Abstract
Organ infiltration by T cells depends on the adhesion molecules expressed in these sites and on homing receptors expressed by the T cells. Here, we have studied which form of priming can enable T cells to home to pancreatic islets. To this end, we have used transgenic mice expressing the model autoantigen ovalbumin in pancreatic islets and transgenic ovalbumin-specific CD4 and CD8 T cells. We demonstrate that these T cells were imprinted with homing receptor patterns characteristic for the site of priming, such as α4β7 integrin for mucosal antigen delivery or functionally active α4β1 integrin for islet autoantigens. The adhesion molecules corresponding to these receptors were found to be constitutively expressed in islets, enabling T cells bearing these receptors to infiltrate the islets and to cause diabetes. Disease was prevented only by blockade of the endothelial adhesion molecule, ligand of homing receptors with which the T cells were imprinted. Thus, different priming locations induced different homing mechanisms, allowing T cells to target the islets. This may contribute to the susceptibility of islets to T-cell-mediated attack. Furthermore, it may pertain to the design of adhesion-modulating therapies alone or in combination with external autoantigen administration.
Circulating T cells use homing receptors to interact with adhesion molecules on vascular endothelium and to egress from vasculature. These homing receptors are acquired during priming in secondary lymphatics1–3 to help guide T cells preferentially into the tissue where the priming antigen was derived and where a causative pathogen is most likely to reside.4 Furthermore, preferential T-cell homing may avert activated effector lymphocytes from other tissues where they might cause unwanted immune reactions. Important determinants of tissue-selective lymphocyte homing identified thus far include homing receptors α4β7 integrin, P-selectin ligand PSGL-1, and L-selectin5 and chemokine receptors CCR9, CCR4, and CCR10.6 In addition to these, numerous other adhesion molecules contribute to lymphocyte homing from blood into tissues.
Several adhesion molecules, most convincingly very late antigen-4 (VLA-4, integrin α4), have been implicated in the pathogenesis of diabetes in the nonobese diabetic (NOD) mouse.7–10 VLA-4 consists of two integrin chains, α4 and β1, linked together to form a heterodimer (α4β1). Integrin α4 also forms a heterodimer with the β7 chain, termed α4β7 (lymphocyte Peyer’s patch adhesion molecule, LPAM-1; mucosal homing receptor), which binds to mucosal addressin cell adhesion molecule-1 (MAdCAM-1),11 a ligand distinct from that of α4β1 (VCAM-1, vascular cell adhesion molecule-1). In addition, α4β7 and its ligand MAdCAM-1 have been implicated in the development of diabetes in the NOD mouse.12–14
The principal site of activation of diabetogenic T cells is the pancreatic lymph node (PaLN).15 However, the expression of the two heterodimers, α4β1 and α4β7, or that of other homing determinants has not been studied on T cells primed in this lymph node. It is also unclear to what extent various homing receptors contribute to homing of T cells into islets. To study this, we used a transgenic mouse model expressing ovalbumin (OVA) as a model autoantigen in islet β-cells and allowing activation of naïve, OVA-reactive, and thus β-cell-reactive T cells in the PaLN.16 Furthermore, we used transgenic mice expressing OVA in islet β-cells but unable to present it to T cells in the PaLN.17 In these mice, we modeled the situation of T-cell activation in response to antigens that enter the body by the gastrointestinal or the subcutaneous route, giving rise to islet-reactive T cells via, eg, antigenic mimicry. Our findings imply more than one receptor-ligand pair in T-cell homing into islets and that the receptor-ligand pair that mediates homing depends on the site of activation of effector T cells.
Materials and Methods
Mice
OT-I, OT-II, RIP-mOVA, and RIP-OVAlo mice,17 backcrossed >12 times to C57/BL 6, were generated and provided by Dr. W.R. Heath and Dr. F.R. Carbone, Melbourne, Australia, and were bred and maintained in the animal facilities of Turku and Bonn Universities. All experiments were approved by the Institutional Board of Animal Experiments.
Adoptive Cell Transfers and Induction of Immune-Mediated Diabetes
Lymph node and spleen cells of OT-II and OT-I mice were isolated using standard techniques. Their number was determined by staining for TCR Vα2 and Vβ5 chains and CD8 or CD4, respectively. OT-I cells were also detected by staining for CD8 and the H2-Kb-SIINFEKL-specific tetramer (Beckman Coulter, Fullerton, CA). Diabetes was induced in RIP-mOVA and RIP-OVAlo mice by adoptive transfer of a mixture of 0.3 × 106 OT-II and 0.2 × 106 OT-I cells or with 2.0 × 106 OT-I cells, as indicated in figure legends. OVA [lipopolysaccharide (LPS) (grade V; Sigma, St. Louis, MO) concentration ≥1.2 U/ml as measured by the LAL assay (BioWhittaker, Walkersville, MD), which corresponds to ∼100 ng/ml LPS from Escherichia coli18] was applied either intragastrically via a plastic feeding gauge (3 mg in 300 μl of phosphate-buffered saline at day 0 and 2) or subcutaneously (200 μg in incomplete Freund’s adjuvant at day 0) in the base of the tail. Blood glucose values were measured on day 10 after cell transfer using a MediSense glucometer, and mice with a reading more than 14.3 mmol/L were considered diabetic. Blood glucose levels were measured again on day 14 and similar results were obtained (not shown).
Determination of Homing Receptor Expression on T Lymphocytes Dividing in Lymph Nodes
Lymph node and spleen lymphocytes from OT-I and OT-II mice were labeled with carboxyfluorescein succinimidyl ester (Molecular Probes, Eugene, OR). OT-I and OT-II cells (4 × 106) were injected intravenously into RIP-mOVA or RIP-OVAlo mice. After adoptive cell transfer, RIP-OVAlo mice recipients were either fed OVA once or immunized subcutaneously with OVA. Cells were stained with Alexa 647-conjugated anti-CD4 and PerCP-Cy5.5-conjugated anti-CD8 and with phycoerythrin (PE)-conjugated anti-integrin α4 monoclonal antibody (mAb) (R1-2), anti-α4β7 heterodimer (DATK-32), or with biotinylated anti-integrin β1 chain mAb (Ha2/5), or anti-CD62L mAb (MEL-14), or with isotype-matched control antibodies, all from Becton, Dickinson and Company, San Jose, CA. In case of biotinylated mAbs, phycoerythrin-conjugated streptavidin (SA-PE; Becton, Dickinson and Company) was used as the second-step reagent. For detection of P-selectin ligand, cells were stained using chimeric P-selectin-human IgG fusion protein (Becton, Dickinson and Company) in a buffer with divalent cations, followed by biotinylated anti-human IgG and SA-PE. Flow cytometry was performed on an LSR-II flow cytometer, and data were analyzed with WinMDI2.8 software.
Immunohistochemistry of Pancreatic Islets
Pancreata were isolated from unmanipulated C57BL/6 mice to study constitutive expression of adhesion molecules. To study their expression during insulitis and to study homing receptor expression of infiltrating CD4 and CD8 T cells, pancreata were isolated from RIP-mOVA and RIP-OVAlo mice 10 days after adoptive transfer of OT-I and OT-II cells (and antigen exposure of RIP-OVAlo mice). Cryosections of normal pancreata were stained for VCAM-1 using the rat IgG1 mAb 6C7 (gift from Dr. D. Vestweber, Max-Planck Institute of Molecular Biomedicine, Münster, Germany), for MAdCAM-1 using MECA-367 (rat IgG2a), and for P-selectin using polyclonal rabbit anti-human P-selectin (Becton, Dickinson and Company). As controls, we used anti-human CD44 (hermes-1 prepared in our laboratory) for VCAM-1 and MAdCAM-1 staining or normal rabbit serum for P-selectin staining. Staining was detected by fluorescein isothiocyanate-conjugated goat anti-rat IgG (heavy and light chains; Southern Biotechnology, Birmingham, AL). Pancreatic islets were visualized by staining for insulin using rabbit anti-mouse insulin (Santa Cruz Biotechnology, Santa Cruz, CA), followed by Alexa 546-conjugated anti-rabbit IgG (Molecular Probes). Cryosections of prediabetic pancreata were stained for VCAM-1, MAdCAM-1, and P-selectin as described above and for infiltrating T cells using PE-conjugated anti-CD4 and anti-CD8. To detect homing receptor expression on infiltrating T cells, PE-conjugated anti-integrin α4 or α4β7 heterodimer or biotinylated anti-CD62L were used, followed by a mixture of fluorescein isothiocyanate-conjugated anti-CD4 and CD8 mAbs. Biotinylated anti-CD62L was revealed by SA-PE.
Role of Vascular Adhesion Molecules in Lymphocyte Accumulation in Pancreatic Islets
The following antibodies were raised from hybridomas, precipitated with ammonium sulfate, purified using protein G columns (Pharmacia Amersham, Piscataway, NJ) and filtered sterile: anti-VCAM-1, anti-MAdCAM-1, anti-CD62L (MEL-14, rat IgG2a), anti-P-selectin (RB40.34, rat IgG1) and anti-PSGL-1 (4RA10, rat IgG1), the last two obtained as gifts from Drs. D. Vestweber and M.K. Wild (see above). Isotype controls included antibodies 2D10 (anti-human VAP-1, rat IgG1 produced in our laboratory) and hermes-1 (rat IgG2a). Antibody treatment was started 2 days after adoptive transfer of OT cells, and in RIP-OVAlo mice, simultaneous exposure to antigen occurred (oral OVA or subcutaneous OVA with adjuvant). Mice received intraperitoneal injections of 200 μg of mAb (purified as indicated below) or isotype control every other day until 14 days after cell transfer. Anti-P-selectin antibody was used at 100 μg per injection, a dose previously shown to block macrophage accumulation after vascular injury.19 Blood glucose values were measured at 10 and 14 days after transfer. To analyze insulitis, nonparallel sections of paraffin-embedded pancreata were stained with hematoxylin and eosin (H&E). Each pancreas was assigned an insulitis score by analyzing the level of lymphocytic infiltration in each of 50 to 100 islets (0, no insulitis; 1, peri-insulitis or scant intra-islet insulitis; 2, insulitis covering <50% of islet area; 3, insulitis covering >50% of islet area) per pancreas. Islets were counted in a blinded manner by the same reader.
Statistical Methods
Statistical significance for the differences in incidence of diabetes between treated and control mice in each experiment was analyzed using χ2 test. Differences in insulitis scores were calculated using two-tailed, unpaired Student’s t-test with Welch’s correction.
Results
Expression of Homing Receptors by Diabetogenic CD4 and CD8 T Cells Depends on the Priming Site and Route of Antigen Entry
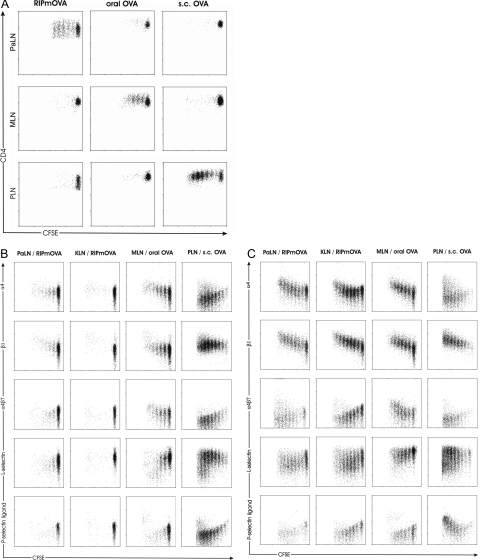
To study expression of homing receptors by autoreactive T cells, a 1:1 mixture of carboxyfluorescein succinimidyl ester-labeled OVA-specific CD4 (OT-II) and CD8 (OT-I) T cells was transferred into transgenic recipient mice expressing this model antigen in pancreatic islets. As recipients, we used either RIP-mOVA or RIP-OVAlo mice, in which OVA is expressed on islet β-cells and presented in pancreatic (and kidney)-draining lymph nodes sufficient to drive T-cell activation (RIP-mOVA) or expressed on islet β-cells in amounts too low to result in such activation but sufficient to permit CTL-mediated lysis of islet β-cells (RIP-OVAlo).17 This allowed study of homing receptor expression by T cells activated by autoantigen expressed in islet β-cells (RIP-mOVA) or introduction of autoantigen in other locations (RIP-OVAlo), for example subcutaneously or orally, and study of islet-directed CTL effectors resulting from such priming. Priming via these routes resulted in antigen presentation to OT cells, as shown for OT-I cells (Figure 1A) in the corresponding lymph nodes (subcutaneous or mesenteric).
Figure 1.
Expression of homing receptors on T cells is determined by the site of T-cell activation. A: Proliferation of OT-I cells in antigen-draining lymph nodes after injection of 4 × 106 carboxyfluorescein succinimidyl ester-labeled OT-I cells into RIP-mOVA or RIP-OVAlo mice. The latter mice were given OVA orally (3 mg) or subcutaneously (0.2 mg). OT-I cells were collected from lymph nodes (PaLN, pancreatic; MLN, mesenteric; IngLN, inguinal lymph node) 48 hours (RIP-mOVA) or 60 hours (RIP-OVAlo) later. B: Expression of α4-, β1-, and α4β7 integrin and of L-selectin and P-selectin binding activity (P-selectin ligand) on OT-II cells proliferating in PaLN and KLN (kidney LN) of RIP-mOVA mice and on OT-II cells proliferating in MLN and PLN of RIP-OVAlo mice. C: OT-I cells simultaneously transferred into the same recipient mice were analyzed for the expression of the same adhesion molecules.
Homing receptor expression in consecutive generations of dividing cells was evaluated separately for OT-II and OT-I cells 4 days (96 hours) after adoptive co-transfer. Only few OT-II cells were driven into cell cycle by OVA expressed as nonlymphoid autoantigen in RIP-mOVA mice (Figure 1B) as previously reported.20 On these few dividing OT-II cells, integrin α4 and β1 chains (which form the α4β1 heterodimer VLA-4) were slightly up-regulated, whereas significant up-regulation occurred on OT-I cells (Figure 1C). Expression of the α4β7 heterodimer remained low on both OT-II and OT-I cells responding to pancreatic autoantigen. L-selectin expression declined slightly but remained on an intermediate level, whereas P-selectin ligand activity decreased on both OT-II and OT-I cells (Figure 1, B and C).
To study expression of homing receptors on autoreactive T cells activated by orally consumed autoantigen, OVA was fed to RIP-OVAlo mice expressing low-dose OVA in pancreatic islets. In mesenteric lymph nodes of these mice, integrin α4 and β1 chains and α4β7 heterodimer were up-regulated both on dividing OT-II and OT-I cells (Figure 1, B and C). To exclude that up-regulation of α4β7 heterodimer was attributable to LPS contaminations in the OVA preparations used, we injected 100 μg of LPS intraperitoneally into RIP-mOVA mice. This did not induce expression of α4β7 heterodimer or otherwise change expression of homing receptors by OT-I cells proliferating in PaLNs (data not shown), indicating that factors other than LPS were responsible for the differences in homing receptor expression. In addition, the antigen dose did not affect the pattern of homing receptor expression, as revealed by titration of orally administered antigen to the smallest possible dose that induced proliferation of OT-II or OT-I cells.
Only in response to autoantigen administered subcutaneously, OT-I cells clearly up-regulated P-selectin ligand activity after several rounds of proliferation, although this was true for some, but not the majority, of OT-II cells. L-selectin expression remained high in the majority of both OT-II and OT-I cells, but in contrast to cells being activated elsewhere, OT-II and OT-I cells activated by subcutaneous autoantigen down-regulated integrin α4 chain (Figure 1, B and C). Neither the dose of subcutaneous antigen nor its combination with LPS or incomplete Freund’s adjuvant affected this expression pattern (data not shown).
Homing Receptor Expression on Islet-Infiltrating T Cells Reflects the Site of Activation of OT-II and OT-I Cells
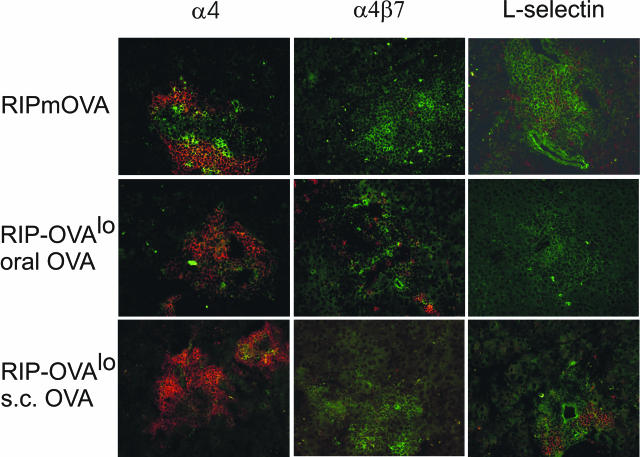
For homing to pancreatic islets, the acquired differences of homing receptor expression should persist until these T cells reach target tissue. We therefore studied expression of integrin α4, α4β7 heterodimer, and of L-selectin in islet-infiltrating T cells by immunohistochemistry. Islet infiltrating T cells (Figure 2) expressed the type of homing receptors that was induced during the respective way of priming (Figure 1) with a few exceptions. These exceptions were the expression of integrin α4 on infiltrating T cells also when OT-II and OT-I cells were activated in peripheral lymph nodes, expression of L-selectin on scattered T cells after T-cell activation in PaLN, and relative scarcity of L-selectin-expressing cells after T-cell activation in response to oral antigen. Importantly, T cells expressing α4β7 heterodimer were detected only if OT-II and OT-I cells were activated in response to oral antigen, and L-selectin was expressed most frequently and at highest intensity on islet-infiltrating T cells when OT-II and OT-I cells were activated in peripheral lymph nodes (Figure 2). These findings indicate that the homing receptor expression profile acquired during activation was at least partly maintained during recirculation and islet homing of activated T cells.
Figure 2.
Expression of homing receptors α4 and α4β7 integrin and L-selectin in islets. OT-II (0.3 × 106) and OT-I (0.2 × 106) cells were co-transferred into RIP-mOVA mice and into RIP-OVAlo mice given oral or subcutaneous OVA. After 10 days, mice were sacrificed and pancreata analyzed by fluorescence microscopy. Homing receptors (red fluorescence) were detected with PE-conjugated or biotinylated antibodies and SA-PE. T cells were detected with anti-CD4 and anti-CD8 antibodies directly conjugated to fluorescein isothiocyanate (green fluorescence). Original magnifications, ×200.
Endothelial Adhesion Molecules Are Expressed Constitutively on Pancreatic Vasculature and Are Induced on Islet-Associated Vessels during Insulitis
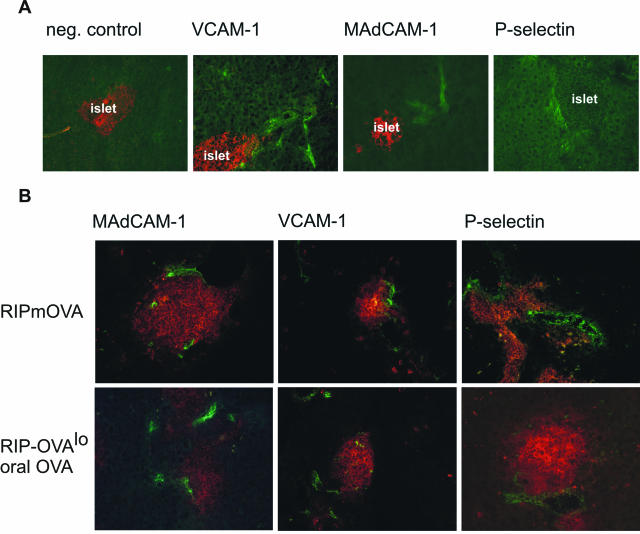
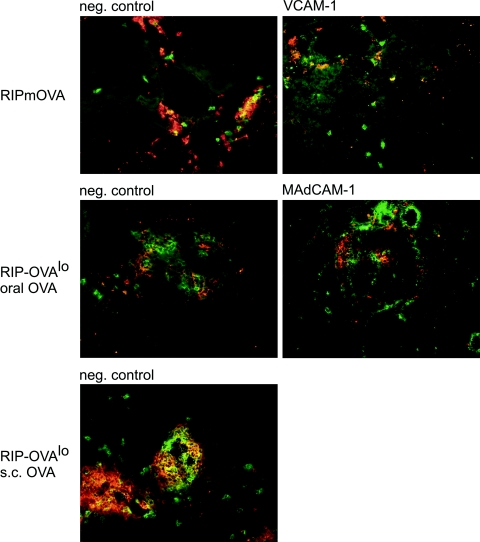
To evaluate the ability of T cells expressing various homing receptors to interact with pancreatic vasculature, we determined expression of the endothelial ligands VCAM-1, MAdCAM-1, and P-selectin on pancreatic vasculature in normal C57BL/6 mice and in RIP-mOVA and RIP-OVAlo mice during insulitis. Intriguingly, constitutive low-level expression of all these ligands was detected even in pancreas tissue from nontransgenic C57BL/6 control mice (Figure 3A). This expression was not located directly on islet vasculature but on several small- and middle-sized vessels close to islets. During insulitis, all of these molecules were expressed on islet vessels irrespective of the type of activation of OT-II and OT-I cells (Figure 3B). Thus, pancreatic and islet vessels expressed the adhesion molecules required to permit entry of autoreactive T cells activated in all locations tested.
Figure 3.
Expression of endothelial adhesion molecules VCAM-1, MAdCAM-1, and P-selectin on pancreatic vasculature. A: Sections from the pancreas of a normal C57BL/6 mouse were stained for expression of indicated adhesion molecules or control IgG (green fluorescence). Islets are highlighted by simultaneous insulin staining (red fluorescence) except for P-selectin staining (see Materials and Methods). B: Sections were stained for expression of the indicated adhesion molecules (green) and CD4 and CD8 T cells (red) from pancreas of RIP-mOVA or RIP-OVAlo mice fed OVA as above. Mice were sacrificed 6 days after transfer of OT-I and OT-II cells.
Infiltration of Islets by Mononuclear Cells and Diabetes Occur after Different Forms of Antigen Challenge
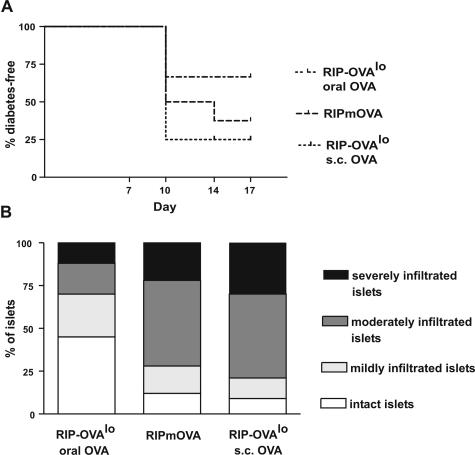
We next tested the efficacy by which each form of priming induced diabetes and mononuclear cell accumulation into islets in the experimental settings described above. A combination of 0.3 × 106 CD4 and 0.2 × 106 cells CD8 T cells was used because both cell types contribute to the pathogenesis of diabetes.21 These cell numbers created a better window for intervention and were closer to T-cell numbers in a normal repertoire than those previously used in this system.22 In one experiment, mice were sacrificed at day 6 for pancreatic histology and, in another, followed until day 17 for the occurrence of hyperglycemia. Subcutaneous priming was most effective, followed by endogenous priming (RIP-mOVA), both of which induced diabetes in the majority of mice. Oral priming was least effective and induced milder insulitis than subcutaneous or endogenous priming (Figure 4). In conclusion, all priming routes tested were able to induce immune-mediated diabetes, albeit at different extents.
Figure 4.
A: Incidence of diabetes in RIP-mOVA mice and in RIP-OVAlo mice after different forms of OVA priming (n = 8–12 mice per group). B: Severity of insulitis in pancreatic sections of RIP-mOVA and RIP-OVAlo mice killed 6 days after OT transfer and OVA priming (n = 5 mice per group).
T Cells Activated in Response to Islet-Derived OVA Use VCAM-1 but Not MAdCAM-1 to Infiltrate Pancreatic Islets and Induce Diabetes
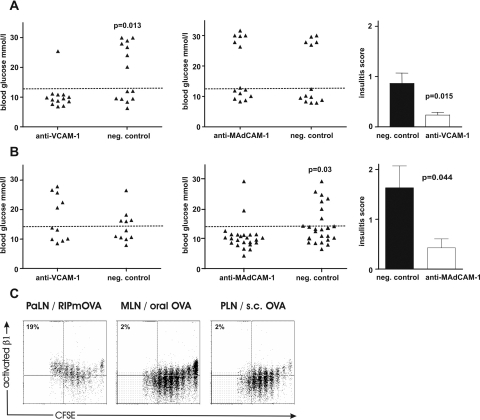
To test whether the adhesion molecules expressed in the islets were relevant for infiltration by autoreactive T cells activated in the three situations tested, we treated RIP-mOVA mice with function-blocking anti-VCAM-1 or isotype control mAb from 2 days after adoptive transfer of OT cells. To test that antibody treatment did not affect expansion of effector cells in response to priming, we determined in separate experiments the numbers of OT-I cells in lymph nodes and the spleen 10 days after adoptive transfer using the H2-Kb-SIINFEKL-tetramer and flow cytometry and found that numbers of OT-I cells were not affected by anti-VCAM-1 treatment (not shown). Borderline hyperglycemia (diabetes) developed in only 1 of 13 recipients treated with anti-VCAM-1, whereas 7 of 14 recipients treated with control antibody developed diabetes (P = 0.012; Figure 5A). Anti-MAdCAM-1 was not able to inhibit development of diabetes in RIP-mOVA mice, as 6 of 14 recipients became diabetic compared with 5 of 13 control recipients. The mean level of insulitis in pancreata of mice that were normoglycemic at the end of the experiment was significantly lower in the group of mice that had received anti-VCAM-1 compared with those receiving control antibody. These findings imply a functional role of VCAM in islet homing of diabetogenic T cells when these were activated in the pancreatic LN.
Figure 5.
VCAM-1, but not MAdCAM-1, blockade prevents islet-destruction after effector T-cell activation in PaLNs. RIP-mOVA mice were treated with function blocking anti-VCAM-1 (A) or anti-MAdCAM-1 (B), or negative control, starting from 2 days after adoptive transfer of OT-I and OT-II cells (in two experiments) or OT-I cells alone (in one experiment). Triangles represent blood glucose values (mmol/L) of individual mice at 10 days after transfer in each group. Dashed line: cutoff value for hyperglycemia. C: Mean insulitis scores ± SEM for pancreata from groups of mice that remained normoglycemic at the end of the experiments in (A) (five mice per group were analyzed). Expression of the high-affinity conformation of β1 integrin (activated β1) on OT-I cells after priming in PaLN in response to islet-derived OVA compared with priming in mesenteric or peripheral lymph node in response to oral or subcutaneous OVA. Percentages indicate the proportion of cells in top left quadrants (ie, cells divided ≥6 times and expressing activated β1).
T Cells Activated in Response to Oral OVA Use MAdCAM-1 but Not VCAM-1 to Infiltrate Pancreatic Islets and Induce Diabetes
To determine whether up-regulation of α4β7 expression was functionally relevant in homing of GALT-activated T cells into the pancreas and whether these cells were able to use also α4β1 for their homing into pancreas, we treated RIP-OVAlo mice with anti-VCAM-1 or anti-MAdCAM-1 antibody starting 2 days after adoptive transfer of OT cells and oral OVA administration. Blockade of VCAM-1 was without an effect, as 5 of 11 anti-VCAM-1-treated mice (and 5 of 11 controls) became diabetic (Figure 5B). However, MAdCAM-1 blockade was effective, as only 2 of 24 anti-MAdCAM-1-treated mice (including one borderline hyperglycemia) (and 8 of 24 control) became diabetic (P = 0.033). Anti-MAdCAM-1 treatment also significantly reduced the accumulation of T cells in islets, implying a functional role of MAdCAM-1 in islet homing of diabetogenic T cells when these were activated in the gut. Anti-MAdCAM-1 treatment did not inhibit expansion of OT-I cells, tested as described above (not shown).
Only T Cells Activated in Response to Islet-Derived OVA Express the Functionally Active Conformation of β1 Integrin
Integrin β1 was up-regulated on OT cells activated in all locations tested (Figure 1), and α4 after priming in PaLN and GALT. Therefore, it was surprising that blocking of the α4β1 ligand VCAM-1 only affected diabetogenesis and insulitis after activation of OT cells in the PaLN. We speculated that expression of α4β1 integrin in its high-affinity state occurring in VLA-423 might be restricted to T cells activated in the PaLN. To test this hypothesis, we used an antibody reacting only with an activation-associated conformational β1 epitope.24 Indeed, activation in response to islet-derived antigen in the PaLN led to appearance of the activated form of β1 on T cells, and after six rounds of cell division most T cells had β1 in its active conformation (Figure 5C). This change in conformation was not observed when T cells were activated by external antigen in other lymphoid tissues.
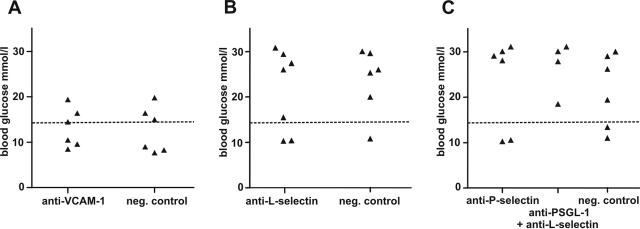
T Cells Activated in Response to Subcutaneous OVA Infiltrate Pancreatic Islets Independent of P- and L-Selectin, PSGL-1, and VCAM-1
After subcutaneous injection of OVA, most OT-II cells lost expression of integrin α4 and α4β7 heterodimer, and only a few OT-I cells up-regulated integrin α4. However, both expressed L-selectin, and all OT-I cells up-regulated P-selectin ligand (Figure 1, B and C). Blockade of VCAM-1 did not inhibit diabetogenesis by these effector cells (Figure 6) nor did blockade of either selectin alone or P-selectin ligand and L-selectin in combination.
Figure 6.
Neither VCAM-1 (A), L-selectin (B), nor P-selectin (C) or a combination of P-selectin ligand and L-selectin blockade prevents islet-destruction after effector T-cell activation in response to subcutaneous antigen and adjuvant. Dashed line: cutoff value for hyperglycemia. Diabetes was induced with a mixture of OT-I and OT-II cells, and antigen was given subcutaneously.
Adhesion Molecule Blockade Affects Infiltration of Islets by Both CD4 and CD8 T Cells
Although we induced diabetes by using both CD4 and CD8 T cells, it was important to examine islets for potential differences in infiltration by these T-cell subsets after different forms of priming and after therapies intervening in adhesion molecule function. Although inhibition of VCAM-1 function diminished insulitis (and partially prevented diabetes) in RIP-mOVA mice and inhibition of MAdCAM-1 function had a similar effect after oral priming in RIP-OVAlo mice, in both cases infiltration of islets by CD4 and CD8 T cells was similarly affected; ie, the remaining infiltrates contained both subsets. Both CD4 and CD8 T cells were also seen in islets of mice primed by subcutaneous OVA, thus excluding major differences in CD4 versus CD8 T-cell homing into islets after different forms of priming (Figure 7).
Figure 7.
Both CD4 (red) and CD8 (green) T cells infiltrate in islets of RIP-mOVA mice after adoptive transfer of OT-II and OT-I cells and in islets of RIP-OVAlo mice irrespective of the form of OVA priming (left panels; “neg. control”). In vivo blockade of VCAM-1 or MAdCAM-1 prevents partially, but in equal efficacy, entry of CD4 and CD8 T cells in islets (right panels).
Discussion
Recent studies have demonstrated that T cells acquire the capacity to home to and infiltrate organs such as the gut mucosa,1–3,25 the brain,26 or the skin27 during their priming in lymph nodes draining these organs. In the present study, we investigated whether priming in the PaLN governed migration of T cells specific for β-cell antigens into islets and what consequences for islet homing and diabetogenicity may result from priming in other lymphoid compartments. We chose a murine model of immune-mediated diabetes that allowed study of pancreatic infiltration by diabetogenic T cells on activation in different organs or by antigen entering the body via different routes. As determined by division of OT-II and OT-I cells in the respective draining lymph nodes, oral or subcutaneous antigen administration resulted in restricted distribution of antigen, as previously reported for islet-antigen in RIP-mOVA mice.17 Consistent with studies on T cells specific for foreign antigen reported by others,1–3,25,27 our results showed that also T cells specific for an islet β-cell-derived autoantigen were imprinted with a characteristic homing receptor pattern during priming.
When we studied the functional consequences of homing receptor patterns, we found that infiltration into pancreatic islets could be facilitated by at least two different endothelial ligands; MAdCAM-1 facilitated islet entry of α4β7 integrin-expressing T cells activated by orally fed autoantigen, whereas VCAM-1 allowed entry of α4β1-imprinted T cells activated by islet-expressed autoantigen. Although β1 was expressed by T cells irrespective of their priming site and α4 after priming in PaLN or GALT, blocking the α4β1 integrin ligand VCAM-1 inhibited infiltration only by T cells activated in PaLN. This paradox could be explained by conformation changes of the α4β1 heterodimer. Generally, integrin-mediated cell-to-cell adhesion can be strengthened by at least two mechanisms: by increasing receptor density (and receptor clustering at contact area) and by altering the conformation of existing receptors. A change in conformation can greatly affect ligand binding affinity, and signals related to T-cell activation can induce a switch from the low- to high-affinity conformation.23 This is particularly true for the family of β1 integrins including α4β1, and thus, also for its ability to bind VCAM-1. In the present study, only activation in response to islet-derived antigen in the PaLN induced expression of the activated form of β1, whereas T cells activated by external antigen in other lymphoid tissues did not express the functionally active conformation23 of β1 integrin. This might explain why OT cells used VCAM-1 for homing into islets only when activated in response to islet-OVA and why OT cells activated in response to oral OVA, imprinted with both α4β7 and α4β1, were not able to use VCAM-1 but were solely dependent on MAdCAM-1 for homing into islets. In addition, β1 could complex with α-chains other than α4 on T cells activated in the mesenteric LNs.
Activation by subcutaneous antigen generated islet-reactive T-cell effectors that used neither VCAM-1, nor MAdCAM-1, but which still homed to pancreatic islets. Blocking L-selectin or P-selectin, the ligand of their tissue-selective homing receptor PSGL-1/CLA,28 alone or even L-selectin and PSGL-1 in combination did not have a significant effect on homing of these T cells into islets and development of diabetes. This could be explained by the ability of such T cells to use E-selectin instead of P-selectin as a ligand for their egress from vasculature, as also observed in other models of inflammation.29 Although several β1 integrins are expressed on T cells, their ligands (collagen, laminin, fibronectin) are mostly expressed in the extracellular matrix and are therefore less likely to have a role in T-cell adhesion to endothelium.30 In contrast, the β2 integrin CD11a/CD18 is known for its important role as an accessory molecule in lymphocyte homing.31 However, administration of a function-blocking antibody against CD11a did not reduce diabetes incidence after subcutaneous priming (data not shown), excluding the possibility of CD11a/CD18 being an important homing receptor for such T-cell entry into pancreatic islets. It is possible that additional adhesion molecules and their ligands affect migration and homing of diabetogenic T cells. In the present study, we focused on adhesion molecules with a known tissue-selective function or a dominant role in islet-infiltration of diabetogenic T cells.
We and others have previously shown that pancreatic islets express a plethora of adhesion molecules during insulitis, such as MAdCAM-1, PNAd, VCAM-1, ICAM-1, and hyaluronic acid (the principal ligand of CD44).7–9,12,13,32,33 However, expression of adhesion molecules in intact pancreatic islets has received less attention so far. Our results revealed constitutive low-level expression of VCAM-1, MAdCAM-1, and P-selectin in pancreatic vasculature of healthy mice. Although these were mostly located in vessels outside islets, they might still contribute to the initial infiltration events after priming of islet-reactive T cells because infiltration of islets is often preceded by lymphocyte infiltration of areas around ducts and extra-islet vessels.34 This, together with the fact that priming of islet-reactive T cells in different lymphoid compartments was able to induce diabetes in our model and this depended on different homing receptor-ligand interactions, suggested that islets can easily be reached by T cells imprinted with various homing patterns. In fact, autoreactive T cells that have escaped thymic censorship are a common feature35 but are normally controlled by peripheral tolerance mechanisms operating deletionally21 or by regulation.36 However, several tissues are targeted by inflammation involving autoreactive T cells, some more often than others. Type 1 diabetes is one of the most prevalent immune-mediated diseases in western countries, with a constantly increasing incidence that cannot be explained entirely on the basis of accumulating susceptibility genes.37,38 One additional mechanism contributing to this increasing prevalence could involve promiscuous islet homing of T cells imprinted with various homing receptors in response to environmental influence of various nature.
In our model, diabetes resulted from an immune attack against a transgenic model antigen (OVA) rather than from spontaneously arising autoimmunity against natural islet-antigens (eg, insulin, GAD-65, IGRP, and others). Thus, it has to be emphasized that our model may not mimic all functional aspects associated with true autoimmunity. Nevertheless, OT-I cells in our model functioned as islet-reactive T cells in the particular environment of RIP-OVA transgenic recipient mice, offering the unique opportunity to examine molecular mechanisms governing islet homing of diabetogenic T cells. Our model does not provide information on the location where priming of diabetogenic T cells in human type 1 autoimmune diabetes occurs or what molecular mechanisms are involved. Nevertheless, our observations allow insights into the mechanisms governing T cell homing to pancreatic islets, which may also be relevant for understanding human type 1 autoimmune diabetes.
Prevention of type 1 diabetes may become feasible by the use of effective immunomodulators adapted specifically to treatment of islet autoimmunity, as exemplified by recent progress in anti-CD3 treatment.39,40 Our results suggest that anti-adhesion molecule therapy targeting α4 integrin could also be effective against islet-inflammation, provided that diabetogenic T cells are always activated in pancreatic lymph nodes (or alternatively, also in gut-associated lymph nodes). Our results regarding homing pathways available for T cells activated in mucosal and subcutaneous sites into islets may also pertain to prevention strategies combining anti-adhesion therapy with autoantigen administration41,42 via external routes.
Acknowledgments
We thank Drs. W.R. Heath and F.R. Carbone for mouse lines, Drs. D. Vestweber and M.K. Wild for antibodies, and Drs. J. Miller and D. Mathis for critically reading the manuscript.
Footnotes
Address reprint requests to Arno Hänninen, Dept. of Medical Microbiology, University of Turku, Kiinamyllynkatu 13, FIN-20520 Turku, Finland. E-mail: arhanni@utu.fi.
Supported by the Finnish Academy, the Juvenile Diabetes Research Foundation, the Sigrid Juselius Foundation, the Finnish Society for Diabetes Research, and the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 704 and Klinische Forschergruppe 115 to C.K.).
R.N., M.M., and J.H. are authors with equal contribution. S.J. and C.K. also contributed equally.
References
- Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003;198:963–969. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- Baron JL, Reich EP, Visintin I, Janeway CA., Jr The pathogenesis of adoptive murine autoimmune diabetes requires an interaction between alpha 4-integrins and vascular cell adhesion molecule-1. J Clin Invest. 1994;93:1700–1708. doi: 10.1172/JCI117153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkly LC, Jakubowski A, Hattori M. Protection against adoptive transfer of autoimmune diabetes mediated through very late antigen-4 integrin. Diabetes. 1994;43:529–534. doi: 10.2337/diab.43.4.529. [DOI] [PubMed] [Google Scholar]
- Yang XD, Michie SA, Tisch R, Karin N, Steinman L, McDevitt HO. A predominant role of integrin alpha 4 in the spontaneous development of autoimmune diabetes in nonobese diabetic mice. Proc Natl Acad Sci USA. 1994;91:12604–12608. doi: 10.1073/pnas.91.26.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XD, Michie SA, Mebius RE, Tisch R, Weissman I, McDevitt HO. The role of cell adhesion molecules in the development of IDDM: implications for pathogenesis and therapy. Diabetes. 1996;45:705–710. doi: 10.2337/diab.45.6.705. [DOI] [PubMed] [Google Scholar]
- Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- Yang XD, Sytwu HK, McDevitt HO, Michie SA. Involvement of beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in the development of diabetes in obese diabetic mice. Diabetes. 1997;46:1542–1547. doi: 10.2337/diacare.46.10.1542. [DOI] [PubMed] [Google Scholar]
- Hänninen A, Taylor C, Streeter PR, Stark LS, Sarte JM, Shizuru JA, Simell O, Michie SA. Vascular addressins are induced on islet vessels during insulitis in nonobese diabetic mice and are involved in lymphoid cell binding to islet endothelium. J Clin Invest. 1993;92:2509–2515. doi: 10.1172/JCI116859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänninen A, Jaakkola I, Jalkanen S. Mucosal addressin is required for the development of diabetes in nonobese diabetic mice. J Immunol. 1998;160:6018–6025. [PubMed] [Google Scholar]
- Gagnerault MC, Luan JJ, Lotton C, Lepault F. Pancreatic lymph nodes are required for priming of beta cell reactive T cells in NOD mice. J Exp Med. 2002;196:369–377. doi: 10.1084/jem.20011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JF, Kurts C, Allison J, Kosaka H, Carbone F, Heath WR. Induction of peripheral CD8+ T-cell tolerance by cross-presentation of self antigens. Immunol Rev. 1998;165:267–277. doi: 10.1111/j.1600-065x.1998.tb01244.x. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Miyazaki Y, Zimmerman GA, Albertine KH, McIntyre TM. Endotoxin contamination of ovalbumin suppresses murine immunologic responses and development of airway hyper-reactivity. J Biol Chem. 2003;278:42361–42368. doi: 10.1074/jbc.M307752200. [DOI] [PubMed] [Google Scholar]
- Phillips JW, Barringhaus KG, Sanders JM, Hesselbacher SE, Czarnik AC, Manka D, Vestweber D, Ley K, Sarembock IJ. Single injection of P-selectin or P-selectin glycoprotein ligand-1 monoclonal antibody blocks neointima formation after arterial injury in apolipoprotein E-deficient mice. Circulation. 2003;107:2244–2249. doi: 10.1161/01.CIR.0000065604.56839.18. [DOI] [PubMed] [Google Scholar]
- Behrens GM, Li M, Davey GM, Allison J, Flavell RA, Carbone FR, Heath WR. Helper requirements for generation of effector CTL to islet beta cell antigens. J Immunol. 2004;172:5420–5426. doi: 10.4049/jimmunol.172.9.5420. [DOI] [PubMed] [Google Scholar]
- Bach JF. Immunotherapy of insulin-dependent diabetes mellitus. Curr Opin Immunol. 2001;13:601–605. doi: 10.1016/s0952-7915(00)00267-3. [DOI] [PubMed] [Google Scholar]
- Kurts C, Carbone FR, Barnden M, Blanas E, Allison J, Heath WR, Miller JF. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J Exp Med. 1997;186:2057–2062. doi: 10.1084/jem.186.12.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Lenter M, Uhlig H, Hamann A, Jeno P, Imhof B, Vestweber D. A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc Natl Acad Sci USA. 1993;90:9051–9055. doi: 10.1073/pnas.90.19.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Calzascia T, Masson F, Di Berardino-Besson W, Contassot E, Wilmotte R, Aurrand-Lions M, Ruegg C, Dietrich PY, Walker PR. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity. 2005;22:175–184. doi: 10.1016/j.immuni.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389:978–981. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- Kanwar S, Steeber DA, Tedder TF, Hickey MJ, Kubes P. Overlapping roles for L-selectin and P-selectin in antigen-induced immune responses in the microvasculature. J Immunol. 1999;162:2709–2716. [PubMed] [Google Scholar]
- Sackstein R. The lymphocyte homing receptors: gatekeepers of the multistep paradigm. Curr Opin Hematol. 2005;12:444–450. doi: 10.1097/01.moh.0000177827.78280.79. [DOI] [PubMed] [Google Scholar]
- Hamann A, Jablonski-Westrich D, Duijvestijn A, Butcher EC, Baisch H, Harder R, Thiele HG. Evidence for an accessory role of LFA-1 in lymphocyte-high endothelium interaction during homing. J Immunol. 1988;140:693–699. [PubMed] [Google Scholar]
- Herold KC, Vezys V, Gage A, Montag AG. Prevention of autoimmune diabetes by treatment with anti-LFA-1 and anti-ICAM-1 monoclonal antibodies. Cell Immunol. 1994;157:489–500. doi: 10.1006/cimm.1994.1244. [DOI] [PubMed] [Google Scholar]
- Weiss L, Slavin S, Reich S, Cohen P, Shuster S, Stern R, Kaganovsky E, Okon E, Rubinstein AM, Naor D. Induction of resistance to diabetes in non-obese diabetic mice by targeting CD44 with a specific monoclonal antibody. Proc Natl Acad Sci USA. 2000;97:285–290. doi: 10.1073/pnas.97.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaccio G, Chieffi-Baccari G, Mezzogiorno V, Esposito V. Extraislet infiltration in NOD mouse pancreas: observations after immunomodulation. Pancreas. 1993;8:459–464. doi: 10.1097/00006676-199307000-00009. [DOI] [PubMed] [Google Scholar]
- Correia-Neves M, Waltzinger C, Mathis D, Benoist C. The shaping of the T cell repertoire. Immunity. 2001;14:21–32. doi: 10.1016/s1074-7613(01)00086-3. [DOI] [PubMed] [Google Scholar]
- Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435:598–604. doi: 10.1038/nature03725. [DOI] [PubMed] [Google Scholar]
- Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 2002;51:3353–3361. doi: 10.2337/diabetes.51.12.3353. [DOI] [PubMed] [Google Scholar]
- Gillespie KM, Bain SC, Barnett AH, Bingley PJ, Christie MR, Gill GV, Gale EA. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet. 2004;364:1699–1700. doi: 10.1016/S0140-6736(04)17357-1. [DOI] [PubMed] [Google Scholar]
- Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- Skyler JS. Diabetes mellitus: pathogenesis and treatment strategies. J Med Chem. 2004;47:4113–4117. doi: 10.1021/jm0306273. [DOI] [PubMed] [Google Scholar]
- Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E. Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial–Type 1. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]