lesions in hypertension. Livolsi VA, editor. Philadelphia: W.B. Saunders Company,; The Renal Biopsy Major Problems in Pathology. 1997:pp 258–268. [Google Scholar]
- Hughes AK, Stricklett PK, Schmid D, Kohan DE. Cytotoxic effect of Shiga toxin-1 on human glomerular epithelial cells. Kidney Int. 2000;57:2350–2359. doi: 10.1046/j.1523-1755.2000.00095.x. [DOI] [PubMed] [Google Scholar]
- Ergonul Z, Clayton F, Fogo AB, Kohan DE. Shigatoxin-1 binding and receptor expression in human kidneys do not change with age. Pediatr Nephrol. 2003;18:246–253. doi: 10.1007/s00467-002-1025-9. [DOI] [PubMed] [Google Scholar]
- Hughes AK, Stricklett PK, Kohan DE. Shiga toxin-1 regulation of cytokine production by human glomerular epithelial cells. Nephron. 2001;88:14–23. doi: 10.1159/000045953. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Jr, Tesh VL, DeBault L, Li A, Chang AC, Kosanke SD, Pysher TJ, Siegler RL. Characterization of the baboon responses to Shiga-like toxin: descriptive study of a new primate model of toxic responses to Stx-1. Am J Pathol. 1999;154:1285–1299. doi: 10.1016/S0002-9440(10)65380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barisoni L, Kopp JB. Update in podocyte biology: putting one’s best foot forward. Curr Opin Nephrol Hypertens. 2003;12:251–258. doi: 10.1097/00041552-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Pavenstädt H. Roles of the podocyte in glomerular function. Am J Physiol Renal Physiol. 2000;278:F173–F179. doi: 10.1152/ajprenal.2000.278.2.F173. [DOI] [PubMed] [Google Scholar]
- Cybulsky AV, Stewart DJ, Cybulsky MI. Glomerular epithelial cells produce endothelin-1. J Am Soc Nephrol. 1993;3:1398–1404. doi: 10.1681/ASN.V371398. [DOI] [PubMed] [Google Scholar]
- Kasinath BS, Fried TA, Davalath S, Marsden PA. Glomerular epithelial cells synthesize endothelin peptides. Am J Pathol. 1992;141:279–283. [PMC free article] [PubMed] [Google Scholar]
- Morigi M, Buelli S, Angioletti S, Zanchi C, Longaretti L, Zoja C, Galbusera M, Gastoldi S, Mundel P, Remuzzi G, Benigni A. In response to protein load podocytes reorganize cytoskeleton and modulate ET-1 gene: implication for permselective dysfunction of chronic nephropathies. Am J Pathol. 2005;166:1309–1320. doi: 10.1016/S0002-9440(10)62350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Hirohama T, Uemura H. Endothelin B receptor-like immunoreactivity in podocytes of the rat kidney. Arch Histol Cytol. 2002;65:245–250. doi: 10.1679/aohc.65.245. [DOI] [PubMed] [Google Scholar]
- Ortmann J, Amann K, Brandes RP, Kretzler M, Munter K, Parekh N, Traupe T, Lange M, Lattmann T, Barton M. Role of podocytes for reversal of glomerulosclerosis and proteinuria in the aging kidney after endothelin inhibition. Hypertension. 2004;44:974–981. doi: 10.1161/01.HYP.0000149249.09147.b4. [DOI] [PubMed] [Google Scholar]
- Mundel P, Reiser J, Zuniga Mejia, Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- Forestal CA, Benach JL, Carbonara C, Italo JK, Lisinski TJ, Furie MB. Francisella tularensis selectively induces proinflammatory changes in endothelial cells. J Immunol. 2003;171:2563–2570. doi: 10.4049/jimmunol.171.5.2563. [DOI] [PubMed] [Google Scholar]
- Oitzinger W, Hofer-Warbinek R, Schmid JA, Koshelnick Y, Binder BR, de Martin R. Adenovirus-mediated expression of a mutant IkappaB kinase 2 inhibits the response of endothelial cells to inflammatory stimuli. Blood. 2001;97:1611–1617. doi: 10.1182/blood.v97.6.1611. [DOI] [PubMed] [Google Scholar]
- Viedt C, Dechend R, Fei J, Hansch GM, Kreuzer J, Orth SR. MCP-1 induces inflammatory activation of human tubular epithelial cells: involvement of the transcription factors, nuclear factor-kappaB and activating protein-1. J Am Soc Nephrol. 2002;13:1534–1547. doi: 10.1097/01.asn.0000015609.31253.7f. [DOI] [PubMed] [Google Scholar]
- Donadelli R, Zanchi C, Morigi M, Buelli S, Batani C, Tomasoni S, Corna D, Rottoli D, Benigni A, Abbate M, Remuzzi G, Zoja C. Protein overload induces fractalkine upregulation in proximal tubular cells through nuclear factor kappaB- and p38 mitogen-activated protein kinase-dependent pathways. J Am Soc Nephrol. 2003;14:2436–2446. doi: 10.1097/01.asn.0000089564.55411.7f. [DOI] [PubMed] [Google Scholar]
- Hannken T, Schroeder R, Zahner G, Stahl RA, Wolf G. Reactive oxygen species stimulate p44/42 mitogen-activated protein kinase and induce p27(Kip1): role in angiotensin II-mediated hypertrophy of proximal tubular cells. J Am Soc Nephrol. 2000;11:1387–1397. doi: 10.1681/ASN.V1181387. [DOI] [PubMed] [Google Scholar]
- Brehm BR, Klaussner M, Wolf SC. Chronic elevated endothelin-1 concentrations regulate mitogen-activated protein kinases ERK 1 and ERK 2 in vascular smooth muscle cells. Clin Sci (Lond) 2002;103(Suppl 48):137S–140S. doi: 10.1042/CS103S137S. [DOI] [PubMed] [Google Scholar]
- Simonson MS, Dunn MJ. Endothelin-1 stimulates contraction of rat glomerular mesangial cells and potentiates beta-adrenergic-mediated cyclic adenosine monophosphate accumulation. J Clin Invest. 1990;85:790–797. doi: 10.1172/JCI114505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinci MC, Visentin B, Cusinato F, Nardelli GB, Trevisi L, Luciani S. Effect of vascular endothelial growth factor and epidermal growth factor on iatrogenic apoptosis in human endothelial cells. Biochem Pharmacol. 2004;67:277–284. doi: 10.1016/j.bcp.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Takekoshi K, Nanmoku T, Ishii K, Isobe K, Kawakami Y. Inhibition of the RhoA/Rho kinase system attenuates catecholamine biosynthesis in PC 12 rat pheochromocytoma cells. Biochim Biophys Acta. 2005;1726:28–33. doi: 10.1016/j.bbagen.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Saadi S, Platt JL. Transient perturbation of endothelial integrity induced by natural antibodies and complement. J Exp Med. 1995;181:21–31. doi: 10.1084/jem.181.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macconi D, Abbate M, Morigi M, Angioletti S, Mister M, Buelli S, Bonomelli M, Mundel P, Endlich K, Remuzzi A, Remuzzi G. Permselective dysfunction of podocyte-podocyte contact upon angiotensin II unravels the molecular target for renoprotective intervention. Am J Pathol. 2006;168:1073–1085. doi: 10.2353/ajpath.2006.050701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quehenberger P, Bierhaus A, Fasching P, Muellner C, Klevesath M, Hong M, Stier G, Sattler M, Schleicher E, Speiser W, Nawroth PP. Endothelin 1 transcription is controlled by nuclear factor-kappaB in AGE-stimulated cultured endothelial cells. Diabetes. 2000;49:1561–1570. doi: 10.2337/diabetes.49.9.1561. [DOI] [PubMed] [Google Scholar]
- Kawana M, Lee ME, Quertermous EE, Quertermous T. Cooperative interaction of GATA-2 and AP1 regulates transcription of the endothelin-1 gene. Mol Cell Biol. 1995;15:4225–4231. doi: 10.1128/mcb.15.8.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Johns EJ, Imaizumi A, Yanagawa Y, Kohsaka T. Activation of beta(2)-adrenoceptor prevents shiga toxin 2-induced TNF-alpha gene transcription. J Am Soc Nephrol. 2001;12:2288–2299. doi: 10.1681/ASN.V12112288. [DOI] [PubMed] [Google Scholar]
- Ryoo SW, Kim DU, Won M, Chung KS, Jang YJ, Oh GT, Park SK, Maeng PJ, Yoo HS, Hoe KL. Native LDL induces interleukin-8 expression via H2O2, p38 kinase, and activator protein-1 in human aortic smooth muscle cells. Cardiovasc Res. 2004;62:185–193. doi: 10.1016/j.cardiores.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Wang T, Hu YC, Dong S, Fan M, Tamae D, Ozeki M, Gao Q, Gius D, Li JJ. Co-activation of ERK, NF-kappa B and GADD45beta in response to ionizing radiation. J Biol Chem. 2005;280:12593–12601. doi: 10.1074/jbc.M410982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression: the role of TATA-binding protein (TBP). J Biol Chem. 1999;274:30858–30863. doi: 10.1074/jbc.274.43.30858. [DOI] [PubMed] [Google Scholar]
- Goebeler M, Gillitzer R, Kilian K, Utzel K, Brocker EB, Rapp UR, Ludwig S. Multiple signaling pathways regulate NF-kappaB-dependent transcription of the monocyte chemoattractant protein-1 gene in primary endothelial cells. Blood. 2001;97:46–55. doi: 10.1182/blood.v97.1.46. [DOI] [PubMed] [Google Scholar]
- Miao L, Dai Y, Zhang J. Mechanism of RhoA/Rho kinase activation in endothelin-1-induced contraction in rabbit basilar artery. Am J Physiol. 2002;283:H983–H989. doi: 10.1152/ajpheart.00141.2002. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nagayama K, Satomura K, Honda T, Okada S. Increased serum IL-10 and endothelin levels in hemolytic uremic syndrome caused by Escherichia coli O157. Nephron. 2000;84:326–332. doi: 10.1159/000045607. [DOI] [PubMed] [Google Scholar]
- Siegler RL, Edwin SS, Christofferson RD, Mitchell MD. Endothelin in the urine of children with the hemolytic uremic syndrome. Pediatrics. 1991;88:1063–1066. [PubMed] [Google Scholar]
- Wilkes BM, Susin M, Mento PF, Macica CM, Girardi EP, Boss E, Nord EP. Localization of endothelin-like immunoreactivity in rat kidneys. Am J Physiol. 1991;260:F913–F920. doi: 10.1152/ajprenal.1991.260.6.F913. [DOI] [PubMed] [Google Scholar]
- Sorokin A, Kohan DE. Physiology and pathology of endothelin-1 in renal mesangium. Am J Physiol. 2003;285:F579–F589. doi: 10.1152/ajprenal.00019.2003. [DOI] [PubMed] [Google Scholar]
- Sakiri R, Ramegowda B, Tesh VL. Shiga toxin type 1 activates tumor necrosis factor-alpha gene transcription and nuclear translocation of the transcriptional activators nuclear factor-kappaB and activator protein-1. Blood. 1998;92:558–566. [PubMed] [Google Scholar]
- Zoja C, Angioletti S, Donadelli R, Zanchi C, Tomasoni S, Binda E, Imberti B, te Loo M, Monnens L, Remuzzi G, Morigi M. Shiga toxin-2 triggers endothelial leukocyte adhesion and transmigration via NF-kappaB dependent up-regulation of IL-8 and MCP-1. Kidney Int. 2002;62:846–856. doi: 10.1046/j.1523-1755.2002.00503.x. [DOI] [PubMed] [Google Scholar]
- Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- Foster GH, Armstrong CS, Sakiri R, Tesh VL. Shiga toxin-induced tumor necrosis factor alpha expression: requirement for toxin enzymatic activity and monocyte protein kinase C and protein tyrosine kinases. Infect Immun. 2000;68:5183–5189. doi: 10.1128/iai.68.9.5183-5189.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato I. Podocyte process effacement in vivo. Microsc Res Tech. 2002;57:241–246. doi: 10.1002/jemt.10082. [DOI] [PubMed] [Google Scholar]
- Ito K, Ger YC, Kawamura S. Actin filament alterations in glomerular epithelial cells of adriamycin-induced nephrotic rats. Acta Pathol Jpn. 1986;36:253–260. doi: 10.1111/j.1440-1827.1986.tb01477.x. [DOI] [PubMed] [Google Scholar]
- Lachapelle M, Bendayan M. Contractile proteins in podocytes: immunocytochemical localization of actin and alpha-actinin in normal and nephrotic rat kidneys. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60:105–111. doi: 10.1007/BF02899534. [DOI] [PubMed] [Google Scholar]
- Takenouchi H, Kiyokawa N, Taguchi T, Matsui J, Katagiri YU, Okita H, Okuda K, Fujimoto J. Shiga toxin binding to globotriaosyl ceramide induces intracellular signals that mediate cytoskeleton remodeling in human renal carcinoma-derived cells. J Cell Sci. 2004;117:3911–3922. doi: 10.1242/jcs.01246. [DOI] [PubMed] [Google Scholar]
- Platek A, Mettlen M, Camby I, Kiss R, Amyere M, Courtoy PJ. v-Src accelerates spontaneous motility via phosphoinositide 3-kinase, phospholipase C and phospholipase D, but abrogates chemotaxis in Rat-1 and MDCK cells. J Cell Sci. 2004;117:4849–4861. doi: 10.1242/jcs.01359. [DOI] [PubMed] [Google Scholar]
- Kawanabe Y, Okamoto Y, Nozaki K, Hashimoto N, Miwa S, Masaki T. Molecular mechanism for endothelin-1-induced stress-fiber formation: analysis of G proteins using a mutant endothelin(A) receptor. Mol Pharmacol. 2002;61:277–284. doi: 10.1124/mol.61.2.277. [DOI] [PubMed] [Google Scholar]
- Imamura T, Huang J, Dalle S, Ugi S, Usui I, Luttrell LM, Miller WE, Lefkowitz RJ, Olefsky JM. beta-Arrestin-mediated recruitment of the Src family kinase Yes mediates endothelin-1-stimulated glucose transport. J Biol Chem. 2001;276:43663–43667. doi: 10.1074/jbc.M105364200. [DOI] [PubMed] [Google Scholar]