Abstract
Type 2 Diabetes is a progressive disease with an insidious onset, including decreased first-phase insulin secretion and mild forms of hyperinsulinemia caused by heterogenous factors such as genetic make-up, obesity and lifestyle. Decreased insulin secretion may already be a first sign of beta-cell damage, which is expected to develop into insulin resistance later and to result in progressive beta-cell deterioration and death. The disease has become highly prevalent in Western countries and is rapidly reaching epidemic proportions in the developing world. While the molecular mechanisms involved in the etiology of this disease remain relatively poorly understood, the United Kingdom Prospective Diabetes Study (UKPDS) has clearly demonstrated in several investigations that the adoption of insulin therapy in early phases of the disease is capable of attenuating or even preventing massive beta-cell loss and thus the severe metabolic syndrome and its late complications. This report aims to highlight the common psychological obstacles healthcare professionals are faced with when they are attempting early introduction of insulin therapy. Many patients typically underrate early signs and repress the fact that the disease is developing while they are vainly attempting to keep to lifestyle changes like New Year’s resolutions. However, there is also reluctance on the part of the healthcare professionals. At the same time, patients’ reservations about needles and fear of hypoglycemia can be removed simply by educating patients about the relatively low risk of hypoglycemia. Coupled with enhanced healthcare services, this strategy can overcome psychological insulin resistance and contribute to the maintenance of good metabolic control.
Keywords: type 2 diabetes, psychological insulin resistance, insulin therapy, lifestyle, hyperinsulinemia
Insulin resistance is both pathological and psychological
Type 2 diabetes is a progressive disease. Worldwide the diabetes epidemic is progressing, in individuals diabetes progresses and for doctors diabetes treatment needs to be progressive. The implications of the progression of diabetes for public and personal health and for professional care have not been fully appreciated. This case report explores some of these implications.
"Okay the diet hasn't worked. I know I have a touch of sugar diabetes, but how long do I have to keep taking these tablets?"
This is a familiar reaction to many physicians caring for patients who have recently been diagnosed with diabetes. People are used to coming to the doctor, getting diagnosed, taking the treatment and being "fixed". Patients try to avoid being labeled as sick and acknowledging a "weakness" like diabetes by taking tablets. Patients and physicians may also think they have "failed" in controlling their diabetes. Patients assume that they could have tried harder (one always can) and physicians may feel that they have not been able to meet their needs and express these feelings by blaming them for their "failure".
Type 2 diabetes is the classic example of a chronic lifestyle-related, progressive disease where there are plenty of opportunities for failure, blame and guilt [1]. Lung disease related to smoking and physical and social problems related to ethanol overuse are others. Insulin therapy has proven advantages compared with solely oral hypoglycemic agents that are aimed at improving insulin resistance and dyslipidemia, but the intention to initiate insulin therapy faces barriers that include patients' fear of disease progression and needle anxiety [2].
Pathological insulin resistance
"Insulin capacity X + 1, insulin resistance X." Result: normoglycemia, quality of life, longevity.
"Insulin capacity X, insulin resistance X + 1." Result: hyperglycemia, discomfort, early mortality.
(After Mr. Micawber. David Copperfield, Charles Dickens.)
We are all born with a certain amount of insulin resistance and a certain capacity to secrete insulin. As we age, our insulin resistance increases and our capacity to secrete insulin decreases [3] (Figure 1). While we are young we have the capacity to make enough insulin to balance the insulin resistance. Later, however, even high insulin levels cannot overcome insulin resistance and blood glucose rises. In addition, hyperglycemia causes oxidative stress in pancreatic beta-cells and reduces insulin secretion [4].
Figure 1. Diabetes progression.
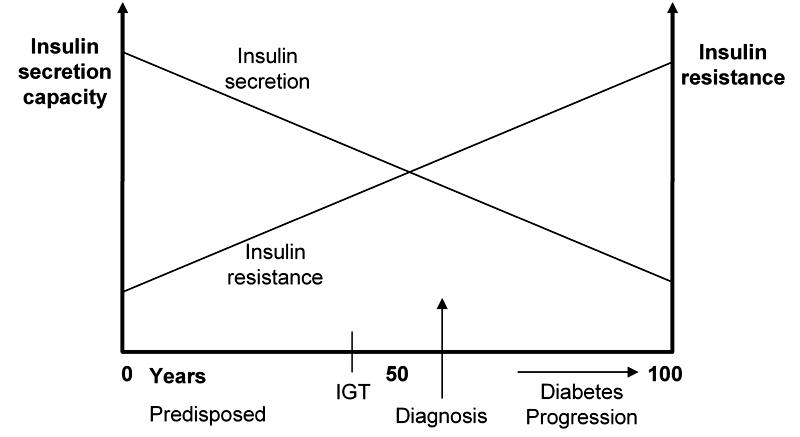
With progression of disease the capacity of β-cells to release insulin and simultaneously the body’s capacity for insulin utilization decrease, suggesting an increase of insulin resistance. Impaired glucose tolerance (IGT) appears when the degree of insulin resistance is almost equivalent to the secretory capacity of pancreatic β-cells (point of intersection). Usually, diagnosis follows shortly after this event.
Insulin resistance and capacity reflect the combination of genes, intrauterine environment, age, fatness, lifestyle and medication, which is collectively known as syndrome X [5]. Unfortunately the most powerful factors controlling blood glucose cannot be controlled – genes, intrauterine environment and age. Changing fatness and lifestyle can decrease insulin resistance and increase insulin capacity but with the passage of time insulin resistance may exceed insulin capacity and blood glucose rises again. At that time a medication is needed to compensate for increasing insulin resistance and decreasing insulin capacity. Later on more medications are needed, including insulin.
Progressive disease, progressive treatment – a case report
The following is a typical case illustrating the progression of diabetes and the need for treatment to progress as well.
1997
May: Mrs. MG aged 58, BMI 28.6 kg/m2 (1.52 m; 66.1 kg; waist 86 cm) presents with tiredness especially after meals, recent nocturia (2-3 times), uncomfortable thrush. Her sister, aged 60, has had type 2 diabetes for 7 years. Mrs. MG’s random BGL was 13.2 mmol/l; fasting BGL 8.3 mmol/l.
June-December: She was reviewed at the Diabetes Center of The Queen Elizabeth Hospital and enrolled in a group education program. Considerable changes in food choices and preparation were carried out. From that time on she walked every day for 30-50 minutes with her sister. She lost 3.6 kg in weight and her waist circumference decreased by 3 cm. The BG profile before meals was 4-7 mmol/l; HbA1c 6.8%.
1998
April: Regained weight but it was still 2.4 kg less than at diagnosis. HbA1c was at 7.4%. She expressed the wish to keep trying lifestyle change.
June: HbA1c was at 8.1%. She agreed to start metformin. Metformin dose increased to 850 mg tds.
August: HbA1c was at 6.6%. Her weight was stable.
1999
February: Her weight was at 65.9 kg; HbA1c at 7.1%. She was disappointed that she was unable to maintain walking program because of pain from heel spur.
July: Her weight increased to 67.4 kg, the HbA1c was at 7.8%.
2000
January: Her HbA1c was at 8.6%. She agreed to start glibenclamide 2.5 mg mane.
November: Her HbA1c improved to 6.9%. Glibenclamide has been raised to 5 mg bd; metformin was maintained at 850 mg tds.
2001
January – December: A gradual increase in weight to 76 kg (BMI 32.9 kg/m2) has been recorded. Subsequently, glibenclamide was enhanced to 10 mg bd. Her HbA1c increased from 6.9 to 7.4%. She expressed a New Year's resolution to "Eat less, walk more".
2002
March: She agreed reluctantly to start night-time isophane insulin and continue oral hypoglycemics.
2003 - 2005
March: Tailoring of glibenclamide, but maintenance of metformin at 850 mg tds. A second insulin dose was added in 2004. Now the insulin dose was: 60 units mane, 30 units nocte. Her weight was 78.2 kg, HbA1c ranged between 6.5% and 8.1% (mean 7.1%).
All diabetes professionals will recognize Mrs. MG. Many of the people they see could tell similar stories.
The progression of type 2 diabetes was shown in the United Kingdom Prospective Diabetes Study (UKPDS; Figure 2) [6]. Results suggest that insulin-treated type 2 diabetic patients are in the near-euglycemic state longer than those treated by oral hypoglycemic agents. In both the conventional and intensive-treated groups, HbA1c progressively rose by 0.15% per year (1% per six years). A sub-study of the UKPDS also showed that this progression of diabetes could be overcome by progressive treatment. To keep HbA1c < 7%, initially lifestyle and/or one oral hypoglycemic agent was enough but by 6 years nearly 50% required insulin therapy (Figure 3) [7]. These data confirm that early insulin therapy of type 2 diabetic patients retains their own insulin secretion capacity and results in lower hemoglobin A1c. In the light of these data, there is a possibility of changing the attitude from "Oh no, not insulin in type 2 diabetes" to the "early rapid-acting insulin analogue treatment" of these patients [8].
Figure 2. Risk progression.
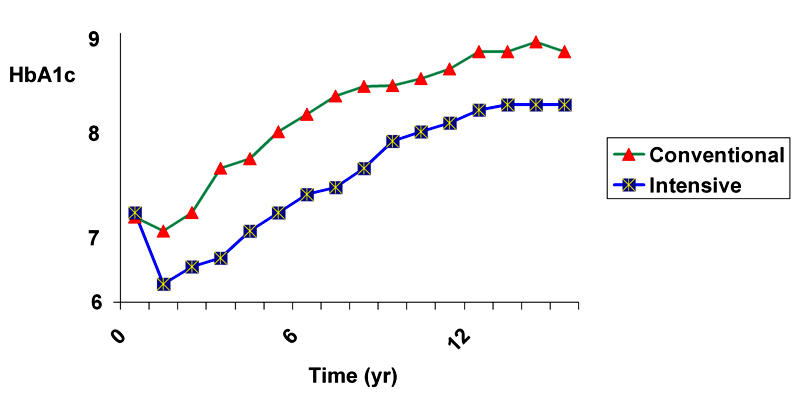
HbA1c values of 3867 newly diagnosed patients with type 2 diabetes were collected within the scope of the UKPDS in a randomized controlled trial to evaluate the risk of late myocardial complications. The data of patients in two groups were compared by means of the effects of intensive blood glucose control with either sulphonylurea or insulin and conventional treatment. HbA1c is continuously lower in the intensive group during follow-up. It turned out that patients in the intensive group showed an 11% reduced HbA1c after ten years of follow-up and a 25% lower risk of developing microvascular complications compared with the conventional group. In contrast, intensive-treated patients suffered significantly more frequently from hypoglycemic episodes (p < 0.001) and weight gain was significantly higher in this group (mean 2.9 kg, p < 0.001) [6].
Figure 3. Progressive treatment.
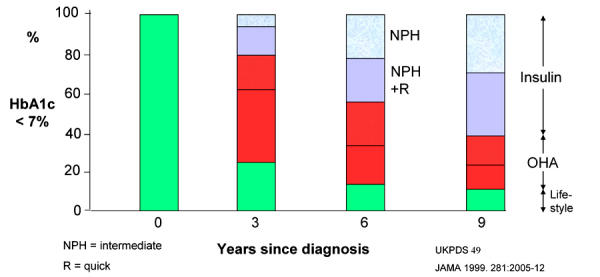
NPH: neutral protamine Hagedorn insulin. NPH+R: NPH plus regular human insulin. OHA: oral hypoglycemic agent. Ordinate: number of patients with HbA1c lower than 7%. In the UKPDS, HbA1c progressively increased in both the conventional and the intensive treatment group. Between 1977 and 1991, 4075 newly diagnosed type 2 diabetes patients were recruited. Approximately 60% of those with HbA1c lower than 7% needed the application of insulin therapy after a follow-up of 9 years. The fraction of the patients who maintained target glycemic levels decreased continuously over the follow-up time and the majority of patients need multiple therapies to attain these glycemic target levels in the longer term [7].
This finding seems extraordinary. There are plenty of patients who have had type 2 diabetes for more than 6 years but nothing like 50% are on insulin. The difference between the findings of the UKPDS and everyday experience is explained by another observation – most of the diabetic patients do not have HbA1c values below 7% [9]. In fact the median HbA1c worldwide is closer to 8%, i.e. in 50% HbA1c exceeds 8% (Diabetes Care targets for HbA1c). Mrs. MG may not have been perfectly adherent to treatment recommendations but, even had she been the “perfect patient”, her diabetes would have progressed. The point is that her treatment should have progressed earlier.
Psychological insulin resistance
The "failure" to achieve the recommended targets (HbA1c < 7%) is also explained by defects in insulin resistance and insulin capacity. Physicians and patients are resistant to the idea of starting insulin and the healthcare systems often lack the capacity to start and monitor insulin therapy satisfactorily.
"Just one more try, doctor - I know my blood glucose has been high over Christmas but one of my New Year's resolutions is to lose the 2 kg I gained over the festive season and get back to my regular walks. My wife wants to start walking too and we'll keep each other motivated."
"Thank goodness" you think, "I really didn't want to start insulin right now. The waiting room is packed, I really need to get away soon and I could do without writing scripts, referrals and all the rest of the paraphernalia. However, I must dig out those notes I made so I know which insulin to use and what dose to start with next time it comes up". [10]
Patients and physicians are both showing insulin resistance so common in type 2 diabetes. In this case, however, they are showing the psychological insulin resistance that stops or delays people starting insulin. Neither the patient nor the doctor wants to take the next step. The problems of starting insulin are immediate and obvious to both (Figure 4) [11]. The problems of not starting are more remote and less obvious – a progressive increase in the risk of diabetes-related complications. The Diabetes Awareness Wishes and Needs (DAWN) study confirmed this psychological insulin resistance [1, 12]. Over 40% of the patients were concerned about starting insulin, only 12% believed insulin therapy would help and 40% believed that starting insulin implied that they had not followed treatment recommendations properly. Many healthcare providers preferred to delay insulin therapy until it was "absolutely essential".
Figure 4. Causes of psychological insulin resistance.
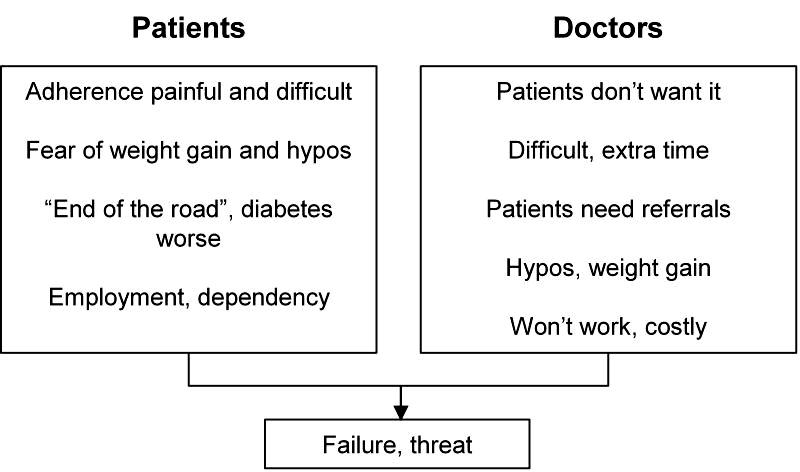
Patients and healthcare professionals both show psychological barriers to starting insulin therapy which frequently results in deferred adoption of insulin and poor metabolic control. The figure shows common kinds of aversions and considerations which both groups foster.
From psychological resistance to acceptance
Both patients and doctors have concerns based on reality (Figure 4) [1, 11]. Some of these concerns can be easily resolved: a demonstration with a "dry" injection, a successful patient and an explanation of the real risk of hypoglycemia (approximately 1/20th that of type 1 diabetes, which can be derived from the UKPDS and DCCT hypoglycemia risk experience) [13], and a weight gain of probably 2-3 kilograms if a simple plan of "eat less…walk more" is adopted [14]. Some other concerns are less easy to resolve: some employers do discriminate (even though this is illegal) and access to some jobs is more difficult (airline pilot, involvement in the active armed forces). Overall, insulin does not limit opportunity much.
For the doctors the message is "Just do it!". Nothing is so persuasive as seeing that starting insulin is not hard, not risky and does work. It is clear that starting with 10 units of intermediate insulin (isophane) at bedtime, using a pen injector and continuing oral hypoglycemic agents is simple. Increasing the night-time dose will control fasting blood glucose and only a minority will need a second morning shot of intermediate insulin to control blood glucose before the evening meal. Most importantly, patients and doctors will feel better and usually wonder why they did not start insulin therapy much earlier.
"Insulin is good" [15]: If physicians feel this way they will not think that they and their patients have failed, no-one will blame anyone and good feelings will replace guilt. Pathological insulin resistance and the progressive nature of diabetes should be matched by psychological insulin acceptance and progressive treatment.
"I feel great. More energy and no more daytime naps. If I had known how easy it was, I would have started years ago" [16].
"But I thought you had to use a basal bolus schedule with four shots a day. I had no idea how to start, what insulins to use and what doses" [17].
References
- 1.DAWN Study. http://www.dawnstudy.com/documents/home_page/document/index.asp
- 2.Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002;26(Suppl 3):S18–S24. doi: 10.1038/sj.ijo.0802173. [DOI] [PubMed] [Google Scholar]
- 3.Muller DC, Elahi D, Tobin JD, Andres R. The effect of age on insulin resistance and secretion: a review. Semin Nephrol. 1996;16(4):289–298. [PubMed] [Google Scholar]
- 4.Kaneto H, Nakatani Y, Kawamori D, Miyatsuka T, Matsuoka TA. Involvement of Oxidative Stress and the NJK Pathway in Glucose Toxicity. Rev Diabetic Stud. 2004;1(4):163–172. doi: 10.1900/RDS.2004.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isomaa B. A major health hazard: the metabolic syndrome. Life Sci. 2003;73(19):2395–2411. doi: 10.1016/s0024-3205(03)00646-5. [DOI] [PubMed] [Google Scholar]
- 6.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 7.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281(21):2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 8.Wittmann I, Soltesz G, Jermendy G, Nagy J. Decreased first-phase secretion of insulin may play a role in the development of insulin resistance. Orv Hetil. 2004;145(45):2267–2272. [PubMed] [Google Scholar]
- 9.Phillipov G, Phillips PJ. Components of total measurement error for hemoglobin A(1c) determination. Clin Chem. 2001;47(10):1851–1853. [PubMed] [Google Scholar]
- 10.Personal communication
- 11.Lauritzen T. DAWN Symposium. London: November 2000. [Google Scholar]
- 12.Peyrot M, Mathews DR, Snoek FJ, Calogiuri R, Kleinebreil L, Rubin R, Ishii H, Lauritzen T, Geelhoed-Duijvestijn PH, Skovlund SE. An international study of physiological insulin resistance to insulin use among persons with diabetes. Paris, France. August 24-29 2003; 18th International Diabetes Federation Congress; 2003. OP Education 5: Psychological insulin resistance. [Google Scholar]
- 13.Phillips P, MacLennan A. HRT – is it for me? Case studies in HRT. RACGP Check Programme. October 1998.
- 14.Phillips P, Carapetis M. Eat less, walk more. Enjoyable eating for type 2 diabetes. Aust Fam Physician. 2002;31(12):1065–1071. [PubMed] [Google Scholar]
- 15.Skyler J. Personal communication
- 16. Mr. BJ 6 weeks after starting insulin.
- 17. Personal communication with a GP colleague after being introduced to the bedtime insulin and daytime hypoglycemic schedule.


