Abstract
Acute myocardial infarction (AMI) associated with unfavorable prognosis is likely to be the consequence of a diffuse active chronic inflammatory process that destabilizes the whole coronary tree and myocardium, suggesting a possible common causal agent underlying both conditions. The main objective of this study was to investigate whether Chlamydia pneumoniae (CP) infection occurred beyond the coronary plaques, namely in the myocardium of individuals who died of AMI. The presence of CP cell wall antigen (OMP-2) and CP-HSP60 was investigated in the myocardium and coronary plaques of 10 AMI and 10 age-matched control patients by immunohistochemistry, electron microscopy, and molecular biology. OMP-2 antigens were found in the unaffected myocardium of 9 of 10 AMI patients. Conversely, only 1 of 10 control patients exhibited a positive staining for CP. Moreover, OMP-2 and CP-HSP60 were detected in the whole coronary tree. CP presence was strongly associated with a T-cell inflammatory infiltrate. Our results suggest that CP may underlie both coronary and myocardial vulnerabilities in patients who died of AMI and corroborate the notion that CP may act by reducing cardiac reserves, thus worsening the ischemic burden of myocardium.
Acute myocardial infarction (AMI) is, at present, the major cause of death in western countries.1 Despite the use of advanced medical and invasive procedures, the mortality rates are up to 17% in the first year.2 Recognition and treatment of common risk factors cannot fully explain and help to prevent the occurrence of acute coronary syndromes.1 Indeed, multiple pathogenetic components are likely to be involved in these events. Hence, a better understanding of the mechanisms underlying the disease process will be of help in optimizing patient treatment.
Clinicopathological and angiographical observations suggest that acute coronary syndromes do not reflect the vulnerability of a single atherosclerotic plaque but the burden of multiple vulnerable plaques studding the entire coronary tree and associated with its diffuse inflammatory infiltration.3–6 Moreover, we have recently demonstrated that activated T lymphocytes infiltrate the myocardium both in the peri-infarctual area and in remote unaffected myocardial regions in patients who died of a first myocardial infarction.7 The simultaneous occurrence of diffuse coronary and myocardial inflammation in these patients further supports the concept that both coronary and myocardial vulnerabilities concur in the pathogenesis of fatal AMI and suggests a possible common causal agent underlying both conditions. Several autoantigens expressed in the atherosclerotic plaque, including oxidized low-density lipoprotein1,8 and infectious and heat shock proteins (HSPs),9–11 are able to elicit an immune response.
In several epidemiological and experimental studies, Chlamydia pneumoniae (CP), among other infectious agents, has been associated with the risk of ischemic heart disease.12,13 Indeed, CP has been detected within the atherosclerotic lesions by immunohistochemistry, electron microscopy, polymerase chain reaction (PCR), and tissue culture.14–17 In fact, considerable evidence demonstrates the association of CP infection with atherosclerosis.18,19 In the light of these considerations, CP may be a potential agent involved not only in coronary vulnerability but also in myocardial vulnerability. Therefore, the main objective of this study was to investigate whether CP infection occurred beyond the coronary plaques, namely in the myocardium of individuals who died of AMI. To detect the presence of CP cell wall antigen (OMP-2)20 and chlamydial-HSP60 (CP-HSP60), an immunohistochemical study has been performed on coronary plaques and on remote unaffected myocardium. The presence of CP in the myocardium was also assessed by molecular biology techniques and electron microscopy.
Materials and Methods
Patient Population
Two patient groups were prospectively collected, forming the study population: 10 patients who died of AMI (AMI group, six males/four females, mean age 72.1 ± 2.1 years) and 10 age-matched control patients without clinical cardiac history (CTRL group, six males/four females, mean age 72.9 ± 2.5 years) and who died of noncardiac causes (Table 1). In the AMI group, the time lapse between symptom onset and death was less than or equal to 72 hours for all cases. Clinical history and standard electrocardiographic findings defined AMI.
Table 1.
Clinical Data
| Case no. | Age | Sex | Cause of death | IRA | Time* | Risk factor | Underlying morbidities |
|---|---|---|---|---|---|---|---|
| 1 | 74 | F | AMI posterior wall | R | 6 to 24 hours | Hyp, Smoke | Emphysema |
| 2 | 75 | M | AMI lateral wall | LC | 6 to 24 hours | Hyp, Lip | Hydrothorax, benign prostatic hyperplasia |
| 3 | 80 | F | AMI anterior wall | LD | 6 to 24 hours | Hyp | Emphysema |
| 4 | 62 | M | AMI anterior wall | LD | 6 to 24 hours | Diab, Lip | |
| 5 | 59 | M | AMI anterior wall | LD | 6 to 24 hours | Smoke, Lip | Acute gastric erosions, benign prostatic hyperplasia |
| 6 | 75 | M | AMI posterior wall | R | 24 to 72 hours | Hyp, Smoke | Emphysema |
| 7 | 76 | M | AMI lateral wall | LC | 6 to 24 hours | Smoke | Emphysema |
| 8 | 77 | F | AMI anterior wall | LD | 6 to 24 hours | Hyp, Diab | Benign prostatic hyperplasia |
| 9 | 74 | M | AMI lateral wall | LC | 24 to 72 hours | Hyp, Lip | |
| 10 | 69 | F | AMI anterior wall | LD | 6 to 24 hours | Hyp, Smoke | Emphysema |
| 11 | 73 | M | Bronchopneumonia | Hyp, Diab | Heart hypertrophy | ||
| 12 | 81 | F | Cirrhosis | Smoke, Lip | Anemia, acute gastric erosions, emphysema | ||
| 13 | 77 | F | Transmural infarction of the small intestine | Diab, Smoke | Pulmonary infarction | ||
| 14 | 64 | M | Bronchopneumonia | Hyp, Lip | Heart hypertrophy and dilatation | ||
| 15 | 72 | M | Pulmonary embolism | Hyp, Lip | Heart hypertrophy, benign prostatic hyperplasia | ||
| 16 | 55 | M | Severe gastrointestinal bleeding | Lip | Anemia, gastric peptic ulcer | ||
| 17 | 75 | F | Intracranial hemorrhage | Hyp, Smoke | Heart hypertrophy | ||
| 18 | 79 | M | Bronchopneumonia | Hyp, Diab | Heart hypertrophy, emphysema, chronic pyelonephritis, benign prostatic hyperplasia | ||
| 19 | 75 | F | Bronchopneumonia | Smoke | Heart hypertrophy and dilatation, acute gastric erosions | ||
| 20 | 78 | M | Bronchopneumonia | Hyp, Lip | Heart hypertrophy and dilatation |
Period after myocardial infarction.
M, male; F, female; AMI, acute myocardial infarction; LD, left descending coronary artery; R, right coronary artery; LC, left circumflex coronary artery; Hyp, hypertension; Diab, diabetes; Lip, hyperlipidemia.
Patients with chronic inflammatory diseases or tumors were excluded from the study to avoid bias attributable to immunological changes. No differences were observed among the three groups in the distribution of the major risk factors (hypertension, hyperlipidemia, smoke, diabetes). All autopsies were performed within 12 to 24 hours of death. This study was conducted in accordance with the Human Research Committee guidelines of our institution.
Tissue Handling and Processing
The hearts were weighed and fixed in 10% neutral-buffered formalin. The three major epicardial coronary arteries (left anterior descending, left circumflex, and right coronary arteries) were carefully dissected from the heart. Coronary segments from patients who died of AMI were subdivided into two additional groups: infarct-related coronary arteries (IRA) and noninfarct-related coronary arteries (non-IRA).
The following segments of the coronary tree were examined: AMI group: in the IRA coronary the culprit segment, including the thrombotic lesion, 2.5 cm long; in the non-IRA a segment 2.5 cm long, in which maximum stenosis was present; and CTRL group: a segment 2.5 cm long, in which maximum stenosis was present. All coronary segments were cut transversely at 5-mm intervals and decalcified if necessary. A total of 165 arterial segments were embedded in paraffin and serially sectioned: 110 from the AMI group (55 from IRA and 55 from non-IRA coronaries) and 55 from the CTRL group. For histopathological examination, arterial sections were stained with hematoxylin and eosin and Movat’s pentachrome stain.
Myocardial slices were then cut transversely at 1.0-cm intervals from apex to base. The myocardium was macroscopically examined to detect the presence and extent of the infarcted area. In all cases, at least one complete transverse heart slice was processed for histopathological examination and the infarction confirmed by light microscopy. We selected for study only those cases affected by infarct in a single coronary artery distribution and in which acute infarct region was correlated with the coronary artery containing the thrombus.
Histopathological and Morphometric Studies
Plaques were classified into three categories, according to the recent consensus document of the American Heart Association:21 1) plaque “culprit,” characterized by the presence of an acute thrombus associated with plaque rupture or plaque erosion; 2) “vulnerable” plaques, including a) “thin fibrous cap atheromata,” characterized by a lesion composed of a lipid-rich core covered by a less than 65-μm-thick fibrous cap containing many lipid-laden macrophage foam cells (>25 per high-power magnification),22 b) plaques with >90% stenosis, and c) superficial calcified nodules. 3) The remaining plaques were classified as “stable plaques.”
Immunohistochemical Stains for Conventional Microscopy
An immunohistochemical study was performed in the unaffected regions of myocardium and coronaries of both experimental groups to evaluate the presence of CP, CP-HSP60, and the inflammatory infiltrate. The presence of CP cell wall antigen, namely OMP-2, was evaluated through an antibody synthesized by the Department of Infectious, Parasitic, and Immune-Mediated Diseases of Istituto Superiore di Sanità (Rome, Italy) and produced from purified recombinant proteins (R-proteins) OMP-2 in mice, as previously described.20 Another commercial antibody directed against the major outer membrane protein of CP (DakoCytomation Ltd., Glostrup, Denmark) was also used.
On the same serial sections where the CP antigens were detected, an immunohistochemical study was performed to evaluate the presence of CP-HSP60 (clone A57-B9; ABR, Golden, CO), the inflammatory infiltrate and their activation status, using the following primary monoclonal antibodies: CD68 (human macrophage; DakoCytomation Ltd.), CD3 (human T cells; DakoCytomation Ltd.), HLA-DR (DakoCytomation Ltd.), and interferon (IFN)-γ (R&D Systems, Minneapolis, MN). The number of positive cells for each antibody was counted at ×40 magnification both in the plaque shoulder region and in the cap region. A mean of 10 randomly selected fields per section was counted.
Multiple Immunostaining for Confocal Microscopy
To study the co-localization of CP cell wall antigen in cardiomyocytes (sarcomeric actin-positive cells) and inflammatory cells, namely T lymphocytes (CD3-positive cells) or macrophages (CD68-positive cells), triple immunostainings were performed. The sections were first incubated with CP cell wall antigen and the fluorescent visualization was obtained with a streptavidin-fluorescein conjugate. After this first reaction, a second reaction with a sarcomeric actin primary antibody (clone α-Sr-1; DAKO, Glostrup, Denmark) revealed with streptavidin-Texas Red fluorescent conjugate was performed. To investigate the CP co-localization with inflammatory cells, a further reaction was performed alternatively with CD68 primary antibody and with CD3 primary antibody, revealed with streptavidin-coumarin fluorescent conjugate.
Furthermore, to assess the presence of dendritic cells and their CP infection in the myocardial interstitium, a quadruple immunostain was performed. In particular, the sarcomeric actin was revealed with Texas Red, CP OMP-2 antigens with a streptavidin fluorescent conjugate, HLA-DR with coumarin and S-100 (DAKO) with Q-Dot 605 streptavidin Tebu-Bio. Myocardial sections were observed by means of conventional fluorescent microscope and confocal microscope. The images were acquired by means of Noran confocal microscope at 60×/1.4 NA immersion oil lens. Three-dimensional stacks were acquired at a resolution of 0.1 μm in x, y, and z axes.
Ultrastructural Study
An ultrastructural study was performed in the unaffected regions of myocardium to investigate CP presence. Myocardial specimens were fixed in 0.1 mol/L phosphate-buffered Karnovsky’s fixative. Tissue samples were postfixed in 1% phosphate-buffered osmium and embedded in epoxy resin (Epon 812; Electron Microscopy Sciences, Hatfield, PA). Ultrathin sections were examined by means of Hitachi H-7100FA electron microscope (Hitachi Software Engineering, Yokohama, Japan).
PCR Analysis
DNA was extracted from sections of paraffin-embedded tissues using the QIAamp DNA mini kit (Qiagen GmbH, Hilden, Germany). Chlamydia touchdown enzyme time release-PCR was performed using a set of primers specific for CP as described by Madico and colleagues.23 The sequences are as follows: CPN 90, 5′-GGTCTCAACCCCATCCGTGTCGG-3′; and CPN 91, 5′-TGCGGAAAGCTGTATTTCTACAGTT-3′.
The total volume of PCR mixture was 50 μl and contained 5 μl of DNA, 100 pmol of each primer, 0.25 mmol/L deoxynucleosides triphosphates (dNTPs), 1 mmol/L MgCl2, PCR buffer, and 2.5 U of Taq polymerase (Qiagen). Cycling times were 5 minutes at 94°C, followed by 60 cycles of denaturation at 94°C for 30 seconds, annealing beginning at 62°C and ending at 52°C for 45 seconds, and extension at 72°C for 30 seconds. The annealing temperature was lowered by 1°C every four cycles until it reached 52°C, and this annealing temperature was kept until the end of the cycling process. PCR products (20 μl) were separated by electrophoresis in a 2.5% agarose gel to detect the 197-bp product.
CP presence was also assessed by a seminested PCR similar to the one described by Skowasch and colleagues.24 The primers used for the first PCR were as follows: hsp1s, 5′-GTTGTTCATGAAGGCCTACT-3′; hsp1AS, 5′-TGCATAACCTACGGTGTGTT-3′. The primers used for the second PCR were hsp1s and hsp2AS, 5′-TTTATTTCCGTGTCGTCCAG-3′. The total volume of the first PCR mixture was 50 μl and contained 5 μl of DNA, 100 pmol of each primer, 0.25 mmol/L deoxynucleosides triphosphates (dNTPs), 6.7 mmol/L MgCl2, PCR buffer, and 2.5 U of Taq Gold polymerase (Perkin Elmer, Norwalk, CT). The reaction was hot-started at 95°C for 10 minutes before the addition of the Taq polymerase and was followed by 45 cycles of denaturation at 95°C for 45 seconds, annealing at 54.5°C for 1 minute, and extension at 72°C for 30 seconds followed by a final 7-minute extension at 72°C. An aliquot (3 μl) of reaction mixture of the first PCR was used for the second amplification. The total volume of the second PCR mixture was 50 μl and was identical to the first PCR. PCR products of the second amplification (25 μl) were separated by electrophoresis in a 2.5% agarose gel.
Nested PCRs were performed for the detection of Helicobacter pylori (HP) and cytomegalovirus (CMV) according to the protocol described by Rassu and colleagues.25 The two sets of primers for HP identify the urease gene of HP DNA. The target of amplification for CMV was located within the morphological transforming region II (mtr II) of the CMV genome. PCR products of the second amplification (20 μl) were separated by electrophoresis in a 2.5% agarose gel.
Statistical Analysis
Statistical analysis of the data were performed with SPSS for Windows (version 11.0; SPSS, Chicago, IL). An unpaired t-test was used to assess intergroup differences for continuous variables. The χ2 test was used to establish differences in the distribution between categorical variables. A P value of <0.05 was considered statistically significant.
Results
Myocardium
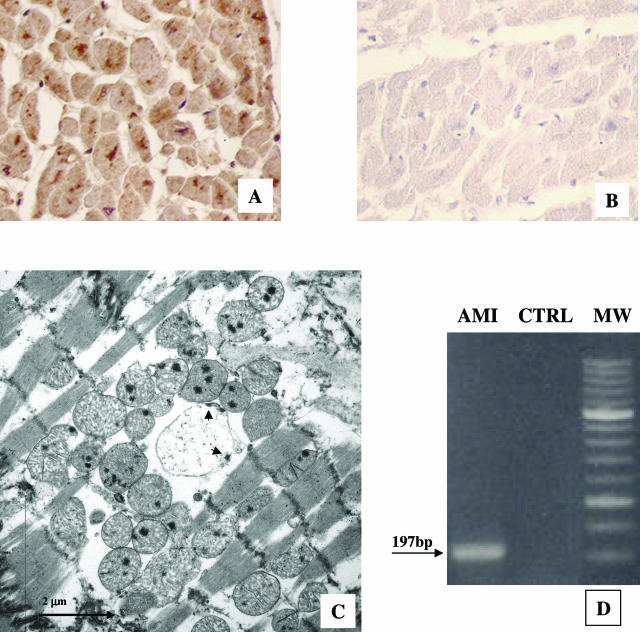
The presence of OMP-2 antigen was demonstrated by immunohistochemistry in the unaffected myocardium, where signs of ongoing or very recent necrotic cell death were absent, in 9 of 10 AMI patients (Figure 1A). Conversely, only 1 of 10 control patients presented a positive staining for CP (Figure 1B). CP formed immunoreactive aggregates within most cardiomyocytes, sometimes restricted to the perinuclear region (Figure 1A). These aggregates showed at electron microscopy the morphology of abnormal reticulate bodies (RBs)26 (Figure 1C) and were characterized by a membrane-bound vesicle, larger than normal RBs, displaying multiple electron-dense sites, possibly representing nucleoid condensation, near the CP cell wall. The ultrastructural study did not provide evidence of necrosis or apoptosis in the infected cardiomyocytes.
Figure 1.
Evidence of C. pneumoniae (CP) in the cytoplasm of myocardiocytes through different converging methods: A: Unaffected myocardium of a patient who died of AMI: immunohistochemistry shows the presence of immunoreactive aggregates constituted by CP cell wall antigen (namely OMP-2), within the cytoplasm, sometimes in the perinuclear region. B: Myocardium of a patient without clinical cardiac history who died of noncardiac causes. No CP cell wall antigen was observed in the cytoplasm of myocardiocytes by immunohistochemistry. C: Transmission electron micrograph of CP within a myocardiocyte of a patient who died of AMI: large abnormal reticulate body, surrounded by a number of mitochondria, displaying multiple electron dense sites, possibly representing nucleoid condensation near the CP cell wall (arrowheads). D: Myocardial CP detection by PCR: CP was detected by myocardial DNA amplification from AMI patients and CTRL group. The expected size of the amplified product was 197 bp as shown by the arrow. MW: 50-bp molecular weight size marker. Original magnifications, ×20 (A, B).
CP infection of unaffected myocardium was also confirmed by the PCR technique using two different nested methods for detection (Figure 1D), demonstrating the presence of CP in the nine CP-positive AMI patients at immunohistochemistry. In all cases a diffuse positivity of myocardium for CP-HSP60 antibody was observed. The immunoreactivity pattern was comparable with that of human-HSP60. Nested PCR also detected HP DNA in the unaffected myocardium of 1 in 10 AMI patients and CMV DNA in one CTRL myocardium.
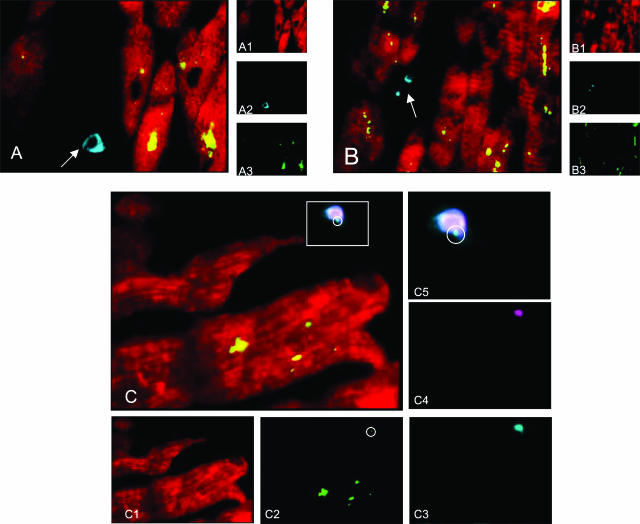
In AMI patients, a variable number of activated T lymphocytes (CD3+/DR+) was found in remote unaffected myocardial regions, in the perivascular compartment as well as in the interstitium, whereas none was found in the control hearts. In situ multiple immunostaining, examined by confocal microscopy, revealed that CP antigens co-localized mainly with cardiomyocytes (Figure 2, A and B). The inflammatory cells (macrophages or T lymphocytes) did not co-localize with CP antigens (Figure 2, A and B). Co-localization of S-100 and HLA-DR reaction demonstrated the presence of few dendritic cells scattered in the interstitium of unaffected myocardium in AMI patients (Figure 2C). Quadruple immunostain revealed the presence of CP antigens in some of the dendritic cells we had observed. HLA-DR reaction was diffusely negative in cardiomyocytes.
Figure 2.
Confocal microscopic examination of the unaffected myocardium of an AMI patient (two-dimensional reconstruction with pseudocolors). A: Triple immunostain for cardiomyocytes (sarcomeric actin, Texas Red; A1), macrophages (CD 68, cumarin; A2), and CP OMP-2 antigens (FITC; A3). Cardiomyocytes observed gave rise a strong positive reaction for CP OMP-2 in the cytoplasm (double positivity, appears as yellow stain), whereas the rare macrophages scattered among cardiomyocytes are negative for CP antigen (arrow). B: Triple immunostain for cardiomyocytes (sarcomeric actin, Texas Red; B1), T lymphocytes (CD3, cumarin; B2), and CP OMP-2 antigens (FITC; B3). Two-dimensional reconstruction shows that T lymphocytes are not infected by CP (arrow). C: Quadruple immunostain for cardiomyocytes (sarcomeric actin, Texas Red; C1), CP OMP-2 antigens (FITC; C2), HLA-DR (cumarin; C3), S-100 (Q-Dot 605 streptavidin Tebu-Bio; C4). Co-localization of S-100 and HLA-DR reaction in the two-dimensional reconstruction demonstrated the presence of few dendritic cells (double positivity revealed by light blue-white stain) some of which were positive also for CP OMP-2 antigens (pale green dot in the circle). HLA-DR reaction was diffusely negative in cardiomyocytes. C5: Magnification of a dendritic cell observed in C.
Coronary Plaques
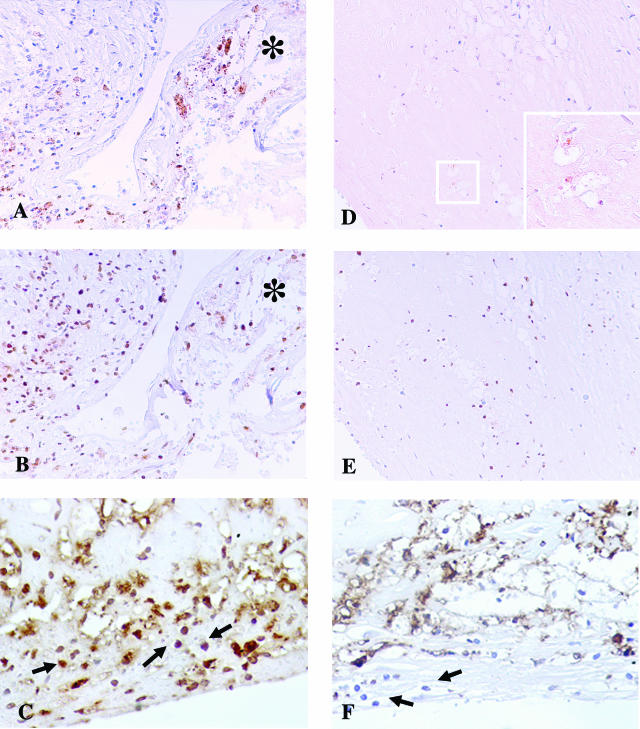
CP OMP-2 antigen was detectable in the coronary plaques of all 10 AMI patients (Figure 3A). In particular, 60 of the 110 coronary segments examined (54.5%), at least one for each patient, showed positive reaction for OMP-2 antigen (Table 2). As for the CTRL group, the positive staining for CP antigens was detected only in 3 of 10 patients, namely in 7 of the 55 coronary segments examined (12.7%) (P = 0.0001). In all cases, the positive staining for CP was associated with positivity for CP-HSP60. In AMI coronaries, the immunoreactivity for CP OMP-2 and CP-HSP60 was unrelated to the plaque type, both in infarct- and noninfarct-related coronary arteries (Table 2). A severe and diffuse inflammation of the coronary tree was observed in patients who died of AMI compared with the CTRL group (121.6 ± 12.4 cell × mm2 versus 37.3 ± 11.9 cell × mm2; P = 0.001). Moreover, in coronaries from AMI patients, immunohistochemical analysis showed the presence of numerous HLA-DR- and IFN-γ-positive T lymphocytes indicating an activated state (Figure 3C), whereas fewer activated lymphocytes were in the CTRL group (Figure 3F).
Figure 3.
Immunohistochemistry evidence of the presence of C. pneumoniae (CP) antigens within coronaries. A–C: Coronary artery of a patient who died of AMI. A strong immunoreactivity to OMP-2 antigens of CP (A) and to CP-HSP60 (B) was detectable in correspondence of the cap rupture. In AMI coronaries, immunohistochemical analysis showed also the presence of numerous inflammatory cells, constituted by macrophagic foam cells and T lymphocytes (arrow), positive for IFN-γ (C), indicating an activated state and a Th1-mediated immune response. *The flap of disrupted cap. D–F: Coronary artery of a patient without clinical cardiac history who died of noncardiac causes. D: A weak positive staining for OMP-2 antigens was detected only in few macrophagic cells. Inset: High-power magnification of the squared area. E: Weaker CP-HSP60 staining compared with AMI coronary plaques. The immunostaining to IFN-γ (F) was almost negative in the few infiltrating lymphocytes (arrow). Original magnifications: ×20 (A–E); ×60 (D, inset).
Table 2.
Immunohistochemical Results
| AMI group | Control group | P | |
|---|---|---|---|
| CP-OMP-2 positivity | |||
| In the unaffected myocardium: | |||
| Cases (no., %) | 9/10 (90%) | 1/10 (30%) | 0.0003 |
| In the coronary tree: | |||
| Cases (no., %) | 10/10 (100%) | 3/10 (30%) | 0.001 |
| Coronary segments (no., %) | 60/110 (54.5%) | 7/55 (12.7%) | 0.0001 |
| Ruptured and vulnerable | |||
| Plaques (no., %) | 15/28 (53.6%) | 0/4 | 0.04 |
| Stable plaques (no., %) | 45/82 (54.9%) | 5/51 (9.4%) | 0.0001 |
| CP-HSP-60 positivity in the coronary tree | |||
| Cases (no., %) | 10/10 (100%) | 6/10 (60%) | 0.02 |
| Coronary segments (no., %) | 79/110 (71.8%) | 8/55 (14.6%) | 0.0001 |
| Plaque inflammation* (mean ± SEM) | 121.6 ± 12.4 | 37.3 ± 11.9 | 0.001 |
CD68- and CD3-positive cells × mm2.
Discussion
Although confirming previous studies on CP presence in vascular tissues of patients affected by coronary atherosclerosis,14,16 this report provides, for the first time, substantial evidence, obtained through different and converging methods, that CP is primarily present in the myocardium of patients who died of AMI (Figures 1 and 2). Moreover, OMP-2 and CP-HSP60 were present in the whole coronary tree, well beyond the culprit lesion and the infarct related artery (Figure 3). Finally, CP presence was strongly associated with a diffuse inflammatory infiltrate consisting of activated T cells, more numerous in the entire coronary tree than in the myocardium.
Studies of CP pathogenesis have shown that this organism can infect several human cytotypes involved in the formation of the atherosclerotic plaques such as macrophages, endothelial cells, and smooth muscle cells.17,27,28 Besides, CP may act as a proatherosclerotic agent, increasing both lipidic peroxidation and local prothrombotic status, modulating the tissue factor expression and the platelet cell adhesion to endothelial cells.29
CP-HSP60 seems to play a key role in the CP infection injury.16 Owing to the molecular mimicry between chlamydial and human HSP epitopes, it is possible that autoimmune and cross-reactive immune responses to HSPs may be elicited. Moreover, under conditions such as the presence of IFN-γ, a cytokine secreted by activated T cells and macrophages within atheroma, CP can achieve a state of intracellular chronic persistent infection. In this state, CP remains viable but metabolically quiescent and does not replicate.27 During such a chronic persistent infection, HSP60 production increases substantially, whereas the major outer membrane protein becomes almost undetectable.27 Biasucci and colleagues30 demonstrated the correlation between high title of antibodies to CP-HSP60 and occurrence of an acute coronary event. High levels of CP-HSP60 were present in 99% of patients with acute coronary syndrome and in 20% of patients with stable angina whereas it was totally lacking in patients of the control group.30 Moreover, Benagiano and colleagues31 have described an infiltration of CP-specific T-cell clones directed against both CP and human HSP60, within atheromatous plaques of the human carotid. It is noteworthy that almost all of these T cells were activated T-helper cells expressing inflammatory Th1 cytokines, suggesting their critical role in the inflammation-dependent plaque destabilization.31,32
Besides the serological and morphological evidence of an association between CP and IMA,10,12,13,30 throughout the last years many pathogenic agents, such as cytomegalovirus33,34 and Helicobacter pylori,35 potentially correlated with acute coronary syndromes, have been identified in the coronary plaques. Ott and colleagues36 recently demonstrated the presence of diverse bacterial signature in atherosclerotic lesions of patients with coronary heart disease. These observations further support the hypothesis that, apart from the etiological agent, modifications of the adaptive immune response elicited by infectious antigens may have a crucial role in coronary vulnerability. However, independently of the pathogenesis of plaque vulnerability and rupture, our data, although observed in a small group of patients, allow us to hypothesize a role of CP in the outcome of AMI.
Previous experimental and human studies have demonstrated the persistence of CP only in the myocardium of patients with CP-induced myocarditis.18,19 Cardiac myosin-induced autoimmune myocarditis is dependent on T cells that recognize a heart muscle-specific peptide.18 Experimental data provide evidence, in vivo as well as in vitro, of molecular mimicry between CP antigens and heart-specific proteins and indicate that bacterial peptides can trigger tissue-specific inflammation of the heart.18,19 Like the inflammation that follows immunization by the endogenous autoantigen derived from α-myosin heavy chain, such as MTA-α, the disease induced by CP-derived peptides is characterized by perivascular and pericardial infiltration of mononuclear cells.19 These data further support the existence of an antigenic mimicry between the endogenous myosin epitopes and CP epitopes that is probably strongly connected with inflammatory heart disease.18 Infections by CP that carry peptides mimicking endogenous heart-specific and heart-pathogenic epitopes lead to activation of autoaggressive lymphocytes. It is relevant that the OMP-2 antigen is CP-specific, and to our knowledge, no human homologous counterpart is present in the heart.20
In our cases, only few activated T lymphocytes have been found in the remote unaffected myocardium (CP-positive), and their presence was not accompanied by signs of apoptosis or ongoing or recent necrotic cell death, as described in CP-induced myocarditis. The absence of signs of myocarditis in the unaffected myocardium has been demonstrated by the ultrastructural study and HLA-DR-negative reaction in cardiomyocytes. In fact previous studies showed that expression of HLA-DR antigens is up-regulated in acute myocarditis and represents a helpful tool to distinguish between inflammatory myocardial disease and noninflammatory disease in patients with congestive heart failure.37,38 Nevertheless, our morphological study does not allow us to assess whether CP directly infects cardiomyocytes or if the infection is mediated by inflammatory cells (infected macrophages and/or T lymphocytes) trafficking through the myocardium.
A condition quite similar to our observations has been reported by Wang and colleagues,39 who described the CP-induced damage of cardiomyocytes isolated from neonatal rats. The infected myocytes contained elementary bodies of CP (ie, the infectious form of the organism). In this condition CP induces damage of myocytes through an increased of release lactate dehydrogenase and production of reactive oxygen species (ROS). However, this organism did not determine nuclear apoptosis of damaged myocytes.
The immune response observed in AMI patients, differently from that of myocarditis, could be attributable to the persistent infection state of CP. Indeed, through electron microscopy studies, we have shown that in AMI patients CP has the morphology of abnormal RBs, corresponding to a nonreproductive persistent infection (Figure 1C). This condition is characterized by a low grade of aggressiveness but also by a highly immunostimulatory and proinflammatory state, according to Hammerschlag.27 There is evidence that CP induces the release of ROS as well of cytokines and adhesion molecules even at this stage.40 Therefore, we could assume that CP may elicit an increase in the intracellular ROS level in the infected cardiomyocytes distant from the infarction. Even if this release does not induce morphological alterations, it could determine a functional damage of ROS with a reduction in cardiac reserve. Although their role in the pathogenesis of clinical heart failure remains unclear, ROS have been implicated in most processes thought to have a significant effect on cardiac function, including hypertrophy, ion flux and calcium handling, extracellular matrix configuration, vasomotor function, metabolism, gene expression, and downstream signaling of several growth factors and cytokines.41
The ability of determining persistent infections is one of the major characteristics of CP and has been implicated in the pathogenesis of many chronic diseases apart from atherosclerosis, including pelvic inflammatory disease, arthritis, and asthma.27 Moreover, during the persistent state, the metabolic activity of the infectious organism is reduced, and it is often refractory to antibiotic treatment.27 This circumstance could explain, at least partially, the variable results obtained in recent clinical trials using the azithromycin or gatifloxacin treatment for secondary prevention of coronary events.42,43 As reported by the same authors, the Azithromycin and Coronary Events Study had several limitations, including the advanced stage of disease, the possibility that the antibiotic does not reach the microorganism in the chronic lesions of atherosclerosis, the possibility that the treatment was not continued long enough or that the dose of antibiotic given was too small, and the possibility that the antibiotic was not sufficiently effective against CP.42 Moreover, Gieffers and colleagues44 found that CP infection in circulating monocytes from patients who had been treated for coronary heart disease was refractory to azithromycin: antibiotic treatment did not inhibit CP growth within monocytes, and after withdrawal of antibiotic therapy, CP could be cultured from monocyte cell lines. This case, as well as ours, dealt with CP persistent infection. Finally, the association of CP infections with mature or advanced lesions raises the possibility that atherosclerotic plaques may secondarily form functional biofilms.45 Therefore, according to Anderson,46 despite the negative results of the recent trials, it is not right to elicit that CP infection may not be anymore considered as the causal agent of coronary atherothrombosis.
The achieved results have demonstrated the presence, in one AMI case, of HP-specific DNA sequences and, in one control case, of CMV DNA. As for the HP-positive case, a double infection can be assumed, as demonstrated recently in coronaries by Ott and colleagues,36 even if it is not possible to exclude contamination during autopsy. With regard to the CMV-positive control case, it must be stressed that the histological examination did not reveal signs of CMV myocarditis. The possibility to find a viral genome in the myocardium in the absence of myocarditis has been recently demonstrated by Kühl and colleagues47 in a study on endomyocardial biopsies of patients with left ventricular dysfunction.
In conclusion, our results suggest that CP may underlie both coronary and myocardial vulnerabilities in patients who died of AMI. The presence of activated T cells and dendritic cells suggests that CP may trigger an immune response that elicits a widespread active inflammation of coronaries and unaffected myocardium. On the other hand, the ROS produced by CP reticular bodies could induce myocardial dysfunction. Together with either of these hypotheses, our observations corroborate the notion that CP may act by reducing cardiac reserve, thus worsening the ischemic burden of myocardium.
Acknowledgments
We thank Antonio Volpe and Alfredo Colantoni for their helpful technical assistance and Prof. Franco Pandolfi for the manuscript revision.
Footnotes
Address reprint requests to Prof. Luigi Giusto Spagnoli, Cattedra di Anatomia ed Istologia Patologica, Dipartimento di Biopatologia e Diagnostica per Immagini, Università di Roma “Tor Vergata,” Via Montpellier 1, 00133 Roma (Italy). E-mail: spagnoli@uniroma2.it.
Supported by Ministero dell’Università e della Ricerca Scientifica e Tecnologica (COFIN 2004 research project) and the Istituto Superiore di Sanità.
References
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Rochon PA, Tu JV, Anderson GM, Gurwitz JH, Clark JP, Lau P, Szalai JP, Sykora K, Naylor CD. Rate of heart failure and 1-year survival for older people receiving low-dose beta-blocker therapy after myocardial infarction. Lancet. 2000;356:639–644. doi: 10.1016/S0140-6736(00)02606-4. [DOI] [PubMed] [Google Scholar]
- Mauriello A, Sangiorgi G, Fratoni S, Palmieri G, Bonanno E, Anemona L, Schwartz RS, Spagnoli LG. Diffuse and active inflammation occurs in both vulnerable and stable plaques of the entire coronary tree a histopathologic study of patients dying of acute myocardial infarction. J Am Coll Cardiol. 2005;45:1585–1593. doi: 10.1016/j.jacc.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Goldstein JA, Demetriou D, Grines CL, Pica M, Shoukfeh M, O’Neill WW. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2000;343:915–922. doi: 10.1056/NEJM200009283431303. [DOI] [PubMed] [Google Scholar]
- Spagnoli LG, Bonanno E, Mauriello A, Palmieri G, Partenzi A, Sangiorgi G, Crea F. Multicentric inflammation in epicardial coronary arteries of patients dying of acute myocardial infarction. J Am Coll Cardiol. 2002;40:1579–1588. doi: 10.1016/s0735-1097(02)02376-8. [DOI] [PubMed] [Google Scholar]
- Libby P. Act local, act global: inflammation and the multiplicity of “vulnerable” coronary plaques. J Am Coll Cardiol. 2005;45:1600–1602. doi: 10.1016/j.jacc.2005.02.058. [DOI] [PubMed] [Google Scholar]
- Abbate A, Bonanno E, Mauriello A, Bussani R, Biondi-Zoccai GG, Liuzzo G, Leone AM, Silvestri F, Dobrina A, Baldi F, Pandolfi F, Biasucci LM, Baldi A, Spagnoli LG, Crea F. Widespread myocardial inflammation and infarct-related artery patency. Circulation. 2004;110:46–50. doi: 10.1161/01.CIR.0000133316.92316.81. [DOI] [PubMed] [Google Scholar]
- Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kol A, Bourcier T, Lichtman AH, Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Invest. 1999;103:571–577. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer OJ, van der Wal AC, Becker AE. Atherosclerosis, inflammation, and infection. J Pathol. 2000;190:237–243. doi: 10.1002/(SICI)1096-9896(200002)190:3<237::AID-PATH541>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Mayr M, Metzler B, Kiechl S, Willeit J, Schett G, Xu Q, Wick G. Endothelial cytotoxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation. 1999;99:1560–1566. doi: 10.1161/01.cir.99.12.1560. [DOI] [PubMed] [Google Scholar]
- Saikku P, Leinonen M, Mattila K, Ekman MR, Nieminen MS, Makela PH, Huttunen JK, Valtonen V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;2:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- Kalayoglu MV, Libby P, Byrne GI. Chlamydia pneumoniae as an emerging risk factor in cardiovascular disease. JAMA. 2002;288:2724–2731. doi: 10.1001/jama.288.21.2724. [DOI] [PubMed] [Google Scholar]
- Kuo CC, Shor A, Campbell LA, Fukushi H, Patton DL, Grayston JT. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993;167:841–849. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- Jackson LA, Campbell LA, Kuo CC, Rodriguez DI, Lee A, Grayston JT. Isolation of Chlamydia pneumoniae from a carotid endarterectomy specimen. J Infect Dis. 1997;176:292–295. doi: 10.1086/517270. [DOI] [PubMed] [Google Scholar]
- Kol A, Sukhova GK, Lichtman AH, Libby P. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation. 1998;98:300–307. doi: 10.1161/01.cir.98.4.300. [DOI] [PubMed] [Google Scholar]
- Boman J, Hammerschlag MR. Chlamydia pneumoniae and atherosclerosis: critical assessment of diagnostic methods and relevance to treatment studies. Clin Microbiol Rev. 2002;15:1–20. doi: 10.1128/CMR.15.1.1-20.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmaier K, Neu N, de la Maza LM, Pal S, Hessel A, Penninger JM. Chlamydia infections and heart disease linked through antigenic mimicry. Science. 1999;283:1335–1339. doi: 10.1126/science.283.5406.1335. [DOI] [PubMed] [Google Scholar]
- Penninger JM, Bachmaier K. Review of microbial infections and the immune response to cardiac antigens. J Infect Dis. 2000;181(Suppl 3):S498–S504. doi: 10.1086/315613. [DOI] [PubMed] [Google Scholar]
- Ciervo A, Visca P, Petrucca A, Biasucci LM, Maseri A, Cassone A. Antibodies to 60-kilodalton heat shock protein and outer membrane protein 2 of Chlamydia pneumoniae in patients with coronary heart disease. Clin Diagn Lab Immunol. 2002;9:66–74. doi: 10.1128/CDLI.9.1.66-74.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- Madico G, Quinn TC, Boman J, Gaydos CA. Touchdown enzyme time release-PCR for detection and identification of Chlamydia trachomatis, C. pneumoniae, and C psittaci using the 16S and 16S–23S spacer rRNA genes. J Clin Microbiol. 2000;38:1085–1093. doi: 10.1128/jcm.38.3.1085-1093.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowasch D, Yeghiazaryan K, Schrempf S, Golubnitschaja O, Welsch U, Preusse CJ, Likungu JA, Welz A, Luderitz B, Bauriedel G. Persistence of Chlamydia pneumoniae in degenerative aortic valve stenosis indicated by heat shock protein 60 homologues. J Heart Valve Dis. 2003;12:68–75. [PubMed] [Google Scholar]
- Rassu M, Cazzavillan S, Scagnelli M, Peron A, Bevilacqua PA, Facco M, Bertoloni G, Lauro FM, Zambello R, Bonoldi E. Demonstration of Chlamydia pneumoniae in atherosclerotic arteries from various vascular regions. Atherosclerosis. 2001;158:73–79. doi: 10.1016/s0021-9150(01)00411-7. [DOI] [PubMed] [Google Scholar]
- Wolf K, Fischer E, Hackstadt T. Ultrastructural analysis of developmental events in Chlamydia pneumoniae-infected cells. Infect Immun. 2000;68:2379–2385. doi: 10.1128/iai.68.4.2379-2385.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschlag MR. The intracellular life of chlamydiae. Semin Pediatr Infect Dis. 2002;13:239–248. doi: 10.1053/spid.2002.127201. [DOI] [PubMed] [Google Scholar]
- Gaydos CA, Summersgill JT, Sahney NN, Ramirez JA, Quinn TC. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect Immun. 1996;64:1614–1620. doi: 10.1128/iai.64.5.1614-1620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer RH, Schwobe EP, Woods ML, Rodgers GM. Chlamydia species infect human vascular endothelial cells and induce procoagulant activity. J Invest Med. 1997;45:168–174. [PubMed] [Google Scholar]
- Biasucci LM, Liuzzo G, Ciervo A, Petrucca A, Piro M, Angiolillo DJ, Crea F, Cassone A, Maseri A. Antibody response to chlamydial heat shock protein 60 is strongly associated with acute coronary syndromes. Circulation. 2003;107:3015–3017. doi: 10.1161/01.CIR.0000078632.76653.6C. [DOI] [PubMed] [Google Scholar]
- Benagiano M, Azzurri A, Ciervo A, Amedei A, Tamburini C, Ferrari M, Telford JL, Baldari CT, Romagnani S, Cassone A, D’Elios MM, Del Prete G. T helper type 1 lymphocytes drive inflammation in human atherosclerotic lesions. Proc Natl Acad Sci USA. 2003;100:6658–6663. doi: 10.1073/pnas.1135726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benagiano M, D’Elios MM, Amedei A, Azzurri A, van der Zee R, Ciervo A, Rombola G, Romagnani S, Cassone A, Del Prete G. Human 60-kDa heat shock protein is a target autoantigen of T cells derived from atherosclerotic plaques. J Immunol. 2005;174:6509–6517. doi: 10.4049/jimmunol.174.10.6509. [DOI] [PubMed] [Google Scholar]
- Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? Lancet. 1997;350:430–436. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- Stassen FR, Vega-Cordova X, Vliegen I, Bruggeman CA. Immune activation following cytomegalovirus infection: more important than direct viral effects in cardiovascular disease? J Clin Virol. 2006;35:349–353. doi: 10.1016/j.jcv.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Danesh J, Peto R. Risk factors for coronary heart disease and infection with Helicobacter pylori: meta-analysis of 18 studies. BMJ. 1998;316:1130–1132. doi: 10.1136/bmj.316.7138.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott SJ, El Mokhtari NE, Musfeldt M, Hellmig S, Freitag S, Rehman A, Kuhbacher T, Nikolaus S, Namsolleck P, Blaut M, Hampe J, Sahly H, Reinecke A, Haake N, Gunther R, Kruger D, Lins M, Herrmann G, Folsch UR, Simon R, Schreiber S. Detection of diverse bacterial signatures in atherosclerotic lesions of patients with coronary heart disease. Circulation. 2006;113:929–937. doi: 10.1161/CIRCULATIONAHA.105.579979. [DOI] [PubMed] [Google Scholar]
- Herskowitz A, Ahmed-Ansari A, Neumann DA, Beschorner WE, Rose NR, Soule LM, Burek CL, Sell KW, Baughman KL. Induction of major histocompatibility complex antigens within the myocardium of patients with active myocarditis: a nonhistologic marker of myocarditis. J Am Coll Cardiol. 1990;15:624–632. doi: 10.1016/0735-1097(90)90637-5. [DOI] [PubMed] [Google Scholar]
- Wojnicz R, Nowalany-Kozielska E, Wodniecki J, Szczurek-Katanski K, Nozynski J, Zembala M, Rozek MM. Immunohistological diagnosis of myocarditis. Potential role of sarcolemmal induction of the MHC and ICAM-1 in the detection of autoimmune mediated myocyte injury. Eur Heart J. 1998;19:1564–1572. doi: 10.1053/euhj.1998.1085. [DOI] [PubMed] [Google Scholar]
- Wang G, Burczynski F, Hasinoff B, Zhong G. Infection of myocytes with chlamydiae. Microbiology. 2002;148:3955–3959. doi: 10.1099/00221287-148-12-3955. [DOI] [PubMed] [Google Scholar]
- Redecke V, Dalhoff K, Bohnet S, Braun J, Maass M. Interaction of Chlamydia pneumoniae and human alveolar macrophages: infection and inflammatory response. Am J Respir Cell Mol Biol. 1998;19:721–727. doi: 10.1165/ajrcmb.19.5.3072. [DOI] [PubMed] [Google Scholar]
- Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayston JT, Kronmal RA, Jackson LA, Parisi AF, Muhlestein JB, Cohen JD, Rogers WJ, Crouse JR, Borrowdale SL, Schron E, Knirsch C. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637–1645. doi: 10.1056/NEJMoa043526. [DOI] [PubMed] [Google Scholar]
- Cannon CP, Braunwald E, McCabe CH, Grayston JT, Muhlestein B, Giugliano RP, Cairns R, Skene AM. Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N Engl J Med. 2005;352:1646–1654. doi: 10.1056/NEJMoa043528. [DOI] [PubMed] [Google Scholar]
- Gieffers J, Fullgraf H, Jahn J, Klinger M, Dalhoff K, Katus HA, Solbach W, Maass M. Chlamydia pneumoniae infection in circulating human monocytes is refractory to antibiotic treatment. Circulation. 2001;103:351–356. doi: 10.1161/01.cir.103.3.351. [DOI] [PubMed] [Google Scholar]
- Katz JT, Shannon RP. Bacteria and coronary atheroma: more fingerprints but no smoking gun. Circulation. 2006;113:920–922. doi: 10.1161/CIRCULATIONAHA.105.607358. [DOI] [PubMed] [Google Scholar]
- Anderson JL. Infection, antibiotics, and atherothrombosis—end of the road or new beginnings? N Engl J Med. 2005;352:1706–1709. doi: 10.1056/NEJMe058019. [DOI] [PubMed] [Google Scholar]
- Kühl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, Poller W, Schultheiss HP. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112:1965–1970. doi: 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]