Abstract
Dentinal repair in the postnatal organism occurs through the activity of specialized cells, odontoblasts, that are thought to be maintained by an as yet undefined precursor population associated with pulp tissue. In this study, we isolated a clonogenic, rapidly proliferative population of cells from adult human dental pulp. These DPSCs were then compared with human bone marrow stromal cells (BMSCs), known precursors of osteoblasts. Although they share a similar immunophenotype in vitro, functional studies showed that DPSCs produced only sporadic, but densely calcified nodules, and did not form adipocytes, whereas BMSCs routinely calcified throughout the adherent cell layer with clusters of lipid-laden adipocytes. When DPSCs were transplanted into immunocompromised mice, they generated a dentin-like structure lined with human odontoblast-like cells that surrounded a pulp-like interstitial tissue. In contrast, BMSCs formed lamellar bone containing osteocytes and surface-lining osteoblasts, surrounding a fibrous vascular tissue with active hematopoiesis and adipocytes. This study isolates postnatal human DPSCs that have the ability to form a dentin/pulp-like complex.
Keywords: odontoblast, dentin, in vivo transplantation
During tooth formation, interactions between epithelial and dental papilla cells promote tooth morphogenesis by stimulating a subpopulation of mesenchymal cells to differentiate into odontoblasts, which in turn form primary dentin. Morphologically, odontoblasts are columnar polarized cells with eccentric nuclei and long cellular processes aligned at the outer edges of dentin (1). After tooth eruption, reparative dentin is formed by odontoblasts in response to general mechanical erosion or disruption, and through dentinal degradation caused by bacteria (2). These odontoblasts are thought to arise from the proliferation and differentiation of a precursor population, residing somewhere within the pulp tissue (3). Despite extensive knowledge of tooth development, and of the various specialized tooth-associated cell types, little is known about the characteristics and properties of their respective precursor cell populations in the postnatal organism.
To date, the identification and isolation of an odontogenic progenitor population from adult dental pulp tissue has never been done. It is known that in certain conditions, cultures of pulp cells derived from early developing dental root tissue and pulp tissue can develop an odontoblast-like appearance with the capacity to form mineralized nodules in vitro (4), a trait normally attributed to cultures of bone or bone marrow cells (5, 6). More is known about the characteristics of multipotent bone marrow stromal cells (BMSCs) and their potential to develop into osteoblasts, chondrocytes, adipocytes, myelosupportive fibrous-stroma, and perhaps even muscle and neural tissues (7–12). They are characterized by their high proliferative capacity ex vivo, whereas maintaining their ability to differentiate into multiple stromal cell lineages. The tissue-specific differentiation of BMSCs seems to be dependent on their state of differentiation and commitment, and the microenvironment in which they are located. By analogy, we speculated that adult dental pulp tissue might also contain a population of multipotential stem cells.
In the present study, clonogenic and highly proliferative cells were derived from enzymatically disaggregated adult human dental pulp, which we have termed DPSCs, and compared with BMSCs, cells with known stem cell character (13). We have previously shown that human bone is generated after xenogeneic transplantation of BMSCs with hydroxyapatite/tricalcium phosphate (HA/TCP) as a carrier vehicle (9). We therefore explored the possibility that isolated ex vivo-expanded human DPSCs would also be capable of regenerating a dentin/pulp-like structure in vivo under similar conditions.
Materials and Methods
Subjects and Cell Culture.
Normal human impacted third molars were collected from adults (19–29 years of age) at the Dental Clinic of the National Institute of Dental and Craniofacial Research under approved guidelines set by the National Institutes of Health Office of Human Subjects Research. Tooth surfaces were cleaned and cut around the cementum-enamel junction by using sterilized dental fissure burs to reveal the pulp chamber. The pulp tissue was gently separated from the crown and root and then digested in a solution of 3 mg/ml collagenase type I (Worthington Biochem, Freehold, NJ) and 4 mg/ml dispase (Boehringer Mannheim) for 1 h at 37°C. Single-cell suspensions were obtained by passing the cells through a 70-μm strainer (Falcon). Bone marrow cells, processed from marrow aspirates of normal human adult volunteers (20–35 years of age), were purchased from Poietic Technologies (Gaithersburg, MD) and then washed in growth medium. Single-cell suspensions (0.01 to 1 × 105/well) of dental pulp and bone marrow were seeded into 6-well plates (Costar) with alpha modification of Eagle's medium (GIBCO/BRL) supplemented with 20% FCS (Equitech-Bio, Kerrville, TX)/100 μM l-ascorbic acid 2-phosphate (Wako Pure Chemicals, Osaka)/2 mM l-glutamine/100 units/ml penicillin/100 μg/ml streptomycin (Biofluids, Rockville, MD), and then incubated at 37°C in 5% CO2. To assess colony-forming efficiency, day 14 cultures were fixed with 4% formalin, and then stained with 0.1% toluidine blue. Aggregates of ≥50 cells were scored as colonies. Conditions for the induction of calcified bone matrix deposition in vitro were as reported (6). The proliferation rate of subconfluent cultures (first passage) of DPSCs and BMSCs was assessed by bromodeoxyuridine (BrdUrd) incorporation for 24 h by using a Zymed BrdUrd staining kit (Vector Laboratories).
Immunohistochemistry.
Primary DPSCs and BMSCs were subcultured into 8-chamber slides (2 × 104 cells/well) (Nunc). The cells were fixed in 4% formalin, and then reacted with saturating levels of primary antibodies and the corresponding control antibodies by using a Zymed broad-spectrum immunoperoxidase kit (Vector Laboratories). Antibodies used were: mouse (IgG) control (Caltag, South San Francisco, CA); and rabbit (Ig) control, TUK4 (anti-CD14), QBEND 10 (anti-CD34), 2B11/PD7 (anti-CD45), M318 (anti-MyoD), 1A4 (anti-α smooth muscle actin), 2F11 (anti-neurofilament) (Dako); H9H11 (anti-CD44), 6G10 (anti-VCAM-1) (Dr. P. J. Simmons, Hanson Centre for Cancer Research, Adelaide, South Australia); CC9 (anti-MUC-18) (S. Gronthos, National Institute of Dental and Craniofacial Research/National Institutes of Health, Bethesda); MAB1343 (anti-COL III), MAB1959 (anti-β1) (Chemicon); LF67 (anti-COL I), LF32 (anti-OC), BON-1 (anti-ON), LF100 (anti-BSP), LF123 (anti-OP) (L. Fisher, National Institute of Dental and Craniofacial Research/National Institutes of Health); MAB1104 (anti-COL II) (Research Diagnostics, Flanders, NJ); E-8 (anti-PPARγ), 147 (anti-FGF-2) (Santa Cruz Biotechnology). Working dilutions of rabbit serum (1/500), monoclonal supernatants (1/4), and purified antibodies (10 μg/ml) were used.
Histochemistry.
Secondary DPSC and BMSC cultures were washed in PBS and then fixed with 4% formalin. Alkaline phosphatase activity was assessed by using a Sigma in vitro alkaline phosphatase substrate kit (85L-2). Calcium deposits were detected by treatment with 2% Alizarin Red S (pH 4.2).
Transplantation.
Approximately 5.0 × 106 of DPSCs and BMSCs (third passage) were mixed with 40 mg of HA/TCP ceramic powder (Zimmer, Warsaw, IN) and then transplanted s.c. into the dorsal surface of 10-week-old immunocompromised beige mice (NIH-bg-nu-xid, Harlan–Sprague–Dawley) as described (9). These procedures were performed in accordance to specifications of an approved small animal protocol (National Institute of Dental and Craniofacial Research no. 97–024). The transplants were recovered at 6 weeks posttransplantation, fixed with 4% formalin, decalcified with buffered 10% EDTA (pH 8.0), and then embedded in paraffin. Sections (5 μm) were deparaffinized and stained with hematoxylin/eosin.
Reverse Transcription–PCR.
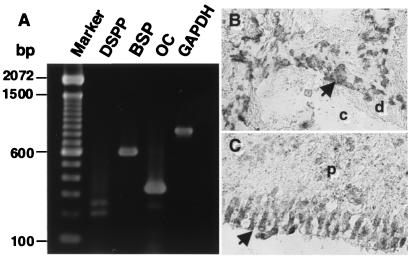
Total RNA was prepared from collagenase/dispase-digested cell suspensions of 6-week-old DPSC transplants by using RNA STAT-60 (Tel-Test, Friendswood, TX). First-strand cDNA synthesis was performed by using a first-strand cDNA synthesis kit (GIBCO/BRL; Life Technologies, Grand Island, NY) by using an oligo-dT primer. First strand cDNA (2 μl) was diluted in a 50-μl PCR reaction of 1X PCR reaction buffer: 1.5 mM MgCl2/200 μM each of dNTP/0.2 units of AmpliTaq DNA Polymerase (Perkin–Elmer)/10 pmol of each human-specific primer sets: bone sialoprotein (sense 5′-CTATGGAGAGGACGCCACGCCTGG-3′ (antisense, 5′-CATAGCCATCGTAGCCTTGTCCT-3′), osteocalcin (sense, 5′-CATGAGAGCCCTCACA-3′; antisense, 5′-AGAGCGACACCCTAGAC-3′), dentin sialophosphoprotein (DSPP) (sense 5′-GGCAGTGACTCAAAAGGAGC-3′; antisense, 5′-TGCTGTCACTGTCACTGCTG-3′), glyceraldehyde-3-phosphate dehydrogenase (sense, 5′-AGCCGCATCTTCTTTTGCGTC-3′; antisense 5′-TCATATTTGGCAGGTTTTTCT-3′). The reactions were incubated in a PCR Express Hybaid thermal cycler (Hybaid, Franklin, MA) at 94°C for 2 min for one cycle and then 94°C/(45 s), 56°C/(45 s), 72°C/(60 s) for 35 cycles, with a final 10-min extension at 72°C. After amplification, 10 μl of each reaction was analyzed by 1.5% agarose gel electrophoresis, and visualized by ethidium bromide staining.
In Situ Hybridization.
A digoxigenin-labeled probe for human-specific alu repetitive sequence was prepared by PCR by using primers for alu as described (13). Similarly, a digoxigenin-labeled probe specific for human DSPP mRNA was also prepared by using the DSPP primers under the same PCR conditions as described above. The specificity of both probes was verified by DNA sequencing. Unstained sections were deparaffinized and hybridized with either the digoxigenin-labeled alu probe (9) or the DSPP probe by using the mRNAlocator-Hyb Kit (catalogue no. 1800; Ambion, Austin, TX). After hybridization, the presence of both alu and DSPP mRNA in tissue sections was detected by immunoreactivity with an anti-digoxigenin alkaline phosphatase-conjugated Fab fragments (Boehringer Mannheim).
Results
Isolation of Clonogenic Populations of DPSCs.
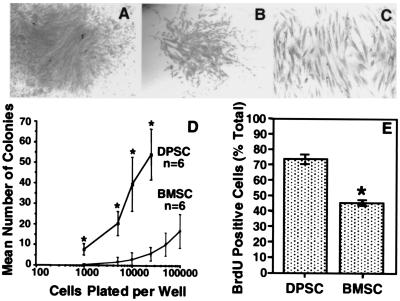
It is well documented that osteoprogenitors can be isolated from aspirates of bone marrow by their ability to adhere to a plastic substratum, and with appropriate stimulation, begin to proliferate (13–15). Each colony originates from a single progenitor cell (colony-forming unit-fibroblast, CFU-F) and displays a wide variation in cell morphology and growth potential (13–18). Here, we demonstrate the presence of a clonogenic cell population in dental pulp tissue (Fig. 1 A and B). The cells within each colony were characterized by a typical fibroblast-like morphology (Fig. 1C) analogous to the progeny of human bone marrow CFU-F (19). The frequency of colony-forming cells derived from dental pulp tissue (22–70 colonies/104 cells plated) was significantly higher in comparison to the incidence of bone marrow CFU-F (2.4–3.1 colonies/104 cells plated) over similar plating densities (0.1–2.5 × 104 cells plated) (Fig. 1D). In addition, the number of proliferating cells in DPSC cultures was also significantly higher (mean 72% BrdUrd-positive cells ± 3.48 SEM, n = 3) when compared with BMSC cultures (46% BrdUrd-positive cells ± 1.96 SEM, n = 3) by using the BrdUrd uptake method (t test P ≤ 0.05) (Fig. 1E).
Figure 1.
Colony-forming efficiency and cell proliferation in vitro. Representative high (A) and low (B) density colonies after 14 days. The morphology is typical of fibroblast-like cells (C). The incidence of colony-forming cells from dental pulp tissue and bone marrow at various plating densities indicates that there are more clonogenic cells in dental pulp than in bone marrow (D). The number of BrdUrd-positive cells were expressed as a percentage of the total number of cells counted for DPSCs and BMSCs (E). Statistical significance (*) was determined by using the Student's t test (P ≤ 0.05).
Characterization of the Immunophenotype of DPSCs in Vitro.
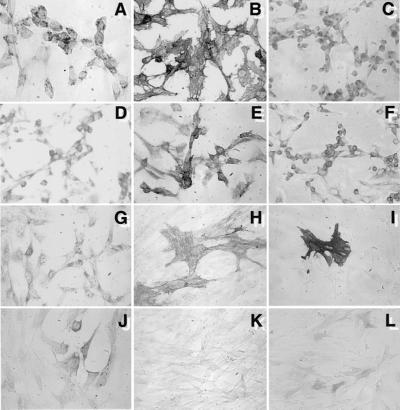
Immunohistochemical studies were performed to characterize the progeny of the DPSC and BMSC clonogenic populations, by using a large panel of antibodies specific to known antigens associated with different phenotypes. Typical immunoreactivity profiles for both cell populations are shown in Table 1. Primary cultures of DPSC and BMSC failed to react with the hematopoietic markers CD14 (monocyte/macrophage), CD45 (common leukocyte antigen), CD34 (hematopoietic stem/progenitor cells/endothelium), and other markers such as MyoD (smooth muscle), neurofilament (nerve), collagen type II (cartilage), and peroxisomal proliferator activated receptor gamma 2 (fat). In general, DPSCs and BMSCs exhibited a similar expression pattern for a variety of markers associated with endothelium [vascular cell adhesion molecule 1 and MUC-18 (CD146)], smooth muscle (α-smooth muscle actin), bone (alkaline phosphatase, type I collagen, osteonectin, osteopontin, and osteocalcin), and fibroblasts (type III collagen and fibroblast growth factor 2). The bone matrix protein, bone sialoprotein, was absent in DPSC cultures, but present at low levels in BMSC cultures. Representative immunoreactivity patterns for DPSC are shown (Fig. 2). Many of the markers were not uniformly expressed, but found in subsets of cells, indicating that the DSPC population is heterogeneous, as has been shown for the BMSC population. The DPSC cultures were also found to be negative for the odontoblast-specific marker, DSPP, by Northern blot analysis, which is suggestive of an undifferentiated phenotype (data not shown).
Table 1.
Immunohistochemical analysis of human DPSCs and BMSCs in vitro
| Marker | DPSC-1 | DPSC-2 | BMSC |
|---|---|---|---|
| CD14 | – | – | – |
| CD34 | – | – | – |
| CD44 | ++ | ++ | ++ |
| CD45 | – | – | – |
| Integrin β1 | ++/+ | ++/+ | ++ |
| VCAM-1 | + | + | ++ |
| MyoD | – | – | – |
| α-SM actin | ++/− | ++/− | ++/+/− |
| Neurofilam. | – | – | – |
| MUC-18 | ++/− | ++/+/− | ++/+/− |
| Collagen-I | + | ++ | ++/+ |
| Collagen-II | – | – | – |
| Collagen-III | ++/+ | ++/+ | ++/+ |
| Osteocalcin | ++/+ | ++/+ | +/− |
| Osteonectin | ++/+ | ++ | ++/+ |
| BSP | – | – | +/− |
| Osteopontin | +/− | +/− | +/− |
| Alk Phos | ++/+/− | ++/+/− | ++/+/− |
| PPARγ | – | – | – |
| FGF-2 | ++/+ | ++ | ++/+ |
++, strong staining; +, weak staining; −, negative; –, subpopulation; VCAM-1, vascular cell adhesion molecule 1; MyoD, myocyte origin; α-SM actin, alpha-smooth muscle actin; MUC-18, CD146; BSP, bone sialoprotein; Alk Phos, alkaline phosphatase; PPARγ, peroxisomal proliferator activated receptor gamma 2; FGF-2, fibroblast growth factor 2.
Figure 2.
Immunophenotype of cultured DPSCs. Studies based on immunoperoxidase reactivity were performed on first passage cultures of DSPCs. Representative staining patterns are shown for: integrin β1 (A); CD44 (B); collagen type I (C); collagen type III (D); fibroblast growth factor-2 (E); osteonectin (F); osteocalcin (G); MUC-18 (CD146) (H); α-smooth muscle actin (I); osteopontin (J); and vascular cell adhesion molecule 1 (K). Endogenous alkaline phosphatase activity is shown in L.
Differentiation Potential of DPSCs in Vitro.
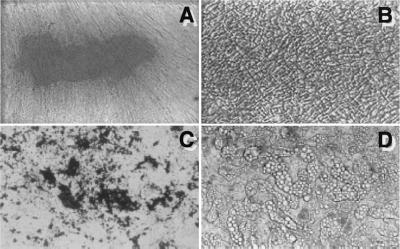
Long-term cultures (5–6 weeks) of DPSCs grown in the presence of l-ascorbate-2-phosphate, the glucocorticoid, dexamethasone, and inorganic phosphate demonstrated the capacity to form Alizirin Red-positive condensed nodules with high levels of calcium (Fig. 3A). The deposits were sparsely scattered throughout the adherent layer as single mineralized zones. In contrast, BMSC cultures produced extensive sheets of calcified deposits over the entire adherent layer after 3–4 weeks of induction (Fig. 3C). After 6 weeks of stimulation with dexamethasone, there was no evidence of adipogenesis in primary DPSC cultures (Fig. 3C), whereas clusters of lipid-containing adipocytes were detected in primary cultures of BMSC as early as 2 weeks (Fig. 3D).
Figure 3.
Developmental potential in vitro. Adherent layers of cultured DPSCs (A and B), and BMSCs (C and D) are shown with Alizarin Red staining as a measure of calcium accumulation after 6 weeks of induction with l-ascorbate-2-phosphate and dexamethasone with inorganic phosphate (A and C). After 6 weeks in the same medium but without inorganic phosphate, lipid accumulation was noted in BMSCs (D), but not in DPSCs (B).
Ex Vivo Expanded DPSCs Can Generate a Dentin/Pulp-Like Structure in Vivo.
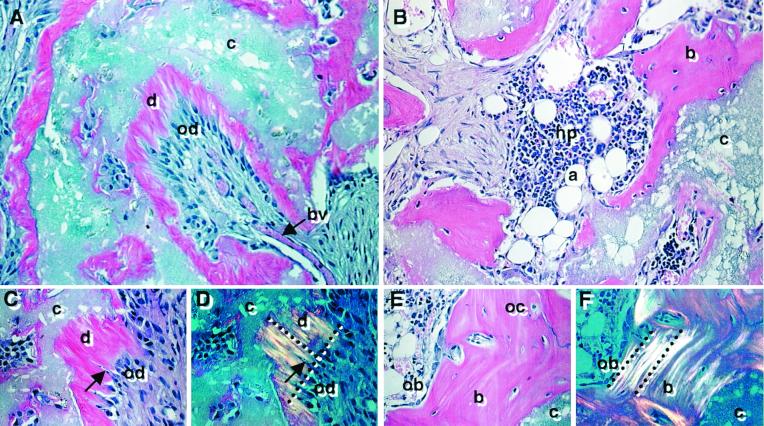
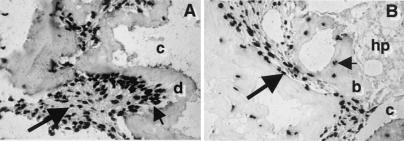
Because complete developmental potential and formation of an appropriate histological structure often cannot be fully realized in vitro, DPSCs were transplanted in conjunction with HA/TCP powder into immunocompromised mice. After 6 weeks posttransplantation, DPSCs generated a dentin-like structure lining the surfaces of the HA/TCP particles, comprised of a highly ordered collagenous matrix deposited perpendicular to the odontoblast-like layer when viewed by polarized light (Fig. 4 A, C, and D). Immunological studies demonstrated that the matrix was predominantly composed of collagen type I (data not shown). The odontoblast-like cells extended cytoplasmic processes into the dentinal matrix, which interfaced with a pulp-like interstitial tissue infiltrated with blood vessels. The pulp and odontoblast-like cells were found to be of donor origin based on their reactivity to the human alu-specific probe (Fig. 5A). Furthermore, the DPSC transplants expressed human-specific transcripts for dentin matrix components, including bone sialoprotein, osteocalcin, and DSPP by reverse transcription–PCR and in situ hybridization (Fig. 6). The corresponding BMSC transplants formed distinct lamellae of bone on the surface of the HA/TCP where the collagen fibers were aligned parallel to the osteoblasts on the bone-forming surfaces (Fig. 4 B, E, and F). Osteocytes, entombed within the bone matrix, and osteoblasts were also found to be of donor origin (Fig. 5B). Newly formed bone surrounded an interstitial tissue that was infiltrated by a sinusoid network resembling a marrow-like organ, with extensive areas of active hematopoiesis and adipocyte accumulation. Interestingly, the DPSC transplants failed to support any hematopoiesis or initiate adipocyte formation even 4 months posttransplantation. In addition, tartrate-resistant acid phosphatase-positive cells were seen lining the new bone surfaces in the BMSC transplants but were not detected in the dental-pulp transplants (data not shown).
Figure 4.
Developmental potential in vivo. Cross sections are representative of DPSC transplants (A, C, and D) and BMSC transplants, (B, E, and F) 6 weeks posttransplantation and stained with hematoxylin and eosin. In the DPSC transplants, the HA/TCP carrier surfaces (c) are lined with a dentin-like matrix (d), surrounding a pulp-like tissue with blood vessels (bv) and an interface layer of odontoblast-like cells (od) (A). A magnified view of the dentin matrix (d) highlights the odontoblast-like layer (od) and odontoblast processes (arrow) (C). Polarized light demonstrates perpendicular alignment (dashed lines) of the collagen fibers to the forming surface (D). In BMSC transplants, lamellar bone (b) is formed on the HA/TCP surfaces (c) and surrounds a vascular, hematopoietic marrow organ (hp) with accumulated adipocytes (a) (B). A magnified view shows that the new bone contains osteocytes (oc), embedded within the calcified matrix, and osteoblasts (ob) lining the bone surfaces (E). With polarized light, collagen fibrils are seen to be deposited parallel with the forming surface (dashed lines) (F).
Figure 5.
In situ hybridization for the human-specific alu DNA sequence. Alu-positive cells in the pulp tissue (large arrow) and odontoblast-like layer (small arrow) adjacent to the dentin matrix (d) are easily recognized in 6-week DPSC transplants (A). Osteocytes encased in the new bone matrix (small arrow) and the osteoblasts lining the bone (b) surfaces (large arrow) show positive reactivity with the alu probe in the BMSC transplants (B). Hematopoietic elements (hp) in the marrow-like organ fail to show reactivity with the alu probe.
Figure 6.
Expression of the human-specific DSPP, osteocalcin (OC), bone sialoprotein (BSP) mRNA in DPSC transplants. Transcripts for DSPP, BSP, OC, and glyceraldehyde-3-phosphate dehydrogenase were detected by reverse transcription–PCR by using total RNA isolated from 6-week-old DPSC transplants (A). DSPP-positive cells were also found in the pulp tissue and odontoblast layer (arrow) adjacent to the dentin matrix (d) by in situ hybridization (B). Specificity of the probe was verified by hybridization in the odontoblast layer (arrow) of human dental pulp (p) tissue (C). No reactivity of the DSPP-specific probe was detected in human bone, bone marrow, and muscle tissue (data not shown).
Discussion
During development, interactions between epithelial cells of the inner enamel organ and mesenchymal cells of the dental papilla lead to the differentiation of ameloblasts and odonotoblasts, each of which deposit specialized mineralized matrices, enamel and dentin, respectively. Once formed, these matrices do not undergo remodeling, unlike bone, which slowly remodels throughout postnatal life. However, after tooth eruption, dentinal damage caused by mechanical trauma, exposure to chemicals or disease processes induces the formation of reparative dentin, a poorly organized mineralized matrix that serves as a protective barrier to the dental pulp. It is thought that progenitors are recruited from dental pulp to develop supportive connective tissue and terminally differentiated odontoblasts (1); yet the origin and nature of these cells have not been characterized, and the possible existence of a postnatal DPSC was never formerly proposed. We speculated that dental pulp might, in fact, be the source of dental stem cells, because bone marrow is a source of stem cells (13).
To determine the existence of such a cell in dental pulp, we applied methodology that had been previously developed for the isolation and characterization of BMSCs. By using a colony-forming efficiency assay that determines the CFU-F number in bone marrow cell suspensions, we demonstrated that a minor population within adult human dental pulp is clonogenic. Colonies of dental pulp cells occurred at an apparently higher frequency in comparison to BMSCs. However, this is most likely because of the basic differences in the composition of the two connective tissues. Whereas dental pulp is comprised largely of fibrous tissue, in bone marrow aspirates, hematopoietic cells constitute the majority of the cell population (>99.9%). When bone marrow core biopsies from normal donors are flushed free of hematopoietic cells and then subjected to collagenase treatment, the incidence of CFU-F dramatically increases to by more than 10-fold compared with marrow aspirates (unpublished observations). Consequently, when considering the number of CFU-F from a connective tissue devoid of hematopoietic cells, the numbers would appear to be fairly equivalent.
Interestingly, DPSCs exhibited a higher proliferation rate compared with BMSCs in vitro. This may be attributed to the developmental state of the respective tissues, because the third molars are the last permanent teeth to fully develop and erupt and are therefore at an earlier state of development compared with adult bone marrow. Current studies indicate that they maintain their high rate of proliferation even after extensive subculturing. Taken together with their clonogenic nature, the cells isolated here satisfy two of the criteria of a postnatal somatic stem cell (14).
Although their localization in situ is quite different, DPSCs and BMSCs share many features. Potent regulators of bone formation such as transforming growth factor-β, bone morphogenic protein (BMP)-2, and BMP-4 have been implicated as promoters of odontoblast development (15, 16). Other growth factors thought to regulate the proliferation and differentiation of odontoblast precursors include basic fibroblast growth factor, platelet-derived growth factor, epidermal growth factor, insulin-like growth factor I tumor necrosis factor-α, and IL-β1 (16–18), all of which influence osteoblastic cells as well. Furthermore, odontoblasts and osteoblasts express similar mineralized matrix proteins, such as dentin matrix protein 1, fibronectin, collagen type I, alkaline phosphatase, osteonectin, osteopontin, bone sialoprotein, and osteocalcin (15, 16, 19–21). Two notable exceptions are the odontoblast-specific gene products, dentin sialoprotein (22) and dentin phosphoprotein (23), encoded by a single gene known as DSPP (24). The expression of both dentin sialoprotein and dentin phosphoprotein occurs after the formation a collagenous predentin matrix and is associated with the process of dentinogenesis (25). Collectively, these studies suggest that biochemical pathways involved in the differentiation of DPSCs into functional odontoblasts are similar to differentiation pathways of BMSCs into osteoblasts, and consequently, we compared these two populations in vitro and in vivo.
The present study demonstrates that human DPSCs do share a similar pattern of protein expression with BMSCs in vitro. However, several subsets of cells expressing markers of bone (alkaline phosphatase, osteopontin, and bone sialoprotein), smooth muscle (α-smooth muscle actin), and endothelial cells (MUC-18) were represented in both DPSCs and BMSCs. Therefore, the heterogeneous nature of DPSCs may reflect differences in their developmental stages or may even represent different pulp cell lineages. More work is clearly needed to assess the full developmental potential of different DPSC clones, in analogy to the proposed marrow stromal hierarchy of cellular differentiation (26), where only a subset of BMSC colonies have the potential to develop into multiple cell lineages in vitro, or the capacity to form new bone and marrow elements in vivo (6, 8, 9, 12, 27).
It is also of interest to note that both DPSCs and BMSCs express smooth muscle and endothelial markers. Based on recent studies in developmental biology, it has been suggested that a number of different stem cells may arise from developing blood vessels (28), and there is evidence that osteoprogenitors are associated with the outer surfaces of vasculature (29, 30). The origin and precise location of DPSCs are unknown because of a lack of specific markers. However, it is possible that they may also be associated with the vasculature. Further characterization of DPSCs by using current molecular technology will hopefully provide novel markers that will be useful in their identification in situ, and isolation and purification ex vivo.
Previous studies have looked at the odontogenic potential of adult human dental pulp by using a number of organ, explant, and cell-culture methods and noted the ability of such cultures to mineralize, at least in part (4, 19, 20, 31). Our data also demonstrates the potential of DPSCs to form calcified deposits in vitro, as do BMSCs (5, 6, 32). However, DPSCs formed sparse and dense calcified nodules, and failed to develop lipid-laden adipocytes, whereas BMSCs developed extensive sheets of calcified deposits and abundant lipid-laden adipocytic clusters. Although in vitro culture systems offer a model to investigate odontoblast development, they fall short in their ability to allow for determination of the ability of a stem cell to generate a functional tissue with the appropriate architecture. Consequently, an in vivo transplantation system, the gold standard, was needed to further characterize the differentiation capacity of DPSCs.
The potential of developing dental cells to generate a dentin-like tissue has previously been described after transplantation of neonatal rodent and bovine dental papilla cells (33–35). However, in another earlier study, intact human dental pulp from a premolar tooth of a 13-year-old male failed to generate a dentin matrix and/or odontoblast-like cells when transplanted beneath the kidney capsule of athymic mice (36). The failure of intact tissue to form dentin suggests that the putative DPSCs remained constrained within the tissue. Our study used clonogenic, ex vivo expanded adult human pulp cells to generate a dentin/pulp-like complex by in vivo transplantation. The HA/TCP used in our transplantation system may be “odonto”-conductive, because it is “osteo”-conductive for BMSCs. The DPSC transplants are characterized by a well-defined layer of aligned odontoblast-like cells expressing the dentin-specific protein DSPP, with their processes oriented in the same direction and extending into tubular structures within newly generated dentin. The collagen matrix mimics the structure of primary dentin with ordered fibers perpendicular to the odontoblast layer, rather than the disorganized matrix characteristic of reparative dentin. The interstitial cellular components of the DPSC transplants is reminiscent of a pulp-like fibrous tissue, infiltrated with blood vessels, and distinct from the hematopoietic, adipocyte-containing marrow formed in the BMSC transplants. Importantly, the odontoblast-like cells and cells within the fibrous tissue were both found to be human in origin. Therefore, even though DPSCs and BMSCs are regulated by similar factors, and share a common protein expression profile, these populations differ significantly in their proliferative ability and developmental potentials in vitro, and more importantly, their ability to develop into distinct tissues representative of the microenvironments from which they were derived in vivo.
The data presented here demonstrate that postnatal dental pulp contains cells that are clonogenic, highly proliferative, and capable of regenerating a tissue, properties that effectively define them as stem cells. Based on recent exciting developments in stem cell biology, it appears that dental pulp is analogous to muscle and nervous tissue (37–39). Although muscle, nervous tissue, and dentin-associated tissue do not remodel during postnatal life, they all contain stem cells that have the ability to differentiate in response to injury. The transplantation of human DPSCs into immunocompromised mice provides a new model by which to further characterize these stem cells. Furthermore, it is noteworthy that the amount of dentin and pulp-like tissue formed in these transplants far exceeds the amount that would be generated in situ during the lifetime of an organism. Consequently, there is a great potential for the isolation of a large number of DPSCs from a single tooth that could be used for dentinal repair of a number of teeth. Further development of carriers with appropriate shape and composition to be used in conjunction with ex vivo expanded DPSCs makes the fabrication of a viable dental implant a real possibility in perhaps the not too distant future.
Acknowledgments
We acknowledge Professor Paolo Bianco, Universita dell'Aquila, Italy for his many thoughtful discussions, and critical evaluation of these studies.
Abbreviations
- DPSC
dental pulp stem cell
- BMSC
bone marrow stromal cell
- HA/TCP
hydroxyapatite/tricalcium phosphate
- BrdUrd
bromodeoxyuridine
- DSPP
dentin sialophosphoprotein
- CFU-F
colony-forming unit-fibroblast
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240309797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240309797
References
- 1.Smith A J, Cassidy N, Perry H, Begue-Kirn C, Ruch J V, Lesot H. Int J Dev Biol. 1995;39:273–280. [PubMed] [Google Scholar]
- 2.Kitamura C, Kimura K, Nakayama T, Terashita M. J Dent Res. 1999;78:673–680. doi: 10.1177/00220345990780020701. [DOI] [PubMed] [Google Scholar]
- 3.Ruch J V. Biochem Cell Biol. 1998;76:923–938. [PubMed] [Google Scholar]
- 4.Couble M L, Farges J C, Bleicher F, Perrat-Mabillon B, Boudeulle M, Magloire H. Calcif Tissue Int. 2000;66:129–138. doi: 10.1007/pl00005833. [DOI] [PubMed] [Google Scholar]
- 5.Cheng S L, Yang J W, Rifas L, Zhang S F, Avioli L V. Endocrinology. 1994;134:277–286. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- 6.Gronthos S, Graves S E, Ohta S, Simmons P J. Blood. 1994;84:4164–4173. [PubMed] [Google Scholar]
- 7.Friedenstein A J, Ivanov-Smolenski A A, Chajlakjan R K, Gorskaya U F, Kuralesova A I, Latzinik N W, Gerasimow U W. Exp Hematol (Charlottesville, VA) 1978;6:440–444. [PubMed] [Google Scholar]
- 8.Bennett J H, Joyner C J, Triffitt J T, Owen M E. J Cell Sci. 1991;99:131–139. doi: 10.1242/jcs.99.1.131. [DOI] [PubMed] [Google Scholar]
- 9.Kuznetsov S A, Krebsbach P H, Satomura K, Kerr J, Riminucci M, Benayahu D, Robey P G. J Bone Miner Res. 1997;12:1335–1347. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 11.Azizi S, Stokes D, Augelli B J, DiGirolamo C, Prockop D J. Proc Natl Acad Sci USA. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pittenger M F, Mackay A M, Beck S C, Jaiswal R K, Douglas R, Mosca J D, Moorman M A, Simonetti D W, Craig S, Marshak D R. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 13.Bianco P, Robey P G. J Clin Invest. 2000;105:1663–1668. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robey P G. J Clin Invest. 2000;105:1489–1491. doi: 10.1172/JCI10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakashima M, Nagasawa H, Yamada Y, Reddi A H. Dev Biol. 1994;162:18–28. doi: 10.1006/dbio.1994.1063. [DOI] [PubMed] [Google Scholar]
- 16.Shiba H, Fujita T, Doi N, Nakamura S, Nakanishi K, Takemoto T, Hino T, Noshiro M, Kawamoto T, Kurihara H, et al. J Cell Physiol. 1998;174:194–205. doi: 10.1002/(SICI)1097-4652(199802)174:2<194::AID-JCP7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 17.Onishi T, Kinoshita S, Shintani S, Sobue S, Ooshima T. Arch Oral Biol. 1999;44:361–371. doi: 10.1016/s0003-9969(99)00007-2. [DOI] [PubMed] [Google Scholar]
- 18.Kettunen P, Karavanova I, Thesleff I. Dev Genet (Amsterdam) 1998;22:374–385. doi: 10.1002/(SICI)1520-6408(1998)22:4<374::AID-DVG7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Kuo M Y, Lan W H, Lin S K, Tsai K S, Hahn L J. Arch Oral Biol. 1992;37:945–952. doi: 10.1016/0003-9969(92)90066-h. [DOI] [PubMed] [Google Scholar]
- 20.Tsukamoto Y, Fukutani S, Shin-Ike T, Kubota T, Sato S, Suzuki Y, Mori M. Arch Oral Biol. 1992;37:1045–1055. doi: 10.1016/0003-9969(92)90037-9. [DOI] [PubMed] [Google Scholar]
- 21.Buurma B, Gu K, Rutherford R B. Eur J Oral Sci. 1999;107:282–289. doi: 10.1046/j.0909-8836.1999.eos107408.x. [DOI] [PubMed] [Google Scholar]
- 22.Ritchie H H, Hou H, Veis A, Butler W T. J Biol Chem. 1994;269:3698–3702. [PubMed] [Google Scholar]
- 23.Gorter de Vries I, Quartier E, Van Steirteghem A, Boute P, Coomans D, Wisse E. Arch Oral Biol. 1986;31:57–66. doi: 10.1016/0003-9969(86)90114-7. [DOI] [PubMed] [Google Scholar]
- 24.MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu T T. J Biol Chem. 1997;272:835–842. doi: 10.1074/jbc.272.2.835. [DOI] [PubMed] [Google Scholar]
- 25.Feng J Q, Luan X, Wallace J, Jing D, Ohshima T, Kulkarni A B, D'Souza R N, Kozak C A, MacDougall M. J Biol Chem. 1998;273:9457–9464. doi: 10.1074/jbc.273.16.9457. [DOI] [PubMed] [Google Scholar]
- 26.Owen M E. In: Marrow Stromal Cell Culture. Owen M A, Beresford J N, editors. Cambridge, U.K.: Cambridge Univ. Press; 1998. pp. 1–9. [Google Scholar]
- 27.Friedenstein A J. Immunology of Bone Marrow Transplantation. Berlin: Springer; 1980. pp. 19–29. [Google Scholar]
- 28.Bianco P, Cossu G. Exp Cell Res. 1999;251:257–263. doi: 10.1006/excr.1999.4592. [DOI] [PubMed] [Google Scholar]
- 29.Bianco P, Riminucci M. In: Marrow Stromal Cell Culture. Owen M A, Beresford J N, editors. Cambridge, U.K.: Cambridge Univ. Press; 1998. pp. 10–25. [Google Scholar]
- 30.Doherty M J, Ashton B A, Walsh S, Beresford J N, Grant M E, Canfield A E. J Bone Miner Res. 1998;13:828–838. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- 31.Shiba H, Nakamura S, Shirakawa M, Nakanishi K, Okamoto H, Satakeda H, Noshiro M, Kamihagi K, Katayama M, Kato Y. Dev Biol. 1995;170:457–466. doi: 10.1006/dbio.1995.1229. [DOI] [PubMed] [Google Scholar]
- 32.Majors A K, Boehm C A, Nitto H, Midura R J, Muschler G F. J Orthop Res. 1997;15:546–557. doi: 10.1002/jor.1100150410. [DOI] [PubMed] [Google Scholar]
- 33.Ishizeki K, Nawa T, Sugawara M. Anat Rec. 1990;226:279–287. doi: 10.1002/ar.1092260303. [DOI] [PubMed] [Google Scholar]
- 34.Holtgrave E A, Donath K. Biomaterials. 1995;16:155–159. doi: 10.1016/0142-9612(95)98280-r. [DOI] [PubMed] [Google Scholar]
- 35.Lyaruu D M, van Croonenburg E J, van Duin M A, Bervoets T J, Woltgens J H, de Blieck-Hogervorst J M. J Oral Pathol Med. 1999;28:293–296. doi: 10.1111/j.1600-0714.1999.tb02043.x. [DOI] [PubMed] [Google Scholar]
- 36.Prime S S, Sim F R, Reade P C. Transplantation. 1982;33:561–562. [PubMed] [Google Scholar]
- 37.Schultz E, McCormick K M. Rev Physiol Biochem Pharmacol. 1994;123:213–257. doi: 10.1007/BFb0030904. [DOI] [PubMed] [Google Scholar]
- 38.Johansson C B, Svensson M, Wallstedt L, Janson A M, Frisen J. Exp Cell Res. 1999;253:733–736. doi: 10.1006/excr.1999.4678. [DOI] [PubMed] [Google Scholar]
- 39.Doetsch F, Caille I, Lim D A, Garcia-Verdugo J M, Alvarez-Buylla A. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]