Abstract
Rheumatoid arthritis is a chronic autoimmune disease of unknown etiology characterized by chronic inflammation in the joints and subsequent destruction of the cartilage and bone. The present study proposes a new strategy for the treatment of arthritis: the administration of the immunomodulatory neuropeptide adrenomedullin. Treatment with adrenomedullin significantly reduced incidence and severity of collagen-induced arthritis, an experimental model of rheumatoid arthritis, completely abrogating joint swelling and destruction of cartilage and bone. The therapeutic effect of adrenomedullin was associated with a striking reduction of the two deleterious components of the disease, ie, the Th1-driven autoimmune and inflammatory responses. Adrenomedullin also induced the generation and/or activation of efficient CD4+CD25+ regulatory T cells in arthritis with capacity to suppress autoreactive response and restore immune tolerance, which could play a pivotal role in the therapeutic effect of adrenomedullin on experimental arthritis contributing to the restoration of immune tolerance.
Collagen-induced arthritis (CIA), a murine experimental disease model induced by immunization with type II collagen (CII), shares a number of clinical, histological, and immunological features with rheumatoid arthritis (RA).1 Although its etiology is unknown, the initial stages of RA and CIA involve multiple steps. They can be divided into two main phases: initiation and establishment of autoimmunity to collagen-rich joint components, and later events associated with the evolving destructive inflammatory processes.1–3 Progression of the autoimmune response involves the development of autoreactive Th1 cells, their entry into the joint tissues, and future recruitment of inflammatory cells through multiple mediators. The fact that the inflammatory process in RA is chronic suggests that immune regulation in the joint is disturbed. This disturbance is probably caused by an excessive inflammatory response together with a deficiency in the mechanisms that control the immune response. Available therapies are based on immunosuppressive agents that inhibit the inflammatory component of RA and either reduce the relapse rate or delay disease onset. However, they have multiple effects, some of which are undesirable, and in the long term they do not suppress progressive clinical disability.4 This illustrates the need for novel therapeutic approaches to prevent the inflammatory and autoimmune components of the disease and to promote immune tolerance restoration.
Adrenomedullin (AM) is a neuropeptide structurally related to the calcitonin gene-related peptide (CGRP), initially known by its cardiovascular protective activities.5 However, much evidence suggests that AM could act as an endogenous immunomodulatory factor, with predominant anti-inflammatory effects. AM and their receptors are detected in several immune cells, including macrophages/monocytes and T cells, in lymphoid organs and in the gastrointestinal tract, and their expressions increase under inflammatory conditions.6–8 In addition, AM inhibits tumor necrosis factor (TNF)-α production by activated macrophages, prevents sepsis-induced mortality, and abrogates colitis in a model of inflammatory bowel disease.6,9–11 These effects seem to be mediated through an inhibitory effect on activated macrophages and on Th1-mediated function. Therefore, the aim of this study was to investigate the potential therapeutic action of AM in CIA. Here we show that treatment of CIA mice with AM has great benefit at the clinical and pathological levels. The therapeutic effect of AM was exerted at multiple levels, being associated with the down-regulation of both inflammatory and Th1-mediated autoimmune components of the disease. The induction and emergence of regulatory T cells (Tregs) with suppressive activity on autoreactive T cells also contributes to restoration of immune tolerance by AM in this disease.
Materials and Methods
Arthritis Induction and Treatment
To induce CIA, DBA/1J mice (7 to 10 weeks old; Jackson Laboratories, Bar Harbor, ME) were injected subcutaneously in the base of the tail with 200 μg of chicken CII (Sigma, St. Louis, MO) emulsified in complete Freund’s adjuvant containing 200 μg of Mycobacterium tuberculosis H37 RA (Difco, Detroit, Michigan). At day 21 after primary immunization, mice were boosted subcutaneously with 100 μg of CII in complete Freund’s adjuvant. AM (American Peptides Co., Sunnyvale, CA) treatment consisted in the intraperitoneal administration of 2 nmol (6.6 μg/mouse/day; or other doses when indicated) on 5 consecutive days or of a unique pulse starting at 25 days after immunization when all mice showed established arthritis (clinical score >2). In each experiment, a control group of mice was injected intraperitoneally with phosphate-buffered saline (PBS) (untreated). For adoptively transferred CIA, draining lymph node (DLN) cells were purified at day 35 after immunization from untreated or VIP-treated (2 nmol) arthritic mice and stimulated in vitro with inactivated CII (20 μg/ml) for 72 hours. After stimulation, CD4 T cells were isolated and depleted of CD25+ cells by immunomagnetic selection (Miltenyi Biotech, Bergisch Gladbach, Germany) following the manufacturer’s recommendations and then injected intravenously (5 × 106 cells) into CIA mice at day 24 after immunization. In some experiments, AM-treated CIA mice received intravenous injections of neutralizing anti-interleukin (IL)-10 polyclonal antibody and/or neutralizing anti-transforming growth factor (TGF)-β monoclonal antibody (mAb) or preimmune rat IgG used as control Ig (500 μg of Ab/mouse) on 5 alternate days.
Mice were analyzed by two independent, blinded examiners every other day and monitored for signs of arthritis onset using two clinical parameters, paw swelling and clinical score. Paw swelling was assessed by measuring the thickness of the affected hind paws with 0- to 10-mm calipers. Clinical arthritis was assessed by using the following system: grade 0, no swelling; grade 1, slight swelling and erythema; grade 2, moderate swelling and edema; grade 3, extreme swelling and pronounced edema; grade 4, joint rigidity. Each limb was graded, giving a maximum possible score of 16 per animal. For histological analysis, mice were sacrificed by cervical dislocation at day 45 after primary immunization, and the paws from four to six animals were randomly collected by two independent experimenters, fixed in 4% buffered formaldehyde, decalcified in Bouin Liquor DC (Panreac, Barcelona, Spain), and embedded in paraffin, and 4-μm sections were stained with hematoxylin and eosin (H&E) or Masson-Goldner trichromic stain. Histopathological changes were scored in a blinded manner based in cell infiltration, cartilage destruction, and bone erosion parameters as described.12
Determination of Cytokines, CII-Specific Abs, and Myeloperoxidase Activity
For cytokine determination in joints, protein extracts were isolated by homogenization of joints (50 mg tissue/ml) in 50 mmol/L Tris-HCl, pH 7.4, with 0.5 mmol/L dithiothreitol, and 10 μg/ml of a cocktail of proteinase inhibitors containing phenylmethyl sulfonyl fluoride, pepstatin, and leupeptin (Sigma). Samples were centrifuged at 30,000 × g for 20 minutes and stored at −80°C until cytokine determination. Serum samples were collected at day 35 after immunization and the levels of anti-CII IgG, IgG1, and IgG2a Abs were measured by enzyme-linked immunosorbent assay as described.12 Cytokine and chemokine levels in the serum and joint protein extracts prepared 35 days after primary immunization were determined by specific sandwich enzyme-linked immunosorbent assays using capture/biotinylated detection Abs from BD Pharmingen (San Diego, CA) and Preprotech (Rocky Hill, NJ) according to the manufacturers’ recommendations. Neutrophil infiltration in the joint was monitored by measuring myeloperoxidase activity as previously described in joint extracts isolated at day 35 after immunization.13 All of the animal experimental protocols were reviewed and approved by the ethical committee of the Spanish Council of Scientific Research.
Assessment of T-Cell Autoreactive Response
At 30 days after primary immunization, spleen, DLN, and synovial membrane were removed. Single-cell suspensions were obtained from spleen and DLN. Synovial cells were isolated by enzymatic digestion of the synovium of the knee joints as previously described.12 Antigen-presenting cells (APCs) were prepared by T-cell depletion of DBA/1J spleen cells using anti-CD3 mAb and complement-mediated lysis as described.14 T cells were isolated from DLN and synovial membrane by immunomagnetic selection. DLN and synovial cells (106 cells/ml) were stimulated in complete medium (RPMI 1640 containing 10% fetal calf serum, 2 mmol/L l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin) with different concentrations of heat-inactivated CII for 48 hours (for cytokine determination) or for 72 hours (for proliferative response). Cell proliferation (expressed as A450) was evaluated by using a cell proliferation assay (BrdU) from Roche Diagnostics GmbH (Mannheim, Germany). Cytokine content in culture supernatants was determined by specific sandwich enzyme-linked immunosorbent assays as above. For intracellular analysis of cytokines in DLNs and synovial cells, cells were stimulated with inactivated CII (10 μg/ml) for 8 hours in the presence of monensin (Sigma), and then stained with PerCP-anti-CD4 mAbs for 30 minutes at 4°C, washed, fixed, saponin-permeabilized, stained with 0.5 μg/sample of fluorescein isothiocyanate- and phycoerythrin-conjugated anti-cytokine-specific mAbs (BD Pharmingen), and analyzed on a FACSCalibur flow cytometer (Becton, Dickinson and Company, San Jose, CA). To distinguish between monocyte/macrophage and T cell sources, intracellular cytokine analysis was done exclusively in the PerCP-labeled CD4 T-cell population.
On the other hand, synovial cells (106 cells/ml) isolated from CIA mice at day 30 after primary immunization were stimulated with inactivated CII (10 μg/ml) in the absence or presence of different concentrations of AM or CGRP (American Peptides). Cytokine levels were determined in supernatants after 48 hours of culture. As a recall antigen control, purified protein derivative (PPD; 30 μg) was injected subcutaneously in the CII-complete Freund’s adjuvant emulsion, and in vitro T-cell function after culture stimulation with 10 μg/ml PPD was assessed as described above.
To determine the suppressive capacity of regulatory T cells, autoreactive T cells (4 × 105 cells/well) were isolated from DLNs of CIA mice as described above, and stimulated with spleen APCs (105 cells/well) and inactivated CII (10 μg/ml) in the absence or presence of DLN T cells isolated from untreated or AM-treated CIA mice (5 × 104 cells/well). After 72 hours, we measured the proliferative response and cytokine production of the autoreactive T cells as above.
Flow Cytometric Analysis
Synovial and DLN cells isolated at day 30 after immunization were incubated with various mAbs (fluorescein isothiocyanate anti-CD25, phycoerythrin anti-CD45RB, PerCP anti-CD4, 2.5 μg/ml final concentration) at 4°C for 1 hour. After extensive washing, cells were fixed, saponin-permeabilized, incubated for 45 minutes at 4°C with phycoerythrin anti-Foxp3 mAb (0.5 μg/sample) diluted in 0.5% saponin, and analyzed on a FACSCalibur flow cytometer. We used isotype-matched Abs as controls and IgG block (Sigma) to avoid nonspecific binding to Fc-receptors.
Data Analysis
All values are expressed as mean ± SD of mice/experiment. The differences between groups were analyzed by Mann-Whitney U-test and, if appropriate, by KruskalWallis analysis of variance test.
Results
AM Decreases Incidence and Severity of CIA
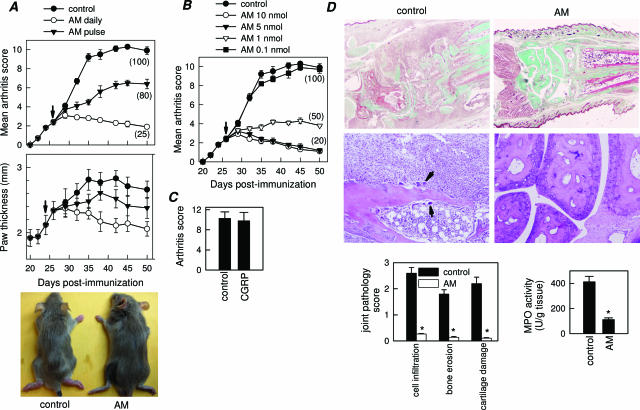
AM treatment of mice with established clinical signs of arthritis progressively decreased incidence and severity of CIA as compared with untreated mice (control), as assessed by clinical score and paw swelling (Figure 1A). Daily administration of 2 nmol of AM for 5 days offered the best protection against disease, although a single injection was enough to significantly ameliorate the pathological signs of arthritis (Figure 1A). The therapeutic effect was dose-dependent, showing an optimal effect with a dose of AM around 6.6 μg/mouse/day (Figure 1B). In contrast to AM, the AM-related peptide CGRP did not show any protective effect on CIA (Figure 1C). Because we observed few differences between the 2- and 10-nmol doses, all further experiments used five administrations of the 2-nmol dose on consecutive days. Histopathological analyses of joints showed that AM treatment completely abrogated CIA characteristic chronic inflammation of synovial tissue (infiltration of inflammatory cells into the joint cavity and periarticular soft tissue, consisting of lymphocytes, plasma cells, macrophages, and neutrophils), pannus formation, cartilage destruction, and bone erosion (Figure 1D). The AM-mediated inhibition of neutrophil infiltration was confirmed with decreased joint myeloperoxidase activity. In addition, AM treatment inhibited the osteoclast-inducing activity observed in the CIA mice with recruitment of osteoclast in basic multicellular units that produce focal subchondral bone erosion (Figure 1D, arrows). We saw no remission in therapeutic effects 3 weeks after cessation of AM treatment (Figure 1A), indicating that no additional neuropeptide is necessary after a short period of AM treatment to maintain protection from the disease. In addition, throughout our study, we did not observe any overt toxicity or lethality caused by daily peptide injection.
Figure 1.
AM decreases CIA incidence and severity. A: Severity of arthritis, assessed by clinical scoring or paw thickness measurement, in mice with established CIA injected intraperitoneally (arrow) either with PBS (control) or with AM (2 nmol/mouse) daily for 5 days or on a unique pulse. Numbers in parentheses represent incidence of arthritis (percentage of mice with arthritis score >2 at day 50). Images show representative examples of the paw swelling in mice of the different experimental groups. n = 8 to 16 mice per group. P < 0.001 versus control after day 32. B: Clinical scoring and disease incidence (parentheses) of CIA mice treated intraperitoneally daily (arrow) for 5 days with either with PBS or with different amounts of AM. n = 8 mice per group. P < 0.001 versus control for 1 to 10 nmol treatments after day 32. C: CIA mice were treated with CGRP (5 nmol/mouse) daily for 5 days from the onset of disease, and arthritis score was determined at day 45. Disease incidence for CGRP-treated mice was 100%. n = 5 mice per group. D: Histological analysis of trichromic-stained (top) or H&E-stained (bottom) sections of joints from CIA mice treated with PBS (control) or AM (2 nmol/mice, daily for 5 days). Arrows point to osteoclasts destroying bone. Scoring of inflammation, cartilage damage, and bone erosion of paws from untreated and AM-treated CIA mice. Myeloperoxidase activity measuring neutrophil infiltration in the joints. *P < 0.001 versus control.
AM Inhibits Inflammatory Response in CIA
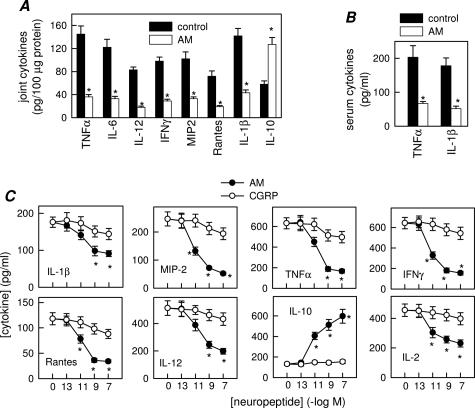
We next investigated the mechanisms underlying the decrease in incidence and severity of CIA after AM treatment. Previous data have demonstrated that inflammatory mediators, such as cytokines and free radicals, produced by infiltrating inflammatory cells, play a critical role in joint damage.1,2 Because, AM has been recently identified as a potential anti-inflammatory factor,6,9–11 we evaluated the effect of AM on the production of inflammatory mediators that are mechanistically linked to CIA severity. AM treatment dramatically reduced expression of inflammatory cytokines (TNF-α, IFN-γ, IL-6, IL-1α, IL-1β, and IL-12) and chemokines [regulated on activation normal T cell expressed and secreted (RANTES) and macrophage inflammatory protein (MIP)-2] in the joints of arthritic mice (Figure 2A). In addition, joints of AM-treated mice showed increased levels of the anti-inflammatory cytokine IL-10 (Figure 2A). The broad anti-inflammatory activity of AM in the inflamed joint was accompanied by down-regulation of the systemic inflammatory response. AM decreased CIA-induced serum levels of the proinflammatory cytokines TNF-α and IL-1β (Figure 2B). The decrease in inflammatory mediators observed in the AM-treated CIA mice could be a consequence of the diminished infiltration of inflammatory cells in the inflamed joints. However, AM inhibited the production of proinflammatory mediators by synovial cells isolated from CIA mice on in vitro CII restimulation (Figure 2C). However, CGRP only showed a slight inhibitory effect (Figure 2C). This suggests that, in addition to the reduction in inflammatory infiltration, AM administration could deactivate the inflammatory response of infiltrating/proliferating synovial cells.
Figure 2.
AM inhibits inflammatory response in CIA. Systemic and local expression of inflammatory mediators in untreated (control) or AM-treated CIA mice assayed at day 35 after immunization. A: Cytokine/chemokine contents in joints. A paw from a nonimmunized mouse was analyzed simultaneously for assessment of the basal response. B: Serum TNF-α and IL-1β levels. n = 6 to 8 mice per group. *P < 0.001 versus controls. C: Cytokine/chemokine production by synovial membrane cells from CIA mice activated with CII in the absence or presence of different concentrations of AM or CGRP. n = 3 experiments performed in duplicate. *P < 0.001 versus control.
AM Down-Regulates Th1-Mediated Autoreactive Response in CIA
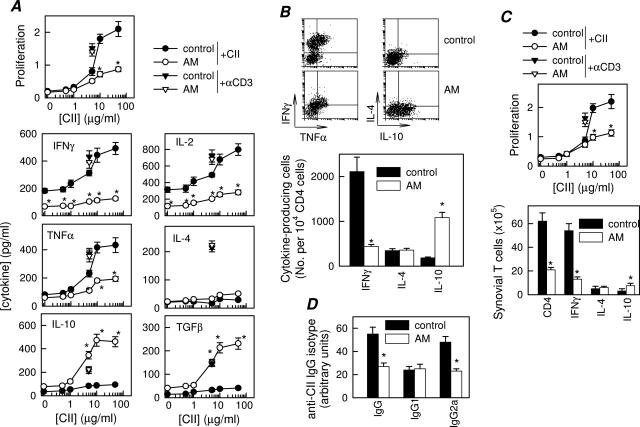
Although macrophages and neutrophils are the major sources of inflammatory mediators, CD4 T cells play a key role in the initiation and perpetuation of CIA by producing IFN-γ, a potent inducer of the inflammatory response. In fact, CIA is considered an archetypal Th1-type cell-mediated autoimmune disease.1,2 Therefore, AM could ameliorate CIA by reducing autoreactive T-cell responses and/or migration to the joints. We determined proliferation and cytokine profile of DLN cells isolated from AM-treated arthritic mice in response to antigen (CII) in vitro. DLN cells obtained from CIA mice showed marked CII-specific proliferation and effector T cells producing high levels of Th1-type cytokines (IFN-γ, IL-2, and TNF-α) and low levels of Th2-type cytokines (IL-4 and IL-10) (Figure 3A). In contrast, DLN cells from AM-treated mice proliferated much less, produced low levels of Th1 cytokines and high amounts of regulatory cytokines (IL-10 and TGF-β1); the Th2-type cytokine IL-4 was not significantly affected (Figure 3A). This effect was antigen-specific, because AM treatment did not affect proliferation and cytokine production by DLN cells stimulated with anti-CD3 or with an unrelated antigen OVA (Figure 3A) or by PPD-stimulated spleen cells from PPD/complete Freund’s adjuvant-immunized CIA mice (not shown). This suggests that AM administration during CIA progression partially inhibits autoreactive Th1 cell clonal expansion. To distinguish whether the decrease in Th1 cytokine production induced by AM treatment is a consequence of either down-regulation of cytokine release or inhibition of Th1 cell differentiation, and whether the macrophages or CD4 T cells are the source of IL-10, we determined the intracellular expression of these cytokines by flow cytometry in sorted CD4 T cells. AM significantly decreased the number of TNF-α-/IFN-γ-producing Th1 cells and increased the number of IL-10-producing CD4 T cells in DLNs (Figure 3B). Thus, AM administration to CIA mice regulates the generation and/or differentiation of autoreactive/inflammatory Th1 cells and, presumably, IL-10-secreting Treg cells. We observed a similar effect on synovial cells, in which synovial cells from AM-treated CIA mice proliferated less than those untreated animals in response to the antigen (Figure 3C, top), and showed less IFN-γ-producing CD4 Th1 cells and higher numbers of IL-10-producing T cells (Figure 3C, bottom).
Figure 3.
AM down-regulates Th1-mediated response in CIA. A: Proliferative response and cytokine production of DLN cells isolated at day 30 from untreated (control) or AM-treated CIA mice and stimulated in vitro with different concentrations of CII. Stimulation of DLN cells with anti-CD3 antibodies (▾, for untreated CIA mice; ▿, for AM-treated CIA mice) was used for assessment of nonspecific stimulation. A pool of three nonimmunized DBA/1 DLN cells was used for assessment of the basal response. No proliferation or cytokine production by T cells was detectable in the presence of an unrelated antigen (OVA). n = 5 mice per group. B: Number of CII-specific cytokine-producing T cells. DLN cells from untreated (control) or AM-treated CIA mice were restimulated in vitro with CII (10 μg/ml) and analyzed for CD4 and intracellular cytokine expression by flow cytometry. Dot plots show representative double staining for IFN-γ/TNF-α or IL-4/IL-10 expression in gated CD4 T cells. The number of IFN-γ-, IL-4-, and IL-10-expressing T cells relative to 104 CD4 T cells is shown in the bottom panel. Data shown represent pooled values from two independent experiments. C: CII-specific proliferative response and number of cytokine-producing CD4 T cells in synovial membrane cells isolated from untreated (control) or AM-treated CIA mice and stimulated in vitro with CII (10 μg/ml) for 48 hours. Data show the results of pooled synovial cells from three animals per group. D: CII-specific IgG, IgG1, and IgG2a levels in serum collected at day 35 from untreated (control) or AM-treated CIA mice (8 to 12 mice per group). *P < 0.001 versus controls.
High levels of circulating antibodies directed against collagen-rich joint tissue invariably accompany the development of RA and CIA, and their production is a major factor in determining susceptibility to the disease.15 AM administration resulted in reduced serum levels of CII-specific IgG, particularly autoreactive IgG2a antibodies, generally reflective of Th1 activity (Figure 3D). These data provide further evidence that AM administration during CIA reduces the Th1 autoreactive responses in both the joint and periphery.
AM Induces the Emergence of Regulatory CD4+CD25+ T Cells in CIA
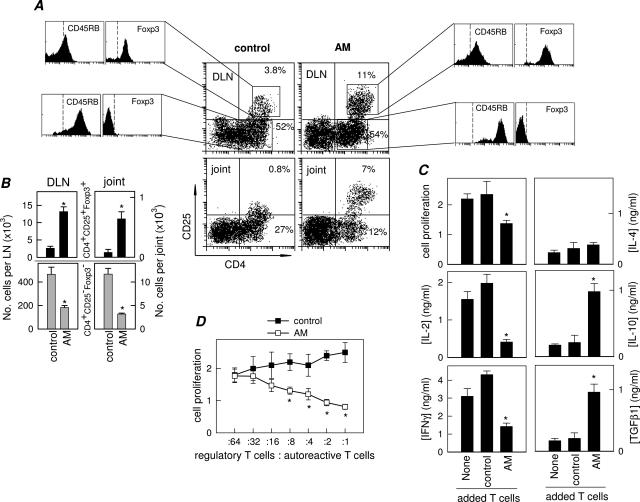
Several studies have indicated that Treg cells confer significant protection against CIA by decreasing the activation and joint homing of autoreactive Th1 cells.16–18 Because the AM treatment also inhibited events in the inflammatory phase of CIA after the activation of antigen-specific Th1 cells and induced the generation of IL-10/TGF-β1-producing T cells, the possibility exists that AM induces Treg with suppressive activity during the progression of the disease. AM-treated CIA mice had significantly higher percentages and numbers of CD4+CD25+ cells in both DLN and synovium compared with control CIA mice (Figure 4, A and B). AM-induced CD4+CD25+ cells additionally exhibited a Treg phenotype,19 ie, CD45RBlowFoxp3+ (Figure 4, A and B).
Figure 4.
AM induces the emergence of regulatory CD4+CD25+ T cells in CIA. A: Flow cytometry analysis of DLN and synovial (joint) CD4+CD25+ cells isolated at day 30 from untreated (control) or AM-treated CIA mice. Numbers represent percentages of CD4+CD25− and CD4+CD25+ cells. Histograms show the expression of CD45RB and Foxp3 in gated CD4+CD25− and CD4+CD25+ cells in DLN. Similar profiles were observed for synovial cells. Dashed lines in histograms are isotype antibody controls. Data are representative of five mice per group. B: Number of CD4+CD25+Foxp3+ cells (black bars) and CD4+CD25−Foxp3− cells (gray bars) per DLN and synovial membrane (joint) isolated from untreated (control) and AM-treated CIA mice (six mice per group). C: Proliferative response and cytokine production of autoreactive T cells isolated from CIA mice stimulated with spleen APCs and CII (10 μg/ml) in the absence (none) or presence of DLN T cells isolated from untreated (control) or AM-treated CIA mice. n = 3 experiments performed in duplicate. D: Proliferative response of autoreactive T cells (4 × 105 cells) isolated from CIA mice co-cultured with increasing numbers of DLN T cells (regulatory T cells) from untreated (control) or AM-treated CIA mice (ratios from 1:64 to 1:1), and stimulated with CII (10 μg/ml) and splenic APCs. n = 3 experiments performed in duplicate. *P < 0.01 versus controls.
When stimulated, Tregs suppress the proliferation and IL-2 production of antigen-specific effector T cells. Several mechanisms have been identified for Treg function, such as surface CTLA-4 and TGF-β expression, co-stimulatory blockade, and IL-10 and/or TGF-β release.18,19 Naturally occurring CD4+CD25+ Tregs exert their suppressive activity primarily through direct cellular contact, whereas peripheral T suppressors act primarily through cytokines.19 To determine whether T cells isolated from AM-treated CIA mice function as suppressive Tregs, we co-cultured T cells isolated from DLNs of arthritic mice treated with PBS (Tcontrol) or AM (TAM) with autoreactive T cells isolated from DLN of CIA mice in the presence of APCs and antigen (CII). Tcontrol did not suppress the proliferation of autoreactive T cells, and slightly up-regulated IL-2 and IFN-γ production in response to CII (Figure 4C). In contrast, TAM suppressed the proliferation of autoreactive T cells and inhibited IL-2 and IFN-γ production while increasing the levels of the regulatory cytokines IL-10 and TGF-β (Figure 4C). The suppression increased with the number of TAM, being effective even at a ratio as low as one TAM to eight autoreactive T cells (Figure 4D). These results demonstrate that AM administration during arthritis development induces the generation and/or activation of Treg that efficiently suppress autoreactive CD4 T cells.
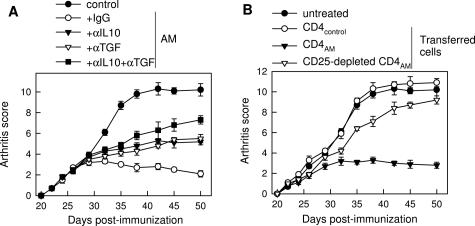
Because AM seems to induce in vivo expansion of IL-10/TGF-β-producing CD4+CD25+ Tregs in CIA mice, we further examined the role of these cells in the therapeutic action of AM in arthritis. In vivo blockade experiments showed that treatment with antibodies against IL-10 and/or TGF-β, major mediators of Treg function, significantly reversed the AM therapeutic effect in arthritis (Figure 5A), suggesting the involvement of Treg. In addition, we assessed the ability of these cells to affect CIA after disease onset. CIA mice were treated 2 days after the onset of the disease with CD4 T cells isolated from arthritic mice treated with medium (CD4control) or AM (CD4AM). In contrast to CD4control, injection of CD4AM prevented development of arthritis, and depletion of CD25+ cells from CD4AM significantly abolished their therapeutic effect (Figure 5B).
Figure 5.
Involvement of Treg in the therapeutic effect of AM in CIA. A: CIA mice were treated with medium (control) or with AM (2 nmol/day) for 5 days and were treated with control Ig, anti-IL-10 (αIL10), anti-TGF-β (αTGFβ), or a combination of both antibodies on alternate days starting with the first AM administration (five to seven mice per group). Severity of arthritis was assessed by clinical scoring. P < 0.001 versus Ig control treatment for αIL10 and/or αTGFβ treatments after day 37. B: CD4+ cells were isolated from DLN of CIA mice treated with medium (CD4control) or AM (CD4AM) at the peak of disease and stimulated in vitro with CII. Some samples were depleted of CD25+ cells. Cells (5 × 106) were injected intravenously into arthritic mice 2 days after disease onset (four to six mice per group). CIA mice without any treatment (untreated) were used as arthritic controls. P < 0.001 versus untreated control for CD4AM treatment after day 32. P < 0.001 versus CD4AM treatment for CD25-depleted CD4AM cells after day 35.
Discussion
In this study, we show that the neuropeptide AM provides a highly effective therapy for CIA. The therapeutic effect of AM is associated with a striking reduction of the two deleterious components of the disease, ie, the autoimmune and inflammatory response. AM treatment decreased the presence of autoreactive Th1 cells in the periphery and the joint. In addition, AM strongly reduced the inflammatory response during CIA progression by down-regulating the production of several inflammatory mediators, such as various cytokines and chemokines in the joints. As a consequence, AM reduced the frequency of arthritis, ameliorated symptoms, and prevented joint damage. From a therapeutic point of view, it is important to consider the ability of delayed administration of AM to ameliorate ongoing disease and that an initial treatment with AM prevented recurrence of the disease.
The capacity of AM to regulate a plethora of inflammatory mediators might offer a therapeutic advantage over neutralizing antibodies and receptor antagonists directed against a single mediator. The infiltration into the joint and activation of various leukocyte populations, which contribute to CIA pathology, are regulated by chemokines.2,3,20 The fact that AM treatment reduced the expression of a wide spectrum of chemokines could partially explain the absence of inflammatory infiltrates in the joint tissues of AM-treated mice. In this sense, chemokines such as MIP-2 (chemotactic for neutrophils) and RANTES (for macrophages and T cells) are especially relevant because they are involved in CIA pathogenesis.20,21 In addition to the regulation of cell recruitment to the joint, AM also regulates the inflammatory cell activation and cytokine production. Thus, AM down-regulated the production of the proinflammatory/cytotoxic cytokines TNF-α, IFN-γ, IL-6, IL-1β, and IL-12 in the inflamed joint. At the same time, AM increased the levels of the anti-inflammatory cytokines IL-10 and TGF-β, which ameliorate the disease.22,23 The decrease in inflammatory mediators could be the consequence of a diminished infiltration of inflammatory cells in the synovium. However, the fact that AM inhibited the production of proinflammatory mediators by synovial cells isolated from CIA mice on in vitro CII-specific Ab treatment argues against this hypothesis. This suggests that, in addition to the reduction in inflammatory infiltration, AM deactivates the inflammatory response. Recent studies demonstrated that AM acts as a macrophage-deactivating factor by down-regulating the production of a wide board of inflammatory mediators.10,11 Therefore, it is plausible that deactivation of resident and infiltrating macrophages is a major mechanism involved in the anti-inflammatory action of AM in CIA.
Although the contribution of Th1 responses in RA is not completely understood, several studies in animal models point to a pathogenic role for Th1-derived cytokines.2,3 Th1 cells react to components of the joint, infiltrate the synovium, release proinflammatory cytokines and chemokines, and promote macrophage and neutrophil infiltration and activation. Our results demonstrate that the treatment of arthritic mice with AM results in a decreased CII-specific Th1-mediated response. It seems that the inhibition of the Th1 response is caused by a direct action on synovial and DLN cells, because synovial and DLN cells obtained from AM-treated animals are refractory to Th1 cell stimulation. In contrast to IFN-γ and TNF-α, AM increased the production of IL-10 and TGF-β1. These effects seem to be independent of the action of the peptide on macrophages, because AM directly affects T cells in the absence of APCs (M.D., submitted for publication). The fact that AM administration increased IL-10, but not IL-4, production in synovial and DLN CD4 T cells argues against a shift toward Th2 responses. IL-10 has been recognized recently as a signature cytokine for a subset of CD4 T cells that exert regulatory functions.24 CD4+CD25+ Tregs have been reported to play a critical function in the regulation of autoimmune diseases, including RA and CIA.16–19 In this sense, our study demonstrated that AM administration to CIA mice induces the appearance of CD4+CD25+Foxp3+ cells with a Treg phenotype in the DLNs and joints. Some evidence indicates that these Tregs play a major role in the therapeutic action of AM in CIA. Our study demonstrates that the unfractionated CD4 cells isolated from AM-treated mice showed an important therapeutic effect on CIA, which was significantly abrogated when they were depleted of CD25+ cells. Furthermore, in vivo blockade of the Treg mediators IL-10 and TGF-β partially reversed the therapeutic effect of AM. Therefore, the Treg induced by AM in the periphery apparently could migrate to the joints and induce local suppression of the self-reactive response. This partially explains that delayed AM treatment also inhibits events in the inflammatory phase of CIA after the activation and/or differentiation of antigen-specific effector Th1 cells. In addition to expanding the CD4+CD25+ population, AM could also induce more efficient Treg in vivo, both in terms of cytokine secretion and suppressive activity. The AM-induced Treg cells produce high levels of IL-10 and TGF-β1, and on a per cell basis, they are very strong suppressors of responder autoreactive T-cell proliferation, particularly at low regulatory T cell/autoreactive T-cell ratios. AM-induced generation of Tregs during CIA correlates with our data showing that AM treatment increases the production of IL-10 and TGF-β1, two of the major mediators of Treg. The mechanisms involved in the generation and/or activation of Tregs by AM during CIA are not fully understood. Whether AM acts directly on T cells inducing the generation or expansion of CD4+CD25+ Treg cells remains to be established. Preliminary experiments indicate that AM induces the differentiation of tolerogenic dendritic cells with capacity to generate IL-10-secreting Treg cells (M.D., unpublished results).
AM is structurally related to CGRP and is an endogenous ligand of the calcitonin-related-like receptor (CRLR).5 Although, increased CGRP levels have been found in patients and animals with arthritis,25,26 and therefore, CGRP has been suspected as immunomodulatory factor,27 CGRP does not efficiently down-regulate the synovial inflammatory response and fails to prevent CIA (Figures 1C and 2D).28 The difference in effectiveness between AM and CGRP could reside in the differential binding of both peptides to different binding complexes, which are formed by the CRLR and various receptor activity-modifying proteins (RAMP1, RAMP2, and RAMP3). The binding specificity for the ligand and the activity of the receptor depend on the RAMP subtype associated to CRLR. Thus, CGRP specifically binds to the CRLR-RAMP1 complex, whereas AM preferentially binds to CRLR associated to RAMP2 and RAMP3.5 Murine macrophages specifically express CRLR-RAMP2/3 complexes (M.D., unpublished data), and CRLR-RAMP2 complexes, specific for AM but not for CGRP, are differentially up-regulated during inflammatory processes.29 This partially explains the higher potency of AM versus CGRP on the inflammatory response.
Of physiological relevance is the observation that the expression of AM is increased in activated inflammatory cells6–8 and in several inflammatory conditions, including RA,30,31 and that AM in synovial fluid from RA patients inhibits IL-6 production by synoviocytes.32 Therefore, it is attractive to speculate that the body responds to an exacerbated inflammatory response by increasing the peripheral production of endogenous anti-inflammatory factors, such as AM, in an attempt to restore the inflammatory homeostasis. Thus, this work adds AM to a list of endogenous anti-inflammatory neuropeptides, such as vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, α-melanocyte-stimulating hormone, urocortin, estrogens, and glucocorticoids among others that have been found to exert anti-arthritic effects.12,33–37 Interestingly, all these neuropeptides show similar efficiencies abrogating arthritic symptoms. This could be explained by the fact that they mainly exert their effect by activating the cAMP/protein kinase A pathway and inhibiting the transactivation of nuclear factor-κB, a transcription factor involved in the regulation of several inflammatory mediators.38 It is important to note that AM has been previously tested in human patients.39 Therefore, AM should be well tolerated in humans in doses similar to those that are able to prevent CIA. In addition, the fact that AM does not completely block the inflammatory/immune response, not inducing a general immunosuppressive state, is an advantage against other agents used in RA.
In summary, we here identify AM as an immunomodulatory factor with the capacity to deactivate the inflammatory response in vivo at multiple levels and to maintain immune tolerance. Therefore, this work provides a powerful rationale for the assessment of the efficacy of AM as an attractive therapeutic approach to the treatment of RA and other chronic inflammatory autoimmune disorders.
Footnotes
Address reprint requests to Elena Gonzalez-Rey, Instituto de Parasitologia y Biomedicina, CSIC, Avd. Conocimiento, PT Ciencias de la Salud, Granada 18100, Spain. E-mail: elenag@ipb.csic.es.
Supported by the Spanish Ministry of Health (grants PI04/0674 and PI06/1291) and the Ramon Areces Foundation.
References
- Anthony DD, Haqqi TM. Collagen-induced arthritis in mice: an animal model to study the pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 1999;17:240–244. [PubMed] [Google Scholar]
- Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- Brand DD, Kang AH, Rosloniec EF. Immunopathogenesis of collagen arthritis. Springer Semin Immunopathol. 2003;25:3–18. doi: 10.1007/s00281-003-0127-1. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21:138–167. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- Elsasser TH, Kahl S. Adrenomedullin has multiple roles in disease stress: development and remission of the inflammatory response. Microsc Res Tech. 2002;57:120–129. doi: 10.1002/jemt.10058. [DOI] [PubMed] [Google Scholar]
- Ueda S, Nishio K, Minamino N, Kubo A, Akai Y, Kangawa K, Matsuo H, Fujimura Y, Yoshioka A, Masui K, Doi N, Murao Y, Miyamoto S. Increased plasma levels of adrenomedullin in patients with systemic inflammatory response syndrome. Am J Respir Crit Care Med. 1999;160:132–136. doi: 10.1164/ajrccm.160.1.9810006. [DOI] [PubMed] [Google Scholar]
- Kubo A, Minamino N, Isumi Y, Katafuchi T, Kangawa K, Dohi K, Matsuo H. Production of adrenomedullin in macrophage cell line and peritoneal macrophage. J Biol Chem. 1998;273:16730–16738. doi: 10.1074/jbc.273.27.16730. [DOI] [PubMed] [Google Scholar]
- Wong LY, Cheung BM, Li YY, Tang F. Adrenomedullin is both proinflammatory and antiinflammatory: its effects on gene expression and secretion of cytokines and macrophage migration inhibitory factor in NR8383 macrophage cell line. Endocrinology. 2005;146:1321–1327. doi: 10.1210/en.2004-1080. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Chorny A, Robledo G, Delgado M. Urocortin and adrenomedullin prevent lethal endotoxemia by downregulating the inflammatory response. Am J Pathol. 2006;168:1921–1930. doi: 10.2353/ajpath.2006.051104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Delgado M. Therapeutic effect of urocortin and adrenomedullin in a murine model of Crohn’s disease. Gut. 2006;55:824–832. doi: 10.1136/gut.2005.084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7:563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- Kasama T, Strieter RM, Lukacs NW, Lincoln PM, Burdick MD, Kunkel SL. Interleukin-10 and chemokine regulation during the evolution of murine type-II collagen-induced arthritis. J Clin Invest. 1995;95:2868–2876. doi: 10.1172/JCI117993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Leceta J, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate-cyclase activating polypeptide stimulate the induction of Th2 responses by up-regulating B7.2 expression. J Immunol. 1999;163:3629–3635. [PubMed] [Google Scholar]
- Seki N, Sudo Y, Yoshioka T, Sugihara S, Fujitsu T, Sakuma S, Ogawa T, Hamaoka T, Senoh H, Fujiwara H. Type II collagen-induced murine arthritis induction and perpetuation of arthritis require synergy between humoral and cell-mediated immunity. J Immunol. 1988;140:1477–1487. [PubMed] [Google Scholar]
- Yudoh K, Matsuno H, Nakazawa F, Yonezawa T, Kimura T. Reduced expression of the regulatory CD4+ T cell subset is related to Th1/Th2 balance and disease severity in rheumatoid arthritis. Arthritis Rheum. 2000;43:617–627. doi: 10.1002/1529-0131(200003)43:3<617::AID-ANR19>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Morgan ME, Sutmuller RP, Witteveen HJ, van Duivenvoorde LM, Zanelli E, Melief CJ, Snijders A, Offringa R, de Vries RR, Toes RE. CD25+ depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–1460. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- Wraith DC, Nicolson KS, Whitlet NT. Regulatory CD4+ T cells and the control of autoimmune disease. Curr Opin Immunol. 2004;16:695–701. doi: 10.1016/j.coi.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answer. Nat Rev Immunol. 2002;2:816–822. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- Koch AE. Chemokines and their receptors in rheumatoid arthritis: future targets? Arthritis Rheum. 2005;52:710–721. doi: 10.1002/art.20932. [DOI] [PubMed] [Google Scholar]
- García-Vicuña R, Gomez-Gaviro MV, Dominguez-Luis MJ, Pec MK, Gonzalez-Alvaro I, Alvaro-Gracia JM, Diaz-Gonzalez F. CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Rheum. 2004;50:3866–3877. doi: 10.1002/art.20615. [DOI] [PubMed] [Google Scholar]
- Kuruvilla AP, Shah R, Hochwald GM, Liggitt HD, Palladito MA, Thorbecke GJ. Protective effect of transforming growth factor beta 1 on experimental autoimmune diseases in mice. Proc Natl Acad Sci USA. 1991;88:2918–2921. doi: 10.1073/pnas.88.7.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley M, Katsikis PD, Abney E, Parry S, Williams RO, Maini RN, Feldmann M. Interleukin-10 inhibition of the progression of established collagen-induced arthritis. Arthritis Rheum. 1996;39:495–503. doi: 10.1002/art.1780390318. [DOI] [PubMed] [Google Scholar]
- Thompson C, Powrie F. Regulatory T cells. Curr Opin Pharmacol. 2004;4:408–414. doi: 10.1016/j.coph.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Larsson J, Ekblom A, Henriksson K, Lundeberg T, Theodorsson E. Concentration of substance P, neurokinin A, calcitonin gene-related peptide, neuropeptide Y and vasoactive intestinal polypeptide in synovial fluid from knee joints in patients suffering from rheumatoid arthritis. Scand J Rheumatol. 1991;20:326–335. doi: 10.3109/03009749109096808. [DOI] [PubMed] [Google Scholar]
- Weihe E, Nohr D, Schafer MK, Persson S, Ekstrom G, Kallstrom J, Nyberg F, Post C. Calcitonin gene related peptide gene expression in collagen-induced arthritis. Can J Physiol Pharmacol. 1995;73:1015–1019. doi: 10.1139/y95-142. [DOI] [PubMed] [Google Scholar]
- Takeba Y, Suzuki N, Kaneko A, Asai T, Sakane T. Evidence for neural regulation of inflammatory synovial cell functions by secreting calcitonin gene-related peptide and vasoactive intestinal peptide in patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:2418–2429. doi: 10.1002/1529-0131(199911)42:11<2418::AID-ANR21>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Hernanz A, Medina S, de Miguel E, Martin-Mola E. Effect of calcitonin gene-related peptide, neuropeptide Y, substance P, and vasoactive intestinal peptide on interleukin-1beta, interleukin-6 and tumor necrosis factor-alpha production by peripheral whole blood cells from rheumatoid arthritis and osteoarthritis patients. Regul Pept. 2003;115:19–24. doi: 10.1016/s0167-0115(03)00127-7. [DOI] [PubMed] [Google Scholar]
- Ono Y, Okano I, Kojima M, Okada K, Kangawa K. Decreased gene expression of adrenomedullin receptor in mouse lungs during sepsis. Biochem Biophys Res Commun. 2000;271:197–202. doi: 10.1006/bbrc.2000.2606. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Matsui N, Yoshiya S, Fujioka H, Kurosaka M. Production of adrenomedullin from synovial cells in rheumatoid arthritis patients. Rheumatol Int. 2004;24:20–24. doi: 10.1007/s00296-003-0315-2. [DOI] [PubMed] [Google Scholar]
- Yudoh K, Matsuno H, Kimura T. Plasma adrenomedullin in rheumatoid arthritis compared with other rheumatic diseases. Arthritis Rheum. 1999;42:1297–1298. doi: 10.1002/1529-0131(199906)42:6<1297::AID-ANR30>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Nanke Y, Kotake S, Yonemoto K, Saito S, Tomatsu T, Kamatani N. Adrenomedullin in synovial fluids from patients with rheumatoid arthritis inhibits interleukin 6 production from synoviocytes. Ann Rheum Dis. 2003;62:82–83. doi: 10.1136/ard.62.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abad C, Martinez C, Leceta J, Gomariz RP, Delgado M. Pituitary adenylate cyclase-activating polypeptide inhibits collagen-induced arthritis: an experimental immunomodulatory therapy. J Immunol. 2001;167:3182–3189. doi: 10.4049/jimmunol.167.6.3182. [DOI] [PubMed] [Google Scholar]
- Ceriani G, Diaz J, Murphree S, Catania A, Lipton JM. The neuropeptide alpha-melanocyte-stimulating hormone inhibits experimental arthritis in rats. Neuroimmunomodulation. 1994;1:28–32. doi: 10.1159/000097087. [DOI] [PubMed] [Google Scholar]
- Josefsson E, Tarkowski A. Suppression of type II collagen-induced arthritis by the endogenous estrogen metabolite 2-methoxyestradiol. Arthritis Rheum. 1997;40:154–163. doi: 10.1002/art.1780400120. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Chorny A, Varela N, O′Valle F, Delgado M: Therapeutic effect of urocortin on collagen-induced arthritis by downregulating inflammatory and Th1 response and inducing regulatory T cells. Arthritis Rheum (in press) [DOI] [PubMed] [Google Scholar]
- Cerinic MM, Konttinen Y, Generini S, Cutolo M. Neuropeptides and steroid hormones in arthritis. Curr Opin Rheumatol. 1998;10:220–235. doi: 10.1097/00002281-199805000-00011. [DOI] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Satoh T, Nishikimi T, Uematsu M, Furuichi S, Sakamaki F, Oya H, Kyotani S, Nakanishi N, Goto Y, Masuda Y, Miyatake K, Kangawa K. Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation. 2000;101:498–503. doi: 10.1161/01.cir.101.5.498. [DOI] [PubMed] [Google Scholar]