Abstract
Because endothelial nitric-oxide synthase (eNOS) is generally considered protective against renal injury, we examined eNOS knockout mice for kidney pathology. In 80% of the adults examined, the renal surface was marked by distinct indented scars containing crowded small glomeruli but lacking attached tubules. Although vasculature was intact in the scars, Bowman’s space was dilated and glomerular tufts were degenerated. The atubular glomeruli were embedded in a dense interstitial matrix composed of cells positive for fibroblast (FSP-1) or macrophage (F4/80) markers, degenerated proximal tubules and collecting ducts, and diffuse fibrotic deposits. Surrounding regions of kidney contained mostly normal-appearing tubules, but enlarged or sclerotic glomeruli were also present. In neonatal animals, apoptosis and necrosis were concentrated in tubules within focal parenchymal zones, with narrowing of the glomerulotubular “neck.” In summary, targeted deletion of eNOS in mice leads to progressive focal renal abnormalities, including glomerular hypoplasia, and tubular cell death, leading to separation of glomeruli from tubules and tubular disruption. These abnormalities begin developing during the normal up-regulation of eNOS in the maturing kidney and are similar to those of a variety of chronic renal disorders. Endogenous renal eNOS production therefore seems critical for the maintenance of nephron maturation and integrity.
Endothelial nitric-oxide synthase (eNOS) is implicated in numerous aspects of renal vascular control and function. It is thought to exert a vasculoprotective effect by modulation of kidney blood flow through counterbalancing the effects of the renin-angiotensin system.1 Following NOS inhibition, the activity of the renin-angiotensin system is increased, with increased expression of fibronectin and α-smooth muscle actin.2 Either angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists ameliorate the consequent renal injury.2 In contrast, ischemic preconditioning increases renal eNOS protein expression and suppresses ischemic injury.3 As its name implies, eNOS is located within the renal vascular endothelium in the adult. However, recent findings indicate that, in neonatal rats, this enzyme is also transiently present in proximal tubules,4 whereas eNOS mRNA is present in the inner medullary collecting duct in the adult.5
The renal anatomy of eNOS knockout mice has been previously reported to be normal in appearance.6,7 In contrast, during our preliminary studies of eNOS gene deletion effects on kidney damage resulting from ureteral obstruction in mice, we repeatedly noted distinct renal surface scarring in adult animals of the Jackson eNOS knockout strain but not in wild-type mice. In the present article, we have investigated the histological, immunocytochemical, and developmental characteristics of this nephropathy, with the intent of determining the pathological process underlying these distinct scars and its relationship to the lack of eNOS in these animals. We also compared the pattern of eNOS expression with that in wild-type mice.
Materials and Methods
Animals
Mice of the incipient congenic Jackson strain B6.129P2-Nos3<tm1Unc>/J, which has gene deletion for eNOS, were purchased from Jackson Laboratories (West Grove, PA) as breeding pairs. The original breeders were subsequently genotyped by Jackson Laboratories and confirmed as being homozygous for the Nos3<tm1Unc> mutation. Additional adult knockout mice descended from the original mutants6 were provided by Dr. Victor E. Laubach of the University of Virginia. For studies of eNOS staining patterns and normal renal morphology, neonatal and adult C57BL (“wild-type”) mice were used.
Tissue Preparation, Staining, and Examination
Paraffin Sections
For paraffin embedding, kidneys examined were removed and fixed by immersion in 10% phosphate-buffered formalin, dehydrated in an ethanol series, and embedded in paraffin. Sections ranging from 4 to 10 μm in thickness were prepared on a Leica RM 2155 microtome (Leica Microsystems, Deerfield, IL). A total of 56 eNOS knockout mice were studied at the following ages: 7 days (n = 16), 14 days (n = 4), and 21 days (n = 11); adults ranging up to a year in age were also examined (n = 25). Histology of kidneys from wild-type (C57Bl) mice (a total of 51) was also studied at 7 days (n = 17), 14 days (n = 5), 21 days (n = 15), and adult (n = 14). Additional neonatal wild-type kidneys were harvested at 0, 3, 5, 7, 8, and 10 days for eNOS immunocytochemistry (see below).
Histochemical and Immunohistochemical Staining
Overall kidney structure was documented with Masson trichrome stain. We have found that picrosirius red staining is particularly effective in revealing both basement membranes and individual collagen fibrils such as found in vascular adventitia. As an additional test for collagen distribution, we used a 20-minute period of staining in 1% aqueous picrosirius red solution followed by a brief (15 seconds) counterstaining with Harris hematoxylin. Glomerular measurements were performed on periodic-acid Schiff (PAS)-stained sections, some of which were prepared in the form of serial arrays so that individual glomeruli could be followed in their entirety to establish their connection to proximal tubules.
Immunohistochemical localization of eNOS was performed with the use of either Abcam’s antibody ab 5589 or Santa Cruz antibody sc-8311, both at dilutions of 1:100, with subsequent development in the DAKO LSAB2 system followed by diaminobenzidine (DAB) (DAKO Corporation, Carpenteria, CA). Additional antibodies used included platelet-endothelial cell adhesion molecule (PECAM)-1 (vascular endothelium; Santa Cruz sc-1506; Santa Cruz Biochemicals, Santa Cruz, CA), fsp-1 (fibroblasts; Lab Vision/Neomarkers, Fremont, CA), F4/80 (macrophages; supernatant derived from an American Type Culture Collection hybridoma; Manassas, VA), renin (a gift from Dr. Tadashi Inagami of Vanderbilt University, Nashville, TN), and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) stain for apoptosis (Apoptag; Chemicon, Temecula, CA). To determine the relative volume contribution of vasculature in different kidney regions, PECAM-stained sections were analyzed by point counting. Because zones of scarring are relatively limited in comparison to the remaining cortical parenchyma, two ×400 fields from each scar were taken from five separate eNOS knockout mouse kidneys and compared with an equal number of cortical fields outside the scar. This generated volume fraction (VV) values as a percentage of the measured area occupied by blood vessels either within the glomeruli or in the surrounding interstitium.
To identify specific tubule segments and their derivatives, sections were exposed to biotinylated lectins and developed with the ABC-DAB development technique as previously described.8 Lectins derived from Lotus tetragonolobus or Dolichos biflorus (Vector Laboratories, Burlingame, CA) were used to stain proximal tubules and collecting ducts, respectively.
Plastic-Embedded Sections
Selected animals were fixed by successively perfusing the vasculature (via the left ventricle) with prewash and fixative. Prewash solution consisted of 3% dextrose plus 3% dextran (47,500 average molecular weight) and 50 mmol/L CaCl2, pH 7.2 to 7.4, and was immediately followed by fixative solution consisting of 1.5% glutaraldehyde added to the same solution used for prewashing. Following perfusion, kidneys were removed, immersed in fresh fixative, and cut into three coronal segments before an additional overnight fixation at 4°C. After washing in carrier solution, the kidney segments were cut into 50-μm sections with a vibrating microtome (DSK Microslicer DTK-3000; Ted Pella, Redding, CA). The protocol for processing free-floating sections for plastic embedment and microscopic examination has previously been described in detail.9 In summary, sections were osmicated, dehydrated, and infiltrated with epoxy resin while still in free-floating state and then flat-embedded on coated slides and cured at 60°C. Survey light micrographs were taken of whole sections, from which areas of interest were cut and mounted on plastic stubs, and “semithin” plastic sections (0.25 μm) were cut with glass knives on a Sorvall MT-2B ultramicrotome (Newton, CT). Sections were stained with warm alkaline toluidine-blue solution. Digital micrographs were captured with a QImaging MicroPublisher camera (Quantitative Imaging Corp., Burnaby, BC, Canada) on a Leica DMLS compound brightfield light microscope.
Quantitation
Measurements of average glomerular areas were made with ImagePro Plus 5.1 Software (Media Cybernetics, Silver Spring, MD). Sagittal paraffin sections (4-μm thickness) prepared from wild-type and knockout mice were analyzed by measuring 50 glomerular profiles per animal at ×400 magnification. For knockout animals, two categories of glomeruli were measured: normal-appearing cortex was measured as for wild type, whereas glomeruli in zones of scarring were measured separately. Average glomerular areas were calculated and expressed as mean ± SEM; measurements of glomeruli from knockout scars, knockout cortex, and wild type were compared by one-way analysis of variance. Statistical significance was considered to exist at P values <0.05.
Urine Protein and Creatine
Urine protein concentration was determined by the method of Bradford10 using dye reagent and immunoglobulin standard purchased from Bio-Rad Laboratories (Hercules, CA). Specimens were assayed in triplicate using from 1 to 5 μl of urine per replicate. Urine creatinine concentration was determined with a colorimetric Quan-tichrome Creatine Assay kit (BioAssay Systems, Hayward, CA) according to the manufacturer’s instructions. Specimens were assayed in duplicate (3 μl of urine/well) in a 96-well plate.
Results
Adult eNOS Knockout Mice
In contrast to wild-type mice, in which no lesions were detected, visual inspection of freshly exposed kidneys in adult eNOS knockout mice often revealed the presence of distinct indentations, indicating zones of parenchymal scarring (Figure 1A). Such scars usually are localized to either renal pole and may exist on either the lateral or medial surface. Scarring can appear in one or both kidneys of an individual and in animals of either sex. Of the 25 adult knockout animals examined, 20 (80% of the total) showed scarring. Seven (28%) had scars on the right kidney alone, four (16%) on the left, and nine (36%) bore scars on both kidneys. Typically, the deepest portion of a scar’s groove is occupied by a deposit of fat (Figure 1B) that is closely adherent to the capsule (Figure 1, C and D). Coronal sections of kidneys showed extreme parenchymal thinning at the level of the scars; distortion of the kidney profile was typical (Figure 1C). Close examination of the thinned parenchyma in the scarred zone revealed that it consisted largely of closely packed clusters of glomeruli (Figure 1D).
Figure 1.
Adult eNOS knockout mouse. A: A deep vertical indentation is present in the lateral caudal portion of the right kidney. B: Detailed view shows supracapsular fat accumulation deep in the groove of the indentation. C: Transverse 50-μm osmicated and plastic-embedded section of kidney from a 12-week male mouse; here, the indentation was medial (note blackened fat deposit at right). The kidney cortex and medulla are noticeably thinned on the side of the scar. D: A semithin (0.25-μm) section taken from the region enclosed in the rectangle in C. Globules of the supracapsular fat pad are seen at right; the thinned parenchymal layer underneath consists largely of crowded small glomeruli embedded in a dense matrix of microvessels and massed small interstitial cells. Scale bars: 2 mm (A); 1 mm (B); 1 mm (C); 100 μm (D).
Within scars, the numerous glomeruli were embedded in a dense matrix (Figures 1D and 3A). Numerous cells in this matrix were positive for the S100A-4 protein (also known as “fsp-1” because of its association with fibroblasts; Figure 2A), and cells positive for F4/80 (specific for macrophages) were concentrated within the scars (Figure 2C). The parenchyma outside the scars contained markedly fewer fibroblasts and macrophages (Figure 2, B and D). Although trichrome staining did not indicate the presence of significant collagen deposits in scars, picrosirius red revealed a diffuse fibrosis consisting of thin strands of connective tissue distributed loosely within the interglomerular interstitium; outside the scars, collagen was restricted to the adventitia of blood vessels (Figure 2, E and F). Renin immunostaining demonstrated the presence of intact juxtaglomerular apparati associated with glomeruli in the scar and surrounding parenchyma (Figure 2, G and H).
Figure 3.
Glomerular features in adult eNOS knockout kidney. A: Scarred region with numerous small glomeruli; lipid droplets are found in some Bowman’s spaces as well as in the adjacent matrix. Glomeruli are embedded in a matrix of microvessels (MV) and tightly packed small interstitial cells of varying morphology. The vasculature of the glomeruli appears intact (Ar, glomerular arteriole), but no clear connection to typical large-diameter proximal tubules is discernible. B and C: High-magnification details of the glomerular cores in hypoplastic glomeruli in scars. Villous projections (arrows) appear on cell surfaces and capillary loops are dilated (B), and in C, a podocyte (P) with its primary and secondary branches appears to be detaching from the adjacent capillary. D: Unscarred parenchyma. Glomerular capillaries are filled with debris. E and F: Serial sections of parenchyma outside the scar, stained with sirius red and PECAM-1, respectively. Although the tubular components appear normal, certain glomeruli (at left of field) are characterized by hyalinization and loss of nuclei within the glomerular core, showing accumulations of sirius red-positive material and a substantial loss of vasculature (see the glomerulus at the right of the field). Scale bars: 50 μm (A); 20 μm (B–D); 100 μm (scale bar in F applies to E and F).
Figure 2.
Comparison in adult eNOS knockout kidney of scarred regions (A, C, E, G, and I) with the surrounding parenchyma (B, D, F, H, and J). Fibroblasts and macrophages are highly concentrated in the scar matrix (A and C), and fibrosis is present in the form of collagenous strands loosely distributed within the interglomerular matrix (E). In comparison, the unscarred kidney contains only scattered positive interstitial cells (B and D), and its collagenous component is concentrated around blood vessels (F). Immunoreactive renin can be found in juxtaglomerular apparati (arrows) of glomeruli both within the scar (G) and in the adjacent parenchyma (H). The vascular distribution is markedly different between eNOS knockout scar (I) and the adjacent tubule-rich parenchyma (J) (PECAM staining). In I, blood vessel profiles are concentrated both in the form of glomerular capillaries (GC) and interstitial vessels (V). In J, vessels are also concentrated in glomeruli but are more widely distributed through the rest of the field because of the presence of renal tubules. K: Cortical vascular volume fractions of eNOS knockout kidney regions, comparing the relative contributions (%) of glomerular capillaries and extraglomerular vessels (peritubular capillaries, afferent and efferent arterioles, etc). The zone of scarring (“atubular”) is represented by black bars, whereas the surrounding parenchyma (“tubular”) is shown as white bars. Within the scar, glomerular and extraglomerular vessels contribute nearly equally to the total volume (because the tubules have largely disappeared within the adult kidney scars), whereas the tubule-containing region of the cortex have the normal distribution in which the relative population of extraglomerular vessels predominates. L: Measurements of glomeruli from adult eNOS knockout (KO) kidneys and normal wild-type (WT) mice. Glomeruli in the eNOS atubular (scar) regions are considerably smaller than their counterparts (*P < 0.05) in either the adjacent tubular (unscarred) eNOS knockout kidney or the normal wild-type mouse kidney. Scale bars: 100 μm (scale bar in D applies to A–D); 50 μm (scale bar in F applies to both E and F); 50 μm (scale bar in H applies to G and H); 100 μm (scale bar in J applies to both I and J).
Staining with PECAM-1 (CD-31) indicated that the vascular supply to these hypoplastic glomeruli within the scar was intact (Figure 2I). A considerable volume of the scarred area was taken up by these blood vessels (Figure 2K), with the contributions of glomerular capillaries and interstitial vessels being roughly equal (Figure 2K). In comparison, the vasculature outside the scars consisted primarily of peritubular capillaries (Figure 2J) and larger vessels, in which total contribution predominated over the glomerular capillaries (Figure 2K). Glomeruli within the scars were abnormal in several respects. First, they were considerably smaller than their counterparts in either the adjacent parenchyma or in wild-type kidneys (Figure 2L). They exhibited a variety of internal alterations, including substantial dilation of the Bowman’s space, the frequent presence of large lipid droplets (Figure 3 , A and C), and shrinkage and sclerosis in some instances. Degeneration of the glomerular core components was common within the scarred zones, including formation of microvilli or plicae (Figure 3B) and dilatation or loss of glomerular tuft vasculature (Figure 3, B and C).
In the cortex outside the scars, abnormalities were frequently observed in glomeruli: the capillary lumina of some of these glomeruli were filled with debris (Figure 3D). In addition (to the degree that over 4% of the glomeruli outside the scar were so affected), glomeruli exhibited “hyalinization” of the tuft components, characterized by a loss of cellularity, an accumulation of sirius red-positive material, and the disappearance of much of the vasculature (Figure 3, E and F).
Serial-section analysis of glomeruli of eNOS knockout kidneys showed that although in the normal-appearing parenchyma glomeruli were routinely found connected to large-diameter proximal tubules, in the scar zones no tubular profiles could be found attached to any of the glomeruli there (Figure 4). When biotinylated lectins were used to stain specific tubule segments in the eNOS knockout kidney, large-diameter tubules with densely stained lumens appeared in most of the parenchyma (Figure 5A). However, small circular profiles with positively stained centers were present within the scars (Figure 5, A–D). These profiles appeared to be degenerated tubules; in semithin plastic sections, they clearly consisted of epithelial cells surrounding reduced lumina (Figure 5, E–G). When serial paraffin sections (5 to 10 μm thick) of lectin-stained scars were analyzed, the small tubule profiles rarely extended over two adjacent sections, suggesting that they represent spherical tubule fragments. Urine protein/creatinine ratio determined in 10 adult eNOS knockout mice averaged 0.20 ± 0.04, whereas the ratio for five wild-type mice was 0.10 ± 0.04 (mg/ml:mg/dl) (p = 0.12).
Figure 4.
Adult eNOS knockout kidney, scarred region. Gallery of consecutive serial 5-μm sections used to trace the appearance and disappearance of three glomeruli (indicated in orange, green, or yellow transparent overlays). Close examination of such series of micrographs revealed no connection of any of the glomeruli to a proximal tubule. Scale bar = 200 μm.
Figure 5.
Scar morphology examined with lectin staining and in semithin plastic sections. A: Survey micrograph of staining pattern with Lotus tetragonolobus lectin, which is specific for proximal tubules. Luminal staining is evident in the majority of large tubule profiles over most of the field (PT), which surrounds a small region of scarring, in which a number of small opaque profiles with similar staining (*) are present. B and C: Serial consecutive sections of an eNOS knockout kidney scar stained with lotus lectin (proximal tubules) and Dolichos biflorus (collecting ducts), respectively. Small stained profiles are present within the scar region in both sections. D: Detail of lotus-stained kidney at the border of a scar. Typical large proximal tubules (PT) show characteristic luminal staining. Similarly stained elements within the scar (arrows) consist of spherical profiles with dense cores. E: Plastic section through a region of scarring. In addition to a sclerotic glomerulus, the field contains numerous small matrix cells, microvessels, and two circular profiles with dense lumina (arrows) that correspond in size and structure to the positively stained bodies in lectin-stained scars. F and G: High-magnification views of the scar structures that appear to be remnants of tubules. Only slightly larger than capillaries (examples of which are indicated by C), they comprise several epithelial cells wrapped around a small dense lumen. Scale bars: 500 μm (A); 300 μm (B, C); 50 μm (D, E); 10 μm (F, G).
Neonatal eNOS Knockout Mice
No lesions were observed in kidneys of 7-day-old neonatal eNOS knockout mice. At 2 weeks of age, eNOS knockout kidneys frequently exhibit discolored regions that were lighter in color than the surrounding kidney parenchyma. Sections of these kidneys stained by the Apoptag TUNEL technique displayed wedges of reactivity extending from the surface deep into the cortex (Figure 6A). Clusters of apoptotic cells also appeared free in the lumina of the medullary-collecting ducts that lie directly beneath the degenerating wedges. Apoptotic nuclei are characterized by their dense punctate appearance, whereas necrotic cells are swollen, lack distinct nuclear profiles, and display a diffuse granular cytoplasmic staining. Although few glomeruli in these regions exhibited examples of either apoptosis or necrosis (Figure 6B), entire proximal tubules appeared to undergo necrosis in these degenerating zones and were readily recognizable by their exclusion of methylene blue counterstain (Figure 6C). Extensive damage was also apparent in semithin sections of such tubules (Figure 6D).
Figure 6.
Kidneys from 14-day-old eNOS knockout mice. A: Survey of a sagittal section through the renal cortex, stained with the Apoptag technique. A discrete wedge of selectively stained tissue is present, which corresponds to a pale area observed on the cortex of the kidney when it was first exposed in the animal. B: Apoptosis in a degenerating zone. Although the glomerulus lacks any staining, an adjacent proximal tubule (shown in serial sections to be continuous with the glomerulus) contains several apoptotic epithelial nuclei (examples at arrows). C: Proximal tubule profiles in the zone that exclude the methylene blue counterstain and also display a fine granular positivity for the Apoptag reagents. Discrete nuclear profiles are absent as well, indicating that these swollen tubular cells are undergoing extensive necrosis. D: Semithin section of similar proximal tubules, illustrating nuclear dissolution and cytoplasmic vacuolation typical of necrosis. Scale bars: 500 μm (A); 25 μm (B–D).
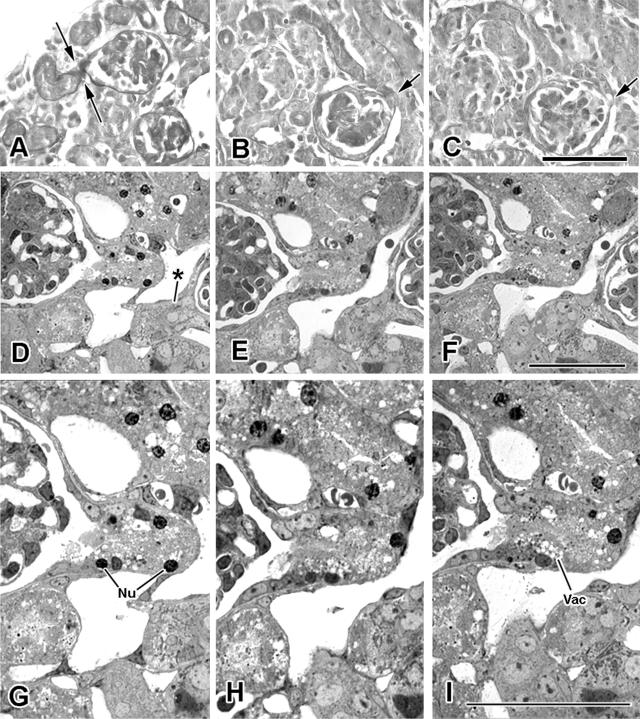
Within the degenerating zones, PAS staining (which accentuates the basement membranes of nephrons, thus tracing out their profiles) revealed glomeruli containing connections with proximal tubules that were reduced to narrow necks (Figure 7, A–C), suggesting that a process of “pinching-off” had occurred. Signs of necrosis, including pyknotic nuclei and vacuolated cytoplasm, were found in some proximal tubules within this neck region (Figure 7, D–I).
Figure 7.
Glomerulotubular junctional changes in zones of degeneration in 14-day eNOS knockout kidneys. A: PAS-stained tissue. The basement membrane of this glomerulus and its associated proximal tubule are clearly stained, revealing a zone of constriction (between arrows) at the neck, where the tubule joins Bowman’s capsule. B and C: Serial consecutive sections of neck constriction. In B, the initial proximal tubule segment appears shrunken; its opening to Bowman’s space (at arrow) is severely constricted. D–I: Consecutive serial semithin sections of glomerulotubular junctions (lower magnification shown above corresponding detail for each of the three sections). The right-hand glomerulus in D leads into a proximal tubule with a pinched neck (*). The glomerulus at left is continuous with a proximal tubule segment that, although of larger diameter, contains evidence of ongoing epithelial-cell necrosis in the form of pyknotic nuclear profiles (Nu) and concentrations of cytoplasmic vacuoles (Vac). Scale bars: 100 μm (A–C); 50 μm (D–F); 25 μm (G–I).
eNOS Localization in Neonatal Wild-Type Mouse Kidney
In the neonatal wild-type mouse, eNOS signal was prominent within proximal tubules from the newborn through 2 weeks of age, reaching its greatest intensity at approximately 7 days postnatal (Figure 8A) but becoming substantially diminished by day 14 (Figure 8B). Close inspection of neonates revealed a spotty pattern of vessel staining that gradually increases in intensity and incidence. Staining largely disappeared from tubules by day 21 in the WT mouse and in adult kidney was limit-ed exclusively to the endothelial linings of all vessel types (Figure 8C), including the glomerular capillaries (Figure 8D).
Figure 8.
Immunostaining patterns for eNOS in wild-type (C57Bl) mice. A: At 7 days postnatal, intense staining is present throughout proximal tubules. B: By 14 days, eNOS staining, although still present in tubules, is substantially fainter. C: In adult kidney, eNOS staining is limited to vascular endothelium, including that of arterioles (Ar), peritubular capillaries (C), and glomerular capillaries (glomerulus shown at G). D: Detail of adult kidney showing endothelial staining in both peritubular and glomerular capillary beds. Scale bars: 100 μm (A, B); 500 μm (C); 50 μm (D).
Discussion
eNOS, on the basis of its generation of nitric oxide, is generally considered protective against renal injury.11 If overactive, however, it plays a role in a variety of pathologies.12 Although heart defects have been found in some studies of eNOS knockout mice,13,14 the histological features of the kidneys when examined were deemed unremarkable.6 In contrast, our observations have revealed focal progressive kidney lesions in the majority of animals examined.
eNOS Patterns in Developing Rodent Kidney
A recent study of postnatal rat kidney4 found eNOS staining in endothelial cells beginning at embryonic day 14 and maintaining a blood vessel-associated location from that time forward. In addition, eNOS appears in proximal tubule epithelial cells, but only at postnatal day 1, when it is concentrated within lysosomes and endocytic vacuoles.4 The authors suggest that this eNOS has been scavenged from glomerular filtrate and report that no immunoreactive eNOS was present in the tubules following that single day of appearance.4
Although we have, in this communication, reported a nonvascular expression of eNOS in postnatal mouse proximal tubules, our observations vary substantially from those of Han et al.4 Rather than a discrete zone of staining in mouse epithelial cells, which would be expected if the enzyme were located in phagocytic vacuoles, there is a generalized distribution of eNOS immunoreactivity throughout epithelial cell cytoplasm in paraffin sections. Moreover, this overall staining pattern is present in the newborn mouse, peaking at 7 days, and continues to be recognizable as late as 21 days of age. Because mRNA for eNOS has been detected in proximal tubules,15 it is likely that the protein is generally distributed there as a result of intracellular synthesis rather than having been sequestered by some phagocytic mechanism. During postnatal development, the transient increase in proximal tubular eNOS staining contrasts with a steady increase in endothelial cell expression of eNOS. This is consistent with a supportive role for eNOS in tubule maturation through the first 2 weeks of life in the wild-type mouse.
The lack of an endothelial enzyme in these animals suggests that some sort of vascular compromise leads to the formation of scars. However, morphometric examination of PECAM-stained material demonstrates that blood vessels constitute a substantial fraction (>20%) of the volume of the scar. The vascular population is roughly evenly divided, furthermore, between glomerular capillaries and extraglomerular vessels. It is unlikely that the vascular population has actually increased, because this concentration of vessels is the result of the disappearance within the scar of most of the tubule mass. Frequently larger vessels are seen to converge on scars, presumably drawn in by the shrinkage of the parenchyma. In any event, there is no lack, per se, of vascular elements within zones of scarring in the adult eNOS knockout mouse kidney.
The Fate of Tubules Within Regions of Scarring
Scars in adult eNOS knockout kidneys revealed the remains of proximal tubules and collecting ducts. Serial-section analysis demonstrates that not only are these tubular segments disconnected from their parent glomeruli, but they are also discontinuous with one another. This suggests that tubular degeneration has resulted in fragmentation rather than tubular atrophy. Atrophic tubules are characterized by a distinctly thickened basement membrane; no such alteration is evident in association with any of the eNOS knockout tubule profiles. Degeneration begins in the neonatal animal, where—in regions destined to become scars in the adult kidney—otherwise normal-appearing tubules become severely “pinched” at their junction with the glomerular capsule. This phenomenon resembles the “swan-neck deformity” seen in other nephropathies such as cystinosis.16 Because there is a considerable incidence of both tubular apoptosis and necrosis within these select parenchymal regions, it is likely that degeneration at the glomerulotubular junction is the initiating event, followed by progressive tubular degeneration. The eventual result is that only spherical remnants of the tubules are left scattered among the clusters of glomeruli that dominate the scars in adult eNOS knockout mice. Consistent with these observations, eNOS has been reported to function as a survival factor, protecting prostate cancer cells from tumor necrosis factor-related apoptosis-inducing ligand-induced apo-ptosis.17 Moreover, eNOS knockout mice develop congenital septal defects during cardiac development, associated with increased cardiomyocyte apoptosis.18
A similar lesion has been described in a model of passive Heymann nephritis in the rat, in which apoptosis develops at the glomerulotubular junction and leads to glomerular-tubule disconnection.19 Interestingly, treatment with an angiotensin converting enzyme inhibitor prevented this glomerular-tubule disconnection.19 A variety of renal tubulointerstitial disorders generate atubular glomeruli, including lithium,20 cisplatin,21 and adriamycin nephropathy,22 ischemic injury,23,24 renal artery stenosis,25 and chronic renal allograft rejection26 as well as polycystic kidney disease.27 The renal parenchymal scars developing in eNOS knockout mice are superficially similar to those of chronic pyelonephritis. In this latter disorder, lesions are also more frequent at either pole of the kidney and result in glomerular crowding and atubular glomeruli.28 A difference between these disorders and eNOS deficiency is the lack of tubular atrophy and interstitial fibrosis in the latter. However, similar to the results in the present study, the size of atubular glomeruli is smaller in disorders that develop before adulthood, although some glomeruli undergo compensatory hypertrophy.29
Glomerulus-tubule disconnection may actually represent a central mechanism in the progression of both glomerular and tubulointerstitial disorders and is a common finding in both experimental and human disease.30–33 Furthermore, it is of historical interest that, based on elegant microdissections of kidneys from patients with chronic renal disease, “aglomerular nephrons” were described in detail by Oliver in his landmark monograph published in 1939.34
The Interstitial Cells of the Scars
The hypoplastic or atrophic glomeruli in regions of scarring are embedded in a complex matrix that contains abundant microvessels, presumably concentrated in these regions because of the reduction of tubular mass. The other identifiable elements are the tubule remnants themselves, as discussed above. The remaining matrix consists of a panoply of small individual cells packed closely in the interstices among the glomeruli. In the definitive adult scar, many of these cells exhibit characteristics either of fibroblasts (fsp-1 staining) or of macrophages (F4/80 positivity). Staining of these zones with an antibody directed against α-smooth muscle actin showed little reaction; this indicates that myofibroblast-like cells are not major components of the scar. Overt renal apoptosis and necrosis are present in the eNOS knockout mouse only within the presumptive scar of the neonatal animal and do not appear in the adult (although abnormal glomeruli are routinely present outside the scars). Thus, it seems unlikely that the presence of fibroblasts and macrophages within the adult scar are necessarily the result of migratory events in response to an inflammatory signal.
Clinical Implications
A number of animal models of renal disorders are characterized by decreased renal eNOS production. These include two-kidney, one-clip hypertension,35 cyclosporin nephropathy,36 renal ablation,37 and unilateral ureteral obstruction.38 Interestingly, although renal cortical eNOS mRNA is increased in children with ureteropelvic junction obstruction, enzyme activity is reduced in proportion to the severity of interstitial fibrosis and the reduction in creatinine clearance.39 Also of note, eNOS gene polymorphisms are associated with reduced enzyme activity and the development of end-stage renal failure.40,41 In males, the Glu298Asp polymorphism is associated with a lower age for the development of renal failure from autosomal dominant polycystic kidney disease.42 Although most reports focus on the role of eNOS in the vasculature, more recently the role of this enzyme in renal tubules has been addressed.43 The present study provides new evidence for the protective role played by endogenous eNOS through the process of renal maturation: a reduction in renal eNOS generation may predispose the individual to renal injury or progression over time.
In summary, mice lacking the eNOS enzyme develop significant focal scarring through a process of segmental tubular degeneration, beginning at a time at which, in wild-type animals, eNOS is strongly expressed by the proximal tubules. The absence of eNOS at critical developmental stages may render tubules, and indeed entire segments of parenchyma, vulnerable to degeneration. A number of parallels exist between the renal lesions appearing in eNOS knockout mice and those described in a wide variety of progressive renal disorders, most notably glomerulus-tubule disconnection and the formation of atubular glomeruli. We speculate that, in addition to its role in vascular function and by serving as a survival factor, eNOS may be necessary for the preservation of tubular integrity in response to nephron injury.
Acknowledgments
We thank Laura Trepanier of The Jackson Laboratory for providing helpful technical information as well performing confirmatory genotyping on the original eNOS knockout breeding pairs used in this study. Thanks also go to Dr. Victor Laubach for providing additional examples of knockout mice from his breeding colony.
Footnotes
Address reprint requests to Robert L. Chevalier, M.D., Department of Pediatrics, University of Virginia, Box 800386, Charlottesville, VA 22908. E-mail: RLC2M@virginia.edu.
Supported by grants from the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases) DK52612, DK45179, and DK62328.
This work has been presented in abstract form at the 38th Annual Meeting of the American Society of Nephrology (November 8–13, 2005; Philadelphia, PA).
References
- Kone BC. Nitric oxide synthesis in the kidney: isoforms, biosynthesis, and functions in health. Semin Nephrol. 2004;24:299–315. doi: 10.1016/j.semnephrol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Shinozaki M, Hirakata H, Tamaki K, Hirano T, Tokumoto M, Goto H, Okuda S, Fujishima M. Locally activated renin-angiotensin system associated with TGF-beta 1 as a major factor for renal injury induced by chronic inhibition of nitric oxide synthase in rats. J Am Soc Nephrol. 2000;11:616–624. doi: 10.1681/ASN.V114616. [DOI] [PubMed] [Google Scholar]
- Yamashita J, Ogata M, Itoh M, Yamasowa H, Shimeda Y, Takaoka M, Matsumura Y. Role of nitric oxide in the renal protective effects of ischemic preconditioning. J Cardiovasc Pharm. 2003;42:419–427. doi: 10.1097/00005344-200309000-00014. [DOI] [PubMed] [Google Scholar]
- Han KH, Lim JM, Kim WY, Kim H, Madsen KM, Kim J. Expression of endothelial nitric oxide synthase in developing rat kidney. Am J Physiol. 2004;288:F694–F702. doi: 10.1152/ajprenal.00085.2004. [DOI] [PubMed] [Google Scholar]
- Wu F, Park F, Cowley AW, Jr, Mattson DL. Quantification of nitric oxide synthase activity in microdissected segments of the rat kidney. Am J Physiol. 1999;276:F874–F881. doi: 10.1152/ajprenal.1999.276.6.F874. [DOI] [PubMed] [Google Scholar]
- Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Nat Acad Sci USA. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa P, Van Goor H, Itoh-Lindstrom Y, Maeda N, Falk RJ, Assmann KJ, Kallenberg CG, Jennette JC. Lack of endothelial nitric oxide synthase aggravates murine accelerated anti-glomerular basement membrane glomerulonephritis. Am J Pathol. 2000;156:879–888. doi: 10.1016/S0002-9440(10)64957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachat F, Lange-Sperandio B, Chang AY, Kiley SC, Thornhill BA, Forbes MS, Chevalier RL. Ureteral obstruction in neonatal mice elicits segment-specific tubular cell responses leading to nephron loss. Kidney Int. 2003;63:564–575. doi: 10.1046/j.1523-1755.2003.00775.x. [DOI] [PubMed] [Google Scholar]
- Forbes MS, Ghribi O, Herman MM, Savory J. Aluminum-induced dendritic pathology revisited: cytochemical and electron microscopic studies of rabbit cortical pyramidal neurons. Ann Clin Lab Sci. 2002;32:75–86. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of micrograph quantities of protein using the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang B, Mathew R, Palmer LS, Valderrama E, Trachtman H. Nitric oxide in obstructive uropathy: role of endothelial nitric oxide synthase. J Urol. 2002;168:1801–1804. doi: 10.1097/01.ju.0000027177.45171.e9. [DOI] [PubMed] [Google Scholar]
- Rolle U, Shima H, Puri P. Nitric oxide, enhanced by macrophage-colony stimulating factor, mediates renal damage in reflux nephropathy. Kidney Int. 2002;62:507–513. doi: 10.1046/j.1523-1755.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- Li W, Mital S, Ojaimi C, Csiszar A, Kaley G, Hintze TH. Premature death and age-related cardiac dysfunction in male eNOS-knockout mice. J Mol Cell Cardiol. 2004;37:671–680. doi: 10.1016/j.yjmcc.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Ortiz PA, Garvin JL. Cardiovascular and renal control in NOS-deficient mouse models. Am J Physiol. 2003;284:R628–R638. doi: 10.1152/ajpregu.00401.2002. [DOI] [PubMed] [Google Scholar]
- Ujiie K, Yuen J, Hogarth L, Danziger R, Star RA. Localization and regulation of endothelial NO synthase mRNA expression in rat kidney. Am J Physiol. 1994;267:F296–F302. doi: 10.1152/ajprenal.1994.267.2.F296. [DOI] [PubMed] [Google Scholar]
- Gahl WA, Thoene JG, Schneider JA. Cystinosis. N Engl J Med. 2002;347:111–121. doi: 10.1056/NEJMra020552. [DOI] [PubMed] [Google Scholar]
- Tong X, Li H. eNOS protects prostate cancer cells from TRAIL-induced apoptosis. Cancer Lett. 2004;210:63–71. doi: 10.1016/j.canlet.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Feng Q, Song W, Lu X, Hamilton JA, Lei M, Peng T, Yee SP. Development of heart failure and congenital septal defects in mice lacking endothelial nitric oxide synthase. Circulation. 2002;106:873–879. doi: 10.1161/01.cir.0000024114.82981.ea. [DOI] [PubMed] [Google Scholar]
- Benigni A, Gagliardini E, Remuzzi A, Corna D, Remuzzi G. Angiotensin-converting enzyme inhibition prevents glomerular-tubule disconnection and atrophy in passive Heymann nephritis, an effect not observed with a calcium antagonist. Am J Pathol. 2001;159:1743–1750. doi: 10.1016/s0002-9440(10)63021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcussen N, Ottosen PD, Christensen S, Olsen TS. Atubular glomeruli in lithium-induced chronic nephropathy in rats. Lab Invest. 1989;61:295–302. [PubMed] [Google Scholar]
- Marcussen N. Atubular glomeruli in cisplatin-induced chronic interstitial nephropathy. APMIS. 1990;98:1087–1097. doi: 10.1111/j.1699-0463.1990.tb05039.x. [DOI] [PubMed] [Google Scholar]
- Javaid B, Olson JL, Meyer TW. Glomerular injury and tubular loss in adriamycin nephrosis. J Am Soc Nephrol. 2001;12:1391–1400. doi: 10.1681/ASN.V1271391. [DOI] [PubMed] [Google Scholar]
- Pagtalunan ME, Olson JL, Tilney NL, Meyer TW. Late consequences of acute ischemic injury to a solitary kidney. J Am Soc Nephrol. 1999;10:366–373. doi: 10.1681/ASN.V102366. [DOI] [PubMed] [Google Scholar]
- Pagtalunan ME, Olson JL, Meyer TW. Contribution of angiotensin II to late renal injury after acute ischemia. J Am Soc Nephrol. 2000;11:1278–1286. doi: 10.1681/ASN.V1171278. [DOI] [PubMed] [Google Scholar]
- Marcussen N. Atubular glomeruli in renal artery stenosis. Lab Invest. 1991;65:558–565. [PubMed] [Google Scholar]
- Pagtalunan ME, Oberbauer R, Haas M, Barlan M, Mayer G, Olson JL, Meyer TW. Atubular glomeruli in patients with chronic allograft rejection. Transplantation. 1996;61:1166–1171. doi: 10.1097/00007890-199604270-00008. [DOI] [PubMed] [Google Scholar]
- Tanner GA, Tielker MA, Connors BA, Phillips CL, Tanner JA, Evan AP. Atubular glomeruli in a rat model of polycystic kidney disease. Kidney Int. 2002;62:1947–1957. doi: 10.1046/j.1523-1755.2002.00689.x. [DOI] [PubMed] [Google Scholar]
- Marcussen N, Olsen TS. Atubular glomeruli in patients with chronic pyelonephritis. Lab Invest. 1990;62:467–473. [PubMed] [Google Scholar]
- Marcussen N. Tubulointerstitial damage leads to atubular glomeruli: significance and possible role in progression. Nephrol Dial Transplant. 2000;15(Suppl 6):74–75. doi: 10.1093/ndt/15.suppl_6.74. [DOI] [PubMed] [Google Scholar]
- Kriz W, Le Hir M. Pathways to nephron loss starting from glomerular diseases—insights from animal models. Kidney Int. 2005;67:404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- Gibson IW, Downie TT, More IAR, Lindop GBM. Atubular glomeruli and glomerular cysts–a possible pathway for nephron loss in the human kidney? J Pathol. 1996;179:421–426. doi: 10.1002/(SICI)1096-9896(199608)179:4<421::AID-PATH616>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Gandhi M, Olson JL, Meyer TW. Contribution of tubular injury to loss of remnant kidney function. Kidney Int. 1998;54:1157–1165. doi: 10.1046/j.1523-1755.1998.00107.x. [DOI] [PubMed] [Google Scholar]
- Marcussen N. Atubular glomeruli and the structural basis for chronic renal failure. Lab Invest. 1992;66:265–284. [PubMed] [Google Scholar]
- Oliver J. New York: Paul B. Hoeber, Inc.; Architecture of the Kidney in Chronic Bright’s Disease. 1939 [Google Scholar]
- Wickman A, Andersson IJL, Jia J, Hedin L, Bergstrom G. Endothelial nitric oxide synthase protein is reduced in the renal medulla of two-kidney, one-clip hypertensive rats. J Hypertens. 2001;19:1665–1673. doi: 10.1097/00004872-200109000-00020. [DOI] [PubMed] [Google Scholar]
- Ling H, Li X, Jha S, Wang W, Karetskaya L, Pratt B, Ledbetter S. Therapeutic role of TGF-beta-neutralizing antibody in mouse cyclosporin A nephropathy: morphologic improvement associated with functional preservation. J Am Soc Nephrol. 2003;14:377–388. doi: 10.1097/01.asn.0000042168.43665.9b. [DOI] [PubMed] [Google Scholar]
- Kim SW, Lee JU, Paek YW, Kang DG, Choi KC. Decreased nitric oxide synthesis in rats with chronic renal failure. J Korean Med Sci. 2000;15:425–430. doi: 10.3346/jkms.2000.15.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein DM, Travis BR, Thornhill BA, Schurr JS, Kolls JK, Leung JC, Chevalier RL. Altered expression of immune modulator and structural genes in neonatal unilateral ureteral obstruction. Kidney Int. 2003;64:25–35. doi: 10.1046/j.1523-1755.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- Vallés P, Pascual L, Manucha W, Carrizo L, Ruttler M. Role of endogenous nitric oxide in unilateral ureteropelvic junction obstruction in children. Kidney Int. 2003;63:1104–1115. doi: 10.1046/j.1523-1755.2003.00833.x. [DOI] [PubMed] [Google Scholar]
- Noiri E, Satoh H, Taguchi J, Brodsky SV, Nakao A, Ogawa Y, Nishijima S, Yokomizo T, Tokunaga K, Fujita T. Association of eNOS Glu298Asp polymorphism with end-stage renal disease. Hypertension. 2002;40:535–540. doi: 10.1161/01.hyp.0000033974.57407.82. [DOI] [PubMed] [Google Scholar]
- Page A, Reich H, Zhou J, Lai V, Cattran DC, Scholey JW, Miller JA. Endothelial nitric oxide synthase gene/gender interactions and the renal hemodynamic response to angiotensin II. J Am Soc Nephrol. 2005;16:3053–3060. doi: 10.1681/ASN.2004110905. [DOI] [PubMed] [Google Scholar]
- Persu A, Stoenoiu S, Messiaen T, Davila S, Robino C, El-Khattabi O, Mourad M, Horie S, Feron O, Balligand JL, Wattiez R, Pirson Y, Chauveau D, Lens XM, Devuyst O. Modifier effect of eNOS in autosomal dominant polycystic kidney disease. Hum Mol Genet. 2002;11:229–241. doi: 10.1093/hmg/11.3.229. [DOI] [PubMed] [Google Scholar]
- Pollock JS, Carmines PK. Renal tubular epithelial cells are not simply large endothelial cells. Hypertension. 2006;47:19–21. doi: 10.1161/01.HYP.0000196276.29211.6f. [DOI] [PubMed] [Google Scholar]