Abstract
The complete resection of pituitary adenomas (PAs) is unlikely when there is an extensive local dural invasion and given that the molecular mechanisms remain primarily unknown. DNA microarray analysis was performed to identify differentially expressed genes between nonfunctioning invasive and noninvasive PAs. Gene clustering revealed a robust eightfold increase in matrix metalloproteinase (MMP)-9 expression in surgically resected human invasive PAs and in the (nonfunctioning) HP75 human pituitary tumor-derived cell line treated with phorbol-12-myristate-13-acetate; these results were confirmed by real-time polymerase chain reaction, gelatin zymography, reverse transcriptase-polymerase chain reaction, Western blot, immunohistochemistry, and Northern blot analyses. The activation of protein kinase C (PKC) increased both MMP-9 activity and expression, which were blocked by some PKC inhibitors (Gö6976, bisindolylmaleimide, and Rottlerin), PKC-α, and PKC-δ small interfering (si)RNAs but not by hispidin (PKC-β inhibitor). In a transmembrane invasion assay, phorbol-12-myristate-13-acetate (100 nmol/L) increased the number of invaded HP75 cells, a process that was attenuated by PKC inhibitors, MMP-9 antibody, PKC-α siRNA, or PKC-δ siRNA. These results demonstrate that MMP-9 and PKC-α or PKC-δ may provide putative therapeutic targets for the control of PA dural invasion.
Pituitary adenoma (PA) accounts for 15 to 20% of primary brain tumors. Surgical resection is the treatment of choice for most symptomatic PAs. Although PAs are rarely malignant, they often invade surrounding structures including the cavernous sinus, diaphragm, and bone. The goal of surgery is complete tumor removal, the success of which is strongly affected by the presence of local invasion. Approximately 40% of PAs have macroscopic evidence of local invasion, and as much as 80% are invasive on microscopic examination.1,2 Complete resection of PA is unlikely when there is extensive local invasion, and surgery for invasive tumors carries increased risks of complications. Failure to achieve surgical cure, incidence of recurrence, and poor outcome are all related to PA invasiveness.
Adjuvant therapy in the form of radiotherapy or medications may be required after incomplete tumor resection, but their indications remain controversial. Not all residual tumors progress or produce symptoms. Response to treatment may also vary between patients and between different tumor subtypes. Conversely, tumors with aggressive behavior may benefit from adjuvant treatment despite apparently complete removal. The pathogenesis of PAs and the factors that determine their proliferation, local invasiveness, and response to adjuvant treatment are incompletely understood. Mutations identified in a significant proportion of pituitary tumors, particularly in growth hormone-secreting adenomas, have been discovered in the gene encoding the α-subunit of Gs G-protein (GNAS1), causing constitutive activation of the cAMP pathway (gsp oncogene).3 A point mutation of protein kinase C (PKC)-α and a higher overall PKC activity and expression have been documented in invasive PAs.4,5 However, other investigators have failed to detect such a change.6 Reduced conventional PKC activity had been observed in some cases of prolactinomas that responded favorably to exogenous dopamine agonists,7 and dose-dependent inhibition of cell growth in pituitary tumor cell culture by hypericin (a PKC inhibitor) also had been demonstrated.8 PKC is a family of ubiquitous phospholipid-dependent enzymes involved in signal transduction pathways associated with a variety of cellular responses including cell growth and invasion in an isozyme-specific manner. The activities of both the conventional (α, βI, βII, γ) and novel (μ, θ, ε, η, δ) PKC isozymes are regulated by phorbol esters, diacylglycerols, and phospholipids. Conventional PKC isozymes (cPKC) require Ca2+ for activity, whereas novel (nPKC) and atypical (λ, ζ) are Ca2+-independent.9 The atypical isozymes (aPKC) are not activated by diacylglycerol, a product of receptor-mediated phospholipid hydrolysis.10
A number of studies have also reported elevated levels of serine proteases and metalloproteinases in PAs,11,12 whereas other researchers have failed to confirm these results.13,14 Other factors elevated in invasive human PAs include matrix metalloproteinase (MMP)-215 and epidermal growth factor receptor.16 The proteases that degrade extracellular matrix and basement membranes are the MMPs and plasmin. Increased levels of these proteases occur in tumor and their levels directly correlate with the tumor grade.17–19 The MMPs are a family of zinc-containing endopeptidases that act on different or overlapping sets of substrates.20,21 The MMP genes are a highly conserved modular structure. Human MMP-9, located on chromosome 20q12-13, degrades extracellular matrix substrates including collagens (IV, V, and IX), gelatin, elastin, fibronectin, and proteoglycan-link protein.22 MMP-9 is activated by MMP-2, MMP-3, and MMP-13, as well as by plasmin.22 The urokinase-type plasminogen activator (uPA) has been implicated in tumor cell migration and invasion that require extensive proteolysis of the cellular matrix.23 Urokinase converts cell-associated plasminogen into plasmin, which degrades several extracellular matrix components including laminin, fibronectin, and possibly type IV collagen and catalyzes the conversion of pro-MMPs to active MMPs.24,25
Phorbol-12-myristate-13-acetate (PMA) activation of PKC has been shown to increase MMP-9 expression in a host of tumor cells, including glioblastomas and hepatocellular and squamous cell carcinomas.26–28 The mechanism by which PKC activates MMP-9 is highly cell-type-specific and the differential expression of PKC isozymes may account for the various underlying signal transduction pathways involved in MMP-9 activation. Therefore, it is important to identify which PKC isozyme(s) is regulating the expression of MMP-9 in PAs.
DNA microarray analysis was used to identify clusters of genes that are either overexpressed or suppressed in invasive and noninvasive PAs. This technology narrowed down the number of significantly altered genes in the PAs and provided us with a platform to test the functional role of one of the up-regulated genes, MMP-9. Our results show increased level and activity of MMP-9 in invasive PAs and the PKC-activated HP75 cell line. PMA treatment of HP75 cells increased the activity and expression of MMP-9, which was blocked by bisindolylmaleimide (BIM) (nonspecific PKC inhibitor), Gö6976 (PKC-α and PKC-β inhibitor), Rottlerin (PKC-δ inhibitor at 5 μmol/L), PKC-α, and PKC-δ siRNA. Likewise, PMA-induced increase in HP75 transwell invasion was blocked by BIM, Gö6976, Rottlerin, MMP-9-neutralizing antibody, and a combined PKC-α and PKC-δ siRNA.
Materials and Methods
Materials
PMA and tubulin antibody (DMA1) were purchased from Sigma Chemical (St. Louis, MO). The PKC inhibitors, BIM, Gö6976, hispidin, Rottlerin, and MMP-9 (Ab-1) are products of Calbiochem (San Diego, CA). Polyclonal antibodies against MMP-2 and MMP-9 were purchased from Cell Signaling (Beverly, MA). Scramble, PKC-α, and PKC-δ siRNA SMART pools (each pool contained four different sequences) were purchased from Dharmacon (Lafayette, CO). PKC-α, δ, βI, and ε antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Noninvasive and Invasive PAs
We used only clinically nonfunctioning adenomas, morphologically characterized by histological, immunohistochemical, and ultrastructural analyses. The PA specimens were collected from the University of Virginia Medical Center under the approval of the Human Investigation Committee. The tissues (five noninvasive and three invasive PAs) were flash frozen in liquid nitrogen immediately after surgical resection and stored at −80°C. The adenomas were subdivided into invasive (one thyrotroph-cell adenoma, positive for thyroid-stimulating hormone; one null-cell adenoma, focal and faintly positive for luteinizing hormone, follicle-stimulating hormone, and α-subunit; and one null-cell adenoma, immunonegative) and noninvasive (one gonadotroph-cell adenoma, positive for follicle-stimulating hormone and α-subunit; one sparsely granulated growth hormone-cell, positive for growth hormone; one null-cell adenoma, focal and faintly positive for α-subunit; one gonadotroph-cell adenoma, positive for follicle-stimulating hormone, luteinizing hormone, and α subunit; and one null-cell adenoma, which was immunonegative) according to the presence or absence of dural invasion by tumor cells on microscopic examination. Although this pathological criterion of invasion may underestimate actual incidence of tumor invasion by radiological and surgical observation, we opted for it because of its objectivity.
Cell Culture
The HP75 cell line is an immortalized human PA cell line developed originally from a nonfunctioning (nonhormone-secreting) PA with a replication-defective recombinant human adenovirus with a SV40 early large T-antigen.29 The HP75 cell line was a generous gift from Dr. Ricardo V. Lloyd (Mayo Clinic, Rochester, MN). HP75 cells were grown to 90 to 100% confluence in Dulbecco’s modified Eagle’s medium with glutamine (2 mmol/L), insulin (10 μmol/L), high glucose (4.5 g/l), and 15% fetal bovine serum.
DNA Microarray Analysis
Total RNA (20 μg/specimen) from five recently resected noninvasive and three invasive PAs, control, and PMA-treated (6 hours) HP75 cells were extracted for analysis. After RNeasy Micro column purification to remove 5s and tRNA contamination, the RNA was used for microarray analysis using Affymetrix HG 133 plus 2.0 gene chips containing 54,613 transcripts through the National Institute of Neurological Disorder and Stroke (National Institutes of Health) Microarray Consortium at the University of California–Los Angeles.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
RNA was extracted from cell lines using Trizol (Invitrogen, Carlsbad, California) according to the manufacturer’s protocol. Total RNA (2 μg) was subjected to cDNA synthesis using the first strand DNA synthesis kit (Bio-Rad, Hercules, CA). Oligonucleotide primers used for MMP-9 detection were: forward, 5′-ACCCAATCTCACCGACAG-3′ and reverse, 5′-CAAAGGCGTCGTCAATCA-3′, producing 317-bp fragments. Primers used for MMP-2 detection were: forward, 5′-TCG CCC ATC ATC AAG TTC-3′; reverse, 5′-GTGATCTGGTTCTTGTCC-3′, product = 309 bp. Other primers used include β-actin (forward, 5′-CACCATGGATGATGATATCG-3′; reverse, 5′-TGGATAGCAACGTACATGG-3′; product = 433 bp), α1A-adrenergic receptor (forward, 5′-GAAGGGCAACACAAGGACAT-3′; reverse, 5′-ACTTCCTCCCCGTTCTCACT-3′; product = 295 bp), MMP-1 (forward, 5′-GATGTGGAGTGCCTGATGTG-3′; reverse, 5′-CTGGTTGAAAAGCATGAGCA-3′; product = 290), MMP-3 (forward, 5′-TGCTTTGTCCTTTGATGCTG-3′; reverse, 5′-GGGAAACCTAGGGTGTGGAT-3′; product = 269), MMP-14 (forward, 5′-CACTGCCTACGAGAGGAAGG-3′; reverse, 5′-TCCCTTCCCAGACTTTGATG-3′, product = 268), and thrombospondin (forward, 5′-TTCTACGAGCTGTGGCAATG-3′; reverse, 5′-TCTTTCTTGCAGGCTTTGGT-3′; product= 287). PCR amplification of cDNA was performed as described previously.30
Real-Time PCR
Real-time PCR assays were performed using the ABI 5700 real-time system (Applied Biosystems, Foster City, CA) with SYBR-green fluorescence and TaqMan method. Direct detection of the PCR product was monitored by measuring the increase in fluorescence caused by the binding of SYBR Green to dsDNA as described previously.30
Immunohistochemistry
Immunohistochemistry for MMP-9 (C-20) (polyclonal antibody at 1:50 dilution from Cell Signaling Technology, Boston, MA) was performed in formalin-fixed, paraffin-embedded tissues. All slides were stained using the automated Nexes IHC stainer (Ventana, Tucson, AZ) for consistency of the experiment conditions. Positive and negative controls were run concomitantly to the cases.
siRNA Transfection
PKC-α siRNA and siRNA PKC-δ were synthesized and purified by Dharmacon Inc. siRNA PKC-α (200 to 400 nmol/L) and PKC-δ (200 to 600 nmol/L) were transfected separately or together into HP75 cells using the Amaxa Nucleofector (Amaxa, Gaithersburg, MD). Briefly, confluent cells were trypsinized and resuspended in Amaxa Nucleofector solution V at a density of 2 × 106 per 100 μl of solution, and PKC-α siRNA or PKC-δ siRNA was added. Cells were transfected using the A23 pulsing program. Immediately after the electroporation, cells were suspended in 4.9 ml of Dulbecco’s modified Eagle’s medium with glutamine (2 mmol/L), insulin (10 μmol/L), and 15% fetal bovine serum.
Gelatin Zymography
The activities of MMP-2 and MMP-9 in the PA specimens and HP75 cells were detected by gelatin zymography. PA specimens were weighed and 10-mg pieces were homogenized in 1% Triton X-100 solubilization buffer described under Western blotting. The homogenized tissues were centrifuged at 3000 × g for 30 minutes at 4°C. Supernatant (25 μg of protein) was assayed for MMP-9 and MMP-2 activity as described by Zhao and colleagues.31 For uPA, fibrinogen was used as a substrate.
Northern Blot Analysis
Total RNA was isolated from adherent cells using Trizol reagent (Life Technologies, Inc., Grand Island, NY) as a monophasic solution of phenol and guanidine isothiocyanate, as modified from the single-step RNA isolation method. Total RNA (20 μg) from control and treated HP75 cells was used and a human MMP-9 cDNA probe (25 ng) was labeled with [α-32P]dCTP (50 μCi) as described previously.32
Western Blot Analysis
For the detection of proteins, cells cultured for 1 hour in serum-free conditions were treated in the presence or absence of PMA (100 nmol/L) for 16 hours. Protein (200 μg) from the supernatant was then boiled for 5 minutes in sodium dodecyl sulfate-polyacrylamide gel electrophoresis buffer and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on polyacrylamide slabs as reported before.32
Cell Invasion Assay
The invasion assay of HP75 cells through type IV collagen was performed as previously reported.31 Briefly, modified Boyden chambers containing polycarbonate filters with 8-μm pores (Becton Dickinson, Boston, MA) were coated with 0.25 mg/ml type IV collagen (Sigma). After 6 hours of incubation, invaded cells were stained with 0.1% crystal violet solution and photographed with a QImaging RETIGA EXi digital camera (Burnaby, BC, Canada) under a Leica DMIRE2 microscope. The invaded cell number was then counted and subjected to statistical analysis.
Statistical Analysis
A representative experiment or average changes with SEM are shown. Statistical differences between results were determined using Student’s t-test for paired or unpaired data as appropriate using Prism GraphPad Software. A P value of 0.05 or less was considered significant.
Results
Microarray DNA Analysis of Noninvasive and Invasive PAs
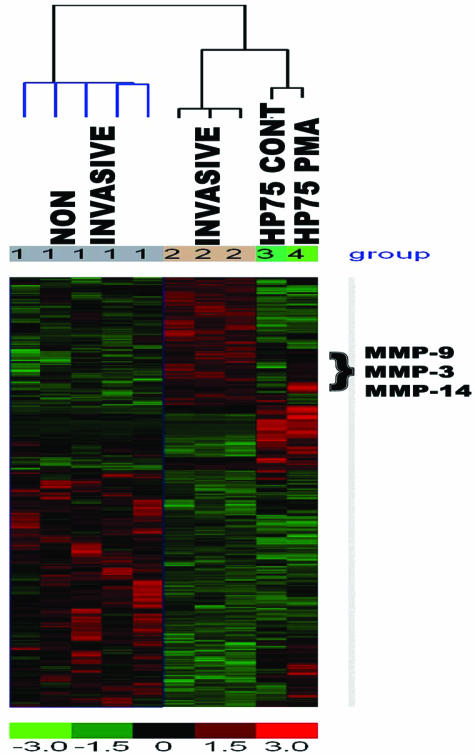
To identify the differentially expressed genes between noninvasive and invasive human PAs, we analyzed gene expression profiles by DNA microarray analysis. After RNeasy Micro column purification to remove 5s and tRNA contamination, total RNA (20 μg/specimen) from five recently resected noninvasive (two null cells, two gonadotrophs, and one sparsely granulated growth hormone cell) and three invasive (two null cells and one thyrotroph cell) PAs were extracted for analysis. Affymetrix HG 133 plus 2.0 gene chips were used in this experiment and each chip contained 54,613 transcripts. The gene expression was extracted with Affymetrix software GCOS 1.1. To filter out significant genes, we compared noninvasive and invasive arrays and a formal statistical test (t-test) was used to filter the genes. We identified 846 significant genes in this comparison. Overexpressed genes (the ratio of TEST to CONTROL >1) are designated as red and underexpressed as green. We also illustrated the hierarchical dendrogram with the selected genes, and reported the gene function categories for the 846 genes by using EASE2. We then compared the differentially expressed genes from the human PA specimens with the established human PA cell line (HP75): control and PKC-activated (treated with PMA) total RNA (Figure 1). The five genes that were significantly (P < 0.001) increased in both invasive PAs and PKC-activated HP75 cells are: thrombospondin (threefold increase), MMP-3 (3.2-fold increase), MMP-9 (8.0-fold increase), and MMP-14 (threefold increase). We used the HP75 cell line so that we can functionally test the role of genes that were differentially expressed between noninvasive and invasive PAs.
Figure 1.
Tumor invasion-associated cDNA microarray analysis. Hierarchical clustering of analysis reveals unique subsets of genes differentially expressed in invasive PAs compared with noninvasive tumors. Affymetrix HG 133 Plus 2.0 gene chips were used in the experiment, and each chip contained 54,613 transcripts. The cluster genes (n = 846) were derived from those greater than 1.5-fold change. The red color indicates up-regulation and the green color indicates genes that were down-regulated.
Validation of DNA Microarray Results with RT-PCR
We used RT-PCR to confirm the microarray data results. The RT-PCR detected significant time-dependent increases in MMP-9 transcripts after PMA treatment but was unable to confirm the microarray results that showed statistically significant changes in levels of thrombospondin, MMP-3, and MMP-14 (data not shown). We used MMP-2 and β-actin as controls because their levels were not changed in invasive PAs when compared with noninvasive tumors or in PMA-treated HP75 cells compared with untreated cells.
MMP-9 Expression and Activity Are Increased in Invasive Human PAs
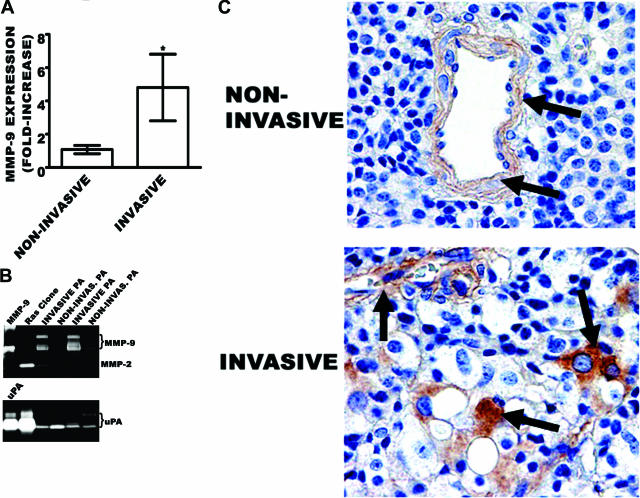
Total RNA from frozen invasive PAs (n = 3) and noninvasive PAs (n = 3) were analyzed for MMP-9 mRNA transcripts by real-time PCR. The MMP-9 mRNA was the only transcript that showed a significant (fivefold increase) expression level to validate the microarray data (Figure 2A). In addition, we analyzed MMP-9 activity in four recently resected frozen nonfunctioning PAs (two invasive and two noninvasive) by zymography. Figure 2B shows an increase in MMP-9 activity in the two invasive PAs and low activity in the noninvasive PAs. The two bands correspond to monomer MMP-9 (92 kd) and lipocalin-bound heterodimer of MMP-9 (125 kd) as described previously.31 There was very low activity of MMP-2 in both invasive and noninvasive PAs. We also determined the activity of another secreted protease, urokinase (uPA), as a control, but its activity was also as low as MMP-2 in both invasive and noninvasive PAs, whereas it was highly active in immortalized normal human astrocytes infected with constitutively active H-Ras. To identify the source of MMP-9 in the invasive PAs, we performed immunohistochemistry with both invasive and noninvasive PAs used in Figure 2B. There was no MMP-9 immunoreactivity of tumors but only a faint positive stain of the vascular structures (arrows) in the noninvasive PAs (Figure 2C). In contrast, the invasive nonfunctioning PAs expressed focal but intense cytoplasmic immunoreactivity of adenomatous cells for MMP-9 (arrows). There was also a weak reactivity of endothelial cells of small capillaries (arrow).
Figure 2.
MMP-9 activity increases in invasive human PAs. A: Real-time PCR. Real-time PCR analysis of MMP-9 was performed with total RNA from frozen three noninvasive and three invasive PAs. y axis represents the fold-change increase of each MMP-9 mRNA in tumor specimens. Each experiment was run in duplicate and the data reported are the average results ± SEM (*P < 0.05). B: Gelatin zymography. MMP-9 activity was determined by gelatin zymography with 25 μg/lane of Triton-extracted, recently resected noninvasive and invasive human PAs (see Materials and Methods for experimental detail). C: Immunohistochemistry. Immunohistochemical stain for MMP-9 in nonfunctioning pituitary adenomas (NFPAs). Top: A noninvasive NFPA shows no MMP-9 reactivity of tumor cells and only weak reactivity of the vascular structures. Bottom: In contrast, an invasive NFPA shows focal but intense cytoplasmic immunoreactivity of adenomatous cells for MMP-9. Note weak reactivity of endothelial cells of small capillaries. MMP-9 polyclonal antibody, ABC immunohistochemistry. Original magnifications, ×40.
Regulation of MMP-9 mRNA Expression by PKC in HP75 Cell Line
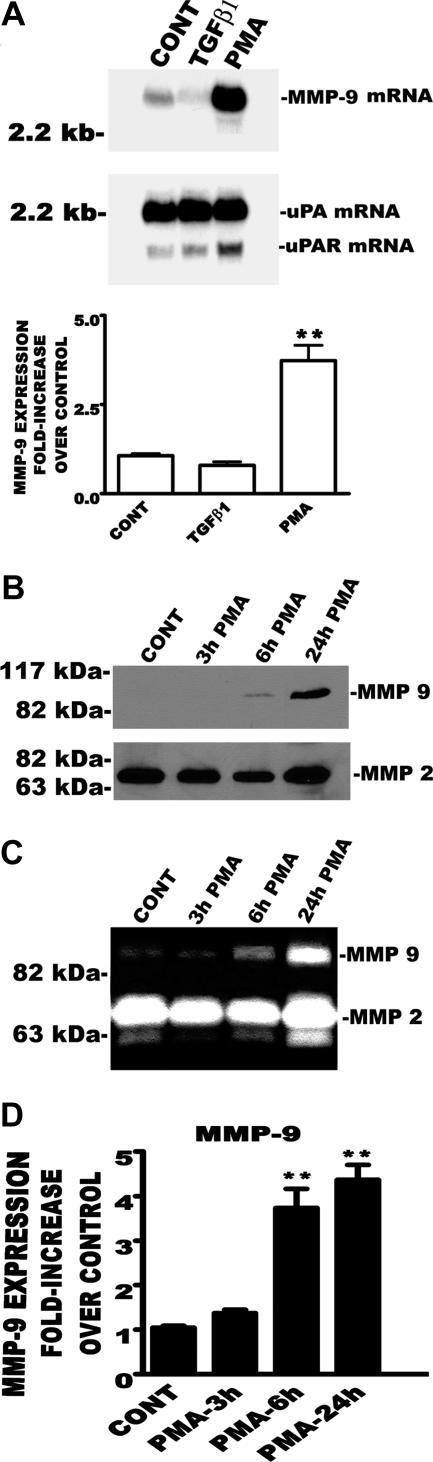
We treated the HP75 cell line with PMA (100 nmol/L) or transforming growth factor (TGF)-β1 (2.5 ng/ml) for 6 hours and determined the effect of the phorbol ester on MMP-9 mRNA expression. Figure 3A shows that PMA up-regulated MMP-9 mRNA expression by 10-fold. There was no significant change in uPA but a slight increase in uPAR level in response to PMA. TGF-β slightly increased the level of uPA. The PMA-evoked increase in MMP-9 mRNA was further confirmed by real-time PCR (Figure 3A, bottom). There was a slight decrease in MMP-9 mRNA transcript after TGF-β1 treatment as observed with Northern blot analysis (Figure 3A, top).
Figure 3.
Expression and activity of MMP-9 in HP75 cells. A: Northern blot and real-time PCR analyses. Total RNA (20 μg) from control, TGF-β1, or PMA-treated cells was subjected to Northern blot analysis using [α-32P]dCTP-labeled probes for MMP-9, uPA, and uPAR. Real-time PCR was performed with the same RNA used in Northern blot analysis (*P < 0.001). B: Western blot analysis. Time-dependent effect of PMA on MMP-9 expression and activity is shown. Western blot was performed with 100 μg of secreted proteins. C: Gelatin zymography. MMP-9 activity was determined using gelatin zymography with 25 μg of conditioned media protein. The study was repeated three times, and we obtained similar results. D: Real-time PCR. MMP-9 mRNA transcript was performed with total RNA from control and PMA-treated (3, 6, and 24 hours) cells (**P < 0.001).
Regulation of MMP-9 Activity and Protein by PKCs in HP75 Cells
We have demonstrated the PKC-regulated increases in the level of MMP-9 mRNA (Figures 1, 2, and 3A). Likewise, we confirmed increased cell-associated MMP-9 protein (Figure 2C) and elevated activity of the protease in invasive PAs (Figure 2B). The next question we asked was whether PKC also affects the activity and level of secreted MMP-9 protein in human PA cell line. In Figure 3, B and C, we treated HP75 cells with PMA (100 nmol/L) for 3, 6, or 24 hours, collected the conditioned media, and assayed for total protein level and activity of MMP-9. PMA produced a time-dependent increase in both MMP-9 protein level and activity without significantly increasing the level of MMP-2. In addition to the elevation of secreted MMP-9 protein, we further confirmed that MMP-9 mRNA was also significantly increased with time after PMA treatment of HP75 cells with real-time PCR (Figure 3D).
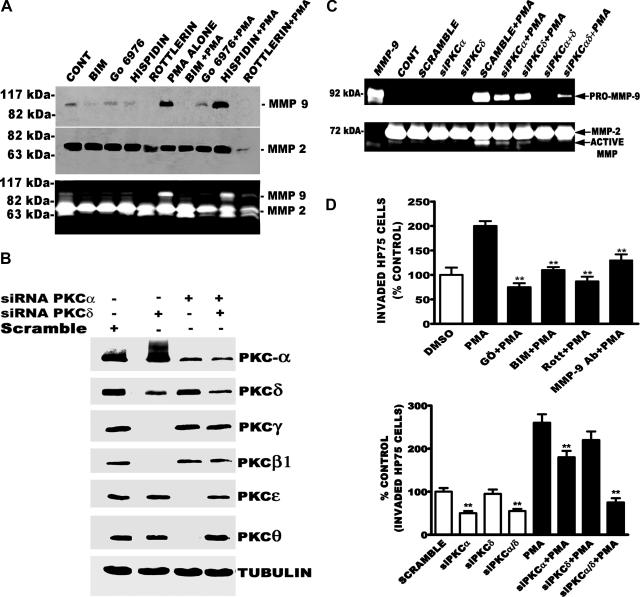
The results presented so far showed that PMA increased MMP-9 mRNA, protein, and activity. Next, we performed a series of studies to identify the classic or novel PKC isozyme(s) mediating the PMA-induced up-regulation of MMP-9 expression and activity. We pretreated HP75 cells for 60 minutes with BIM (1.0 μmol/L), an inhibitor of both classical and novel PKC isozymes; Gö6976 (10 μmol/L), which inhibits only classic PKCs (α and βI/II); hispidin (10 μmol/L), which inhibits only βI/II; or Rottlerin (5 μmol/L), which inhibits only PKC-δ at this concentration, and added PMA or dimethyl sulfoxide (the solvent in which PMA was dissolved) for 23 hours. The conditioned media was collected and assayed for MMP-9 protein expression and activity. As expected, PMA induced a robust increase in MMP-9 expression and activity (Figure 4A). The PMA-evoked elevation in MMP-9 level and activity was blocked by BIM, Gö6976, and Rottlerin but not by hispidin. The fact that hispidin was not effective in blocking PMA-induced increase in MMP-9 level argues against the involvement of PKC-βI/II. Gö6976 is an inhibitor of PKC-α but not PKC-γ, which excludes the involvement of the latter in reducing PMA-evoked increase in MMP-9 expression and activity. The PKC-δ inhibitor (Rottlerin) blocked MMP-9 expression and activity suggesting that, in addition to PKC-α, PKC-δ may be partly mediating the PMA effect.
Figure 4.
Effect of PKC inhibitors on MMP-9 expression and activity. A: Western blot. Western blot was performed with 100 μg of secreted proteins. MMP-9 activity determined using zymography with 25 μg of conditioned media protein. B: Western blot. Western blot analysis was performed with 400 nmol/L siRNA against PKC-α or PKC-δ alone or in combination and PMA alone (100 nmol/L). C: Gelatin zymography. Zymography was performed to test the effectiveness of PKC-α siRNA, PKC-δ siRNA alone, or in combination on MMP-9 gelatinase activity. D: Cell invasion assay. The invasion assay of control and PMA-treated HP75 cells through type IV collagen was performed to determine number of invaded cells. After 6 hours of incubation of cells with PMA, invaded cells were stained with 0.1% w/v crystal violet solution and photographed and counted. The number of invaded cells was used for subsequent comparative analyses. **P < 0.001. The effect of pharmacological inhibitors (Gö6976, BIM, and Rottlerin) of PKC and neutralizing MMP-9 antibody was determined on PMA-evoked increase in invasion. The effect of PKC-α, PKC-δ, siRNA alone, or in combination was determined on PMA-evoked increase in invasion. All treatments were performed in the presence of dimethyl sulfoxide (0.1% v/v), the vehicle in which PMA was dissolved.
To delineate the role of PKC-α and PKC-δ in the increase of MMP-9 expression and activity, we first determined the optimal concentrations of both PKC-α and PKC-δ siRNA sequences that would significantly silence the expression of the PKC isozymes. There was no significant difference between 400 or 600 nmol/L siRNA in silencing PKC-α. Both concentrations produced ∼70% down-regulation of PKC-α protein. Likewise, 200 and 400 nmol/L of PKC-δ siRNA reduced the protein level of the novel PKC isozyme by ∼60%. We then used 400 nmol/L of both PKC-α and PKC-δ siRNA alone or in combination and determined their effects on the expression levels of the two PKC isozymes (Figure 4B). As a control, we used scramble sequences and as expected they did not alter the level of either PKC-α or PKC-δ. To further test the specificity of the siRNA, we probed the same blot with antibodies against two novel PKC isozymes (PKC-ε and PKC-θ), which have 40 to 50% amino acid sequence homology to PKC-δ and there was no significant change in the levels of these PKC isozymes (Figure 4B, lane 2, rows 5 and 6) when PKC-δ siRNA was used. For PKC-α controls, we used antibodies against two classic PKC isozymes (PKC-βI and PKC-γ, which have 75 to 80% amino acid sequence homology to PKC-α) and observed no significant change in their expression levels when PKC-α siRNA was used (Figure 4B, lane 3, rows 3 and 4). We did not probe PKC-α siRNA blot with PKC-ε or PKC-θ antibody, explaining the reason for the blank spaces (Figure 4B, lane 3, rows 5 and 6) in Figure 4B; the same is true for PKC-β and PKC-γ rows 3 and 4 (Figure 4B, lane 2). A third control was also performed with an antibody against the housekeeping gene tubulin (Figure 4B, row 7). This also showed no change in the level of tubulin in siRNA-treated cells compared with scramble sequences (siRNA mismatch).
After establishing the effectiveness of our siRNA in reducing PKC-α and PKC-δ expression, we next determined whether these siRNA sequences would alter MMP-9 activity. Conditioned media (25 μg of protein) was analyzed for MMP-9 activity by gelatin zymography. PMA induced a robust increase in MMP-9 activity. The individual siRNA sequences only partially reduced MMP-9 expression and activation (Figure 4C). However, a combination of siRNA sequences against PKC-α and PKC-δ significantly reduced both basal and PMA-induced increase in the activities of both pro-MMP-9 (92 kd) and active MMP (∼60 kd; below MMP-2) (Figure 4C).
PKC Activation Increases HP75 Invasion
MMPs are proteolytic enzymes that play an important role in various aspects of cancer progression, including invasion. Because PMA increased MMP-9 expression and activity, we next determined whether the phorbol ester would alter invasive phenotype of HP75 in our transwell assay. As shown in Figure 4D, cells treated with PMA (100 nmol/L) for 6 hours invaded through the collagen type IV-coated membranes at a higher rate and greater number than untreated cells. PMA increased the number of invaded cells by twofold when compared with control cells (Figure 4D, top). We chose 6 hours because PMA affects the proliferative capacity of these cells after long-term (24 hours) exposure. Pretreatment of HP75 cells for 60 minutes with Gö6976 (10 μmol/L), BIM (1 μmol/L), or Rottlerin reduced PMA-induced increased invasion by 100, 68.6, or 100%, respectively (Figure 4D, top). Neutralizing MMP-9 antibody (25 μg/ml) also attenuated PMA-evoked increase in HP75 cell invasion by 60% (Figure 4D, top).
Based on the results presented in Figure 4, C and D (top), we hypothesize that PKC-α and PKC-δ increase invasion by activating MMP-9 activity. If this hypothesis is correct, the combined PKC-α and PKC-δ siRNA sequences that significantly reduced MMP-9 activity (Figure 4C) should block PMA-evoked increase in invasion. In Figure 4B, we have established that siRNA against PKC-α and PKC-δ reduced the expression of the PKC isozymes and significantly reduced PMA-induced increase in MMP-9 expression (Figure 4C). To determine the functional importance of these findings, we performed another set of transmembrane invasion assays to dissect the role of PKC-α and PKC-δ. PKC-α but not PKC-δ siRNA significantly (45%, P < 0.001) reduced the basal transmembrane invasion (Figure 4D, bottom). PKC-α siRNA also significantly reduced (30%) PMA-evoked increase in invasion. Likewise, PKC-δ siRNA reduced the effect of PMA but was not statistically significant. The down-regulation of PKC-δ alone with siRNA was not sufficient to significantly alter invasion. Interestingly, combining the PKC-α and PKC-δ siRNA (400 nmol/L each) completely blocked PMA-induced increase in invasion as compared with control scramble siRNA (800 nmol/L).
Discussion
Pituitary tumors are almost always benign, but aggressive local growth and invasion into the dura may occur. The resection of invasive PAs is more difficult than for noninvasive PAs, and tumor recurrence is often observed after surgery. Surgical complications, failure to achieve remission of endocrine disease, and patient outcome are all related to invasiveness. The molecular mechanisms that dictate this local invasive behavior of PAs remain poorly understood. Proteases including MMPs and uPA, protease inhibitors, and growth factors have been shown to correlate with PA invasive phenotype. MMP-2 and MMP-9 mRNA and protein expression correlated well with cavernous sinus invasion of a variety of nonfunctioning and functioning PAs.33 Likewise MMP-9 expression level was found to be significantly higher in invasive macroprolactinomas than noninvasive tumors.11 In addition, invasive nonfunctioning adenomas expressed higher levels of uPA than noninvasive PAs.12 Other DNA microarray studies of PAs have been reported; however, they have not focused on genes related to protease activity nor other aspects of invasiveness.
In our study, we used only nonfunctioning PAs to determine the differential expression of invasion-associated gene profiles. When enough human tumor material is available, we plan to extend these studies to hyperfunctioning PAs as seen in acromegaly and Cushing’s disease. We have demonstrated the differential expression of MMP-9 by invasive and noninvasive primary nonfunctioning PAs by DNA microarray and gelatin zymography. We used DNA microarray analysis to examine gene expression profiles using RNA from noninvasive and invasive nonfunctioning PAs. The increase in the expression levels of MMP-9 and α1AAR were similarly observed in the DNA microarray analysis of the human pituitary tumor cell line (HP75) treated with PMA. Because the human PA specimens were usually not adequate in volume to perform rigorous experiments and functional assays, we next confirmed the patient PA microarray results in the human PA cell line (HP75) using real-time PCR (Figure 3D), gelatin zymography (Figures 3 and 4), and Western (Figures 3 and 4) and Northern (Figure 3A) blot analyses. We also demonstrated that the invasive nonfunctioning PAs have higher MMP-9 mRNA by real-time PCR (Figure 2A), activity by zymography (Figure 2B), and cell-associated protein by immunohistochemistry (Figure 2C) in recently resected specimens that were not part of the samples used for the microarray analysis.
Numerous studies have reported DNA microarray gene expression profiles in human PAs but none of these studies compared invasive and noninvasive nonfunctioning pituitary tumors. Complementary DNA microarray analysis was used to examine gene expression profiles in nonfunctioning, prolactin-, growth hormone-, and adrenocorticotrophin (ACTH)-secreting adenomas, compared with normal pituitary.34,35 Folate receptor was shown to be differentially expressed in PAs.34 Another study using a similar comparison paradigm identified the up-regulation of lysosomal-associated protein membrane-4-β (LAPTM4β) and ACTH when compared with nonfunctioning PAs.36 Furthermore, differential display analysis of rat or human pituitary tumors has identified a host of genes, including pituitary tumor-transforming gene (PTTG), growth arrest and DNA damage induced-45 (GADD45), maternally expressed gene 3a (MEG3a), and bone morphogenetic protein 4 (BMP-4).37–40 A significant increase in the percentage of ACTH tumors expressing Gal-3 in pituitary carcinomas was noted compared with adenomas.41,42 Metallothionein isoforms 3 gene was also identified by microarray to be differentially expressed in nonfunctioning adenomas in comparison to the nonfunctioning pituitary carcinomas that have metastasized to the spinal cord.43
Invasive PAs present a major challenge to clinicians. The most significant biological feature of these tumors is their local invasion into the dura. As stated above, the factors and mechanisms that control the invasive behavior of PAs are poorly documented. The biological characteristics of cell migration and invasion are adhesion to extracellular matrix or cell membranes, motility mechanisms (through uPAR/integrin interaction) to generate migration, and the ability to modify the local environment so that the cells can move. Cell invasion involves a selective remodeling of the extracellular matrix by the secretion of proteases (MMPs and uPA). In Figure 4D, we tested the hypothesis that MMP-9 increases invasion in PAs by modifying collagen IV. PMA-evoked increase in HP75 was blocked by MMP-9-neutralizing antibody and siRNA-targeting PKC-α and PKC-δ. Although we used a high concentration (400 nmol/L) of the siRNA pool, we included a number of controls to make sure that the effect we have observed was not nonspecific. First, we used the same amount of scrambled siRNA sequence as our control and in the presence of PMA. Secondly, we tested the PKC-α and PKC-δ siRNA against other PKC isozymes that have ∼60 to 70% sequence homology and their expression was not altered (Figure 4B). These results suggest that PKC-mediated elevation in MMP-9 expression and activity increase transwell invasion in HP75 cells. PKC has been implicated in the invasion of a number of tumor cells, including astrocytomas,44 urinary bladder carcinomas,45 colon carcinomas,46 and breast cancers.47 Initial observation of a point mutation of PKC-α and a higher overall PKC activity and expression in invasive PAs reported by Alvaro and colleagues4,5 was not confirmed by others.6 However, a host of growth factors (including TGF-α, epidermal growth factor) and their receptors are overexpressed in PA.16,48,49 Activation of these G-protein coupled receptors may lead to the stimulation of phospholipase C (PLC) and subsequent generation of inositol triphosphate (IP3) and diacylglycerol, an activator of PKC.50 It is conceivable that activation of PKC, especially the α and δ isozymes, may cause an increase in the expression and activity of MMP-9 and a subsequent increase in PA invasion into the dura. In bovine capillary endothelial cell, activation of PKC-α has been shown to increase MMP-9 activity and cell invasion.51
In conclusion, we have demonstrated an increase in the expression level and activity of MMP-9 in invasive nonfunctioning PAs and HP75 cell line. In addition, activation of PKCs with PMA increased the activity and expression of MMP-9, which was blocked by PKC inhibitors and siRNA against PKC-α and PKC-δ. Finally, transwell PKC-mediated invasion of HP75 cells was blocked by Gö6976, BIM, Rottlerin, MMP-9 antibody, and siRNA against PKC-α and PKC-δ. These results suggest that PKC and MMP-9 may be attractive therapeutic targets for the control of invasive PAs. Therefore, a combination of MMP-9 and PKC inhibitors would at least make the invasive PA a local disease, making a complete tumor resection more feasible. Though MMP-9, PKC-α, and PKC-δ are implicated in PA invasion, other MMPs (MMP-3 and MMP-14) and proteases (plasmin/uPA) could contribute to invasion.
Footnotes
Address reprint requests to Isa M. Hussaini, Ph.D., MR5 Room 3324, Department of Pathology, 415 Lane Rd., Box 800904, University of Virginia, Charlottesville, VA 22908. E-mail: imh5c@virignia.edu.
Supported by the National Institutes of Health (grants CA90851 and NS35122 to I.M.H.), the S. Ranieri Fund for Pituitary Tumor Research (to E.R.L.), the Umfrid Fund for Pituitary Research (to E.R.L.), and the Page and Otto Marx Foundation (to E.R.L.).
References
- Selman WR, Laws ER, Jr, Scheithauer BW, Carpenter SM. The occurrence of dural invasion in pituitary adenomas. J Neurosurg. 1986;64:402–407. doi: 10.3171/jns.1986.64.3.0402. [DOI] [PubMed] [Google Scholar]
- Meiji BP, Lopes MS, Ellegala DB, Alden T, Laws ER., Jr The long-term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated with transsphenoidal surgery. J Neurosurg. 2002;96:195–208. doi: 10.3171/jns.2002.96.2.0195. [DOI] [PubMed] [Google Scholar]
- Barlier A, Pellegrini-Bouiller I, Caccavelli L, Gunz G, Morange-Ramos I, Jaquet P, Enjalbert A. Abnormal transduction mechanisms in pituitary adenomas. Horm Res. 1997;47:227–234. doi: 10.1159/000185468. [DOI] [PubMed] [Google Scholar]
- Alvaro V, Levy L, Dubray C, Roche A, Peillon F, Querat B, Joubert D. Invasive human pituitary tumors express a point-mutated alpha-protein kinase-C. J Clin Endocrinol Metab. 1993;77:1125–1129. doi: 10.1210/jcem.77.5.8077302. [DOI] [PubMed] [Google Scholar]
- Alvaro V, Touraine P, Raisman Vozari R, Bai-Grenier F, Birman P, Joubert D. Protein kinase C activity and expression in normal and adenomatous human pituitaries. Int J Cancer. 1992;50:724–730. doi: 10.1002/ijc.2910500510. [DOI] [PubMed] [Google Scholar]
- Schiemann U, Assert R, Moskopp D, Gellner R, Hengst K, Gullotta F, Domschke W, Pfeiffer A. Analysis of a protein kinase C-α mutation in human pituitary tumors. J Endocrinol. 1997;153:131–137. doi: 10.1677/joe.0.1530131. [DOI] [PubMed] [Google Scholar]
- Todo T, Buchfelder M, Thierauf P, Fahlbusch R. Immunohistochemical expression of protein kinase C type III in human pituitary adenomas. Neurosurgery. 1993;32:635–642. doi: 10.1227/00006123-199304000-00022. [DOI] [PubMed] [Google Scholar]
- Hamilton HB, Hinton DR, Law RE, Gopalakrishna R, Su YZ, Chen ZH, Weiss MH, Couldwell WT. Inhibition of cellular growth and induction of apoptosis in pituitary adenoma cell lines by the protein kinase C inhibitor hypericin: potential therapeutic application. J Neurosurg. 1996;85:329–334. doi: 10.3171/jns.1996.85.2.0329. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- Resnick MS, Luo X, Vinton G, Sando JJ. Selective up-regulation of protein kinase C beta in phorbol ester-sensitive versus -resistant EL4 mouse thymoma cells. Cancer Res. 1997;57:2209–2215. [PubMed] [Google Scholar]
- Knappe UJ, Hagel C, Lisboa BW, Wilczak W, Ludecke DK, Saeger W. Expression of serine proteases and metalloproteinases in human pituitary adenomas and anterior pituitary lobe tissue. Acta Neuropathol (Berl) 2003;106:471–478. doi: 10.1007/s00401-003-0747-5. [DOI] [PubMed] [Google Scholar]
- Turner HE, Nagy Z, Sullivan N, Esiri MM, Wass JA. Role of matrix metalloproteinase 9 in pituitary tumor behavior. J Clin Endocrinol Metab. 2000;85:2931–2935. doi: 10.1210/jcem.85.8.6754. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Hirano H, Moroki K, Goto M, Imamura S, Kuratsu JI. Are nonfunctioning pituitary adenomas extending into the cavernous sinus aggressive and/or invasive? Neurosurgery. 2001;49:857–862. doi: 10.1097/00006123-200110000-00014. [DOI] [PubMed] [Google Scholar]
- Beaulieu E, Kachra Z, Mousseau N, Delbecchi L, Hardy J, Beliveau R. Matrix metalloproteinases and their inhibitors in human pituitary tumors. Neurosurgery. 1999;45:1432–1440. doi: 10.1097/00006123-199912000-00033. [DOI] [PubMed] [Google Scholar]
- Liu W, Matsumoto Y, Okada M, Miyake K, Kunishio K, Kawai N, Tamiya T, Nagao S. Matrix metalloproteinase 2 and 9 expression correlated with cavernous sinus invasion of pituitary adenomas. J Med Invest. 2005;52:151–158. doi: 10.2152/jmi.52.151. [DOI] [PubMed] [Google Scholar]
- LeRiche VK, Asa SL, Ezzat S. Epidermal growth factor and its receptor (EGFR) in human pituitary adenomas: EGFR correlates with tumor aggressiveness. J Clin Endocrinol Metab. 1996;81:656–662. doi: 10.1210/jcem.81.2.8636285. [DOI] [PubMed] [Google Scholar]
- Kawamoto H, Uozumi T, Kawamoto K, Arita K, Yano T, Hirohata T. Type IV collagenase activity and cavernous sinus invasion in human pituitary adenomas. Acta Neurochir (Wien) 1996;138:390–395. doi: 10.1007/BF01420300. [DOI] [PubMed] [Google Scholar]
- Andreasen P, Kristensen P, Lund LR, Dano K. Urokinase-type plasminogen activator is increased in the involuting ventral prostate of castrated rats. Endocrinology. 1990;126:2567–2576. doi: 10.1210/endo-126-5-2567. [DOI] [PubMed] [Google Scholar]
- Tryggvason K, Hoyhtya M, Salo T. Proteolytic degradation of extracellular matrix in tumor invasion. Biochim Biophys Acta. 1987;907:191–217. doi: 10.1016/0304-419x(87)90006-0. [DOI] [PubMed] [Google Scholar]
- Lauer-Fields JL, Sritharan T, Stack MS, Nagase H, Fields GB. Selective hydrolysis of triple-helical substrates by matrix metalloproteinase-2 and -9. J Biol Chem. 2003;278:18140–18145. doi: 10.1074/jbc.M211330200. [DOI] [PubMed] [Google Scholar]
- Kline T, Torgov MY, Mendelsohn BA, Cerveny CG, Senter PD. Novel anti-tumor prodrugs designed for activation by matrix metalloproteinases-2 and -9. Mol Pharm. 2004;1:9–22. doi: 10.1021/mp0340183. [DOI] [PubMed] [Google Scholar]
- Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- Salo T, Liotta L, Keski-Oja J, Turpeenniemi-Hujanen T, Tryggvason K. Secretion of basement membrane collagen degrading enzyme and plasminogen activator by transformed cells: role in metastasis. Int J Cancer. 1982;30:669–673. doi: 10.1002/ijc.2910300520. [DOI] [PubMed] [Google Scholar]
- Bu G, Williams S, Strickland DK, Schwartz AL. Low-density lipoprotein receptor-related protein/α2-macroglobulin receptor is a hepatic receptor for tissue-type plasminogen activator. Proc Natl Acad Sci USA. 1992;89:7427–7431. doi: 10.1073/pnas.89.16.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Peterson CM, Moller B, Jensen PH, Moestrup SK, Holtet TL, Etzerodt M, Thogersen HC, Munch M, Andreasen PA, Gliemann J. Purified α2-macroglobulin receptor/low density lipoprotein receptor-related protein binds urokinase-plasminogen activator inhibitor type-1 complex: evidence that α2-macroglobulin receptor mediates cellular degradation of urokinase receptor-bound complexes. J Biol Chem. 1992;267:14543–14546. [PubMed] [Google Scholar]
- Hah N, Lee ST. An absolute role of the PKC-dependent NF-kappaB activation for induction of MMP-9 in hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2003;305:428–433. doi: 10.1016/s0006-291x(03)00788-5. [DOI] [PubMed] [Google Scholar]
- Simon C, Simon M, Vucelic G, Hicks MJ, Plinkert PK, Koitschev A, Zenner HP. The p38 SAPK pathway regulates the expression of the MMP-9 collagenase via AP-1-dependent promoter activation. Exp Cell Res. 2001;271:344–355. doi: 10.1006/excr.2001.5374. [DOI] [PubMed] [Google Scholar]
- Arai K, Lee SR, Lo EH. Essential role for ERK mitogen-activated protein kinase in matrix metalloproteinase-9 regulation in rat cortical astrocytes. Glia. 2003;43:254–264. doi: 10.1002/glia.10255. [DOI] [PubMed] [Google Scholar]
- Jin L, Kulig E, Qian X, Scheithauer BW, Eberhardt NL, Lloyd RV. A human pituitary adenoma cell line proliferates and maintains some differential functions following expression of SV40 large T-antigen. Endocr Pathol. 1998;9:168–184. [Google Scholar]
- Abdel-Fattah R, Xiao A, Bomgardner D, Pease CS, Lopes MB, Hussaini IM. Differential expression of HOX genes in neoplastic and non-neoplastic human astrocytes. J Pathol. 2006;209:15–24. doi: 10.1002/path.1939. [DOI] [PubMed] [Google Scholar]
- Zhao YG, Xiao AZ, Newcomer RG, Park HI, Kang T, Chung LW, Swanson MG, Zhau HE, Kurhanewicz J, Sang QX. Activation of pro-gelatinase B by endometase/matrilysin-2 promotes invasion of human prostate cancer cells. J Biol Chem. 2003;278:15056–15064. doi: 10.1074/jbc.M210975200. [DOI] [PubMed] [Google Scholar]
- Hussaini IM, Karns LR, Vinton G, Carpenter JE, Redpath GT, Sando JJ, VandenBerg SR. Phorbol 12-myristate 13-acetate induces protein kinase Cη-specific proliferative response in astrocytic tumor cells. J Biol Chem. 2000;275:22348–22354. doi: 10.1074/jbc.M003203200. [DOI] [PubMed] [Google Scholar]
- Liu W, Kunishio K, Matsumoto Y, Okada M, Nagao S. Matrix metalloproteinase-2 expression correlates with cavernous sinus invasion in pituitary adenomas. J Clin Neurosci. 2005;12:791–794. doi: 10.1016/j.jocn.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Evans CO, Reddy P, Brat DJ, O’Neill EB, Craige B, Stevens VL, Oyesiku NM. Differential expression of folate receptor in pituitary adenomas. Cancer Res. 2003;63:4218–4224. [PubMed] [Google Scholar]
- Moreno CS, Evans CO, Zhan X, Okor M, Desiderio DM, Oyesiku NM. Novel molecular signaling and classification of human clinically nonfunctional pituitary adenomas identified by gene expression profiling and proteomic analyses. Cancer Res. 2005;65:10214–10222. doi: 10.1158/0008-5472.CAN-05-0884. [DOI] [PubMed] [Google Scholar]
- Morris DG, Musat M, Czirjak S, Hanzely Z, Lillington DM, Korbonits M, Grossman AB. Differential gene expression in pituitary adenomas by oligonucleotide array analysis. Eur J Endocrinol. 2005;153:143–151. doi: 10.1530/eje.1.01937. [DOI] [PubMed] [Google Scholar]
- Pei L, Melmed S. Isolation and characterization of a pituitary tumor-transforming gene (PTTG). Mol Endocrinol. 1997;11:433–441. doi: 10.1210/mend.11.4.9911. [DOI] [PubMed] [Google Scholar]
- Zhang X, Sun H, Danila DC, Johnson SR, Zhou Y, Swearingen B, Klibanski A. Loss of expression of GADD45 gamma, a growth inhibitory gene, in human pituitary adenomas: implications for tumorigenesis. J Clin Endocrinol Metab. 2002;87:1262–1267. doi: 10.1210/jcem.87.3.8315. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhou Y, Mehta KR, Danila DC, Scolavino S, Johnson SR, Klibanski A. A pituitary-derived MEG3 isoform functions as a growth suppressor in tumor cells. J Clin Endocrinol Metab. 2003;88:5119–5126. doi: 10.1210/jc.2003-030222. [DOI] [PubMed] [Google Scholar]
- Paez-Pereda M, Giacomini D, Refojo D, Nagashima AC, Hopfner U, Grubler Y, Chervin A, Goldberg V, Goya R, Hentges ST, Low MJ, Holsboer F, Stalla GK, Arzt E. Involvement of bone morphogenetic protein 4 (BMP-4) in pituitary prolactinoma pathogenesis through a Smad/estrogen receptor crosstalk. Proc Natl Acad Sci USA. 2003;100:1034–1039. doi: 10.1073/pnas.0237312100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernicone PJ, Scheithauer BW, Sebo TJ, Kovacs KT, Horvath E, Young WF, Jr, Lloyd RV, Davis DH, Guthrie BL, Schoene WC. Pituitary carcinoma: a clinicopathologic study of 15 cases. Cancer. 1997;79:804–812. doi: 10.1002/(sici)1097-0142(19970215)79:4<804::aid-cncr18>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Riss D, Jin L, Qian X, Bayliss J, Scheithauer BW, Young WF, Jr, Vidal S, Kovacs K, Raz A, Lloyd RV. Differential expression of galectin-3 in pituitary tumor. Cancer Res. 2003;63:2251–2255. [PubMed] [Google Scholar]
- Giorgi RR, Correa-Giannella ML, Casarini AP, Machado MC, Bronstein MD, Cescato VA, Giannella-Neto D. Metallothionein isoform 3 gene is differentially expressed in corticotropin-producing pituitary adenomas. Neuroendocrinology. 2005;82:208–214. doi: 10.1159/000092521. [DOI] [PubMed] [Google Scholar]
- Park MJ, Park IC, Hur JH, Rhee CH, Choe TB, Yi DH, Hong SI, Lee SH. Protein kinase C activation by phorbol ester increases in vitro invasion through regulation of matrix metalloproteinases/tissue inhibitors of metalloproteinases system in D54 human glioblastoma cells. Neurosci Lett. 2000;290:201–204. doi: 10.1016/s0304-3940(00)01358-6. [DOI] [PubMed] [Google Scholar]
- Koivunen J, Aaltonen V, Koskela S, Lehenkari P, Laato M, Peltonen J. Protein kinase C alpha/beta inhibitor Go6976 promotes formation of cell junctions and inhibits invasion of urinary bladder carcinoma cells. Cancer Res. 2004;64:5693–5701. doi: 10.1158/0008-5472.CAN-03-3511. [DOI] [PubMed] [Google Scholar]
- Zhang J, Anastasiadis PZ, Liu Y, Thompson EA, Fields AP. Protein kinase C (PKC) betaII induces cell invasion through a Ras/Mek-, PKC iota/Rac 1-dependent signaling pathway. J Biol Chem. 2004;279:22118–22123. doi: 10.1074/jbc.M400774200. [DOI] [PubMed] [Google Scholar]
- Pan Q, Bao LW, Kleer CG, Sabel MS, Griffith KA, Teknos TN, Merajver SD. Protein kinase C epsilon is a predictive biomarker of aggressive breast cancer and a validated target for RNA interference anticancer therapy. Cancer Res. 2005;65:8366–8371. doi: 10.1158/0008-5472.CAN-05-0553. [DOI] [PubMed] [Google Scholar]
- Finley EL, Ramsdell JS. A transforming growth factor-α pathway is expressed is expressed in GH4C1 rat pituitary tumors and appears necessary to tumor formation. Endocrinology. 1994;135:416–422. doi: 10.1210/endo.135.1.8013379. [DOI] [PubMed] [Google Scholar]
- Jaffrain-Rea ML, Petrangeli E, Lubrano C, Minniti G, Stefano DD, Sciarra F, Frati L, Tamburrano G, Cantore G, Gulino A. Epidermal growth factor binding sites in human pituitary macroadenomas. J Endocrinol. 1998;158:425–433. doi: 10.1677/joe.0.1580425. [DOI] [PubMed] [Google Scholar]
- Balogh A, Csuka O, Teplan I, Keri G. Phosphatidylcholine could be the source of 1,2-DAG which activates protein kinase C in EGF-stimulated colon carcinoma cells (HT29). Cell Signal. 1995;7:793–801. doi: 10.1016/0898-6568(95)02007-1. [DOI] [PubMed] [Google Scholar]
- Park MJ, Park IC, Lee HC, Woo SH, Lee JY, Hong YJ, Rhee CH, Lee YS, Lee SH, Shim BS, Kuroki T, Hong SI. Protein kinase C-alpha activation by phorbol ester induces secretion of gelatinase B/MMP-9 through ERK 1/2 pathway in capillary endothelial cells. Int J Oncol. 2003;22:137–143. [PubMed] [Google Scholar]