Abstract
Relapsing fever is an infection characterized by peaks of spirochetemia attributable to antibody selection against variable serotypes. In the absence of B cells, serotypes cannot be cleared, resulting in persistent infection. We previously identified differences in spirochetemia and disease severity during persistent infection of severe combined immunodeficiency mice with isogenic serotypes 1 (Bt1) or 2 (Bt2) of Borrelia turicatae. To investigate this further, we studied pathogen load, clinical disease, cytokine/chemokine production, and inflammation in mice deficient in B (Igh6−/−) or B and T (Rag1−/−) cells persistently infected with Bt1 or Bt2. The results showed that Igh6−/− mice, despite lower spirochetemia, had a significantly aggravated disease course compared with Rag1−/− mice. Measurement of cytokines revealed a significant positive correlation between pathogen load and interleukin (IL)-10 in blood, brain, and heart. Bt2-infected Rag1−/− mice harbored the highest spirochetemia and, at the same time, displayed the highest IL-10 plasma levels. In the brain, Bt1, which was five times more neurotropic than Bt2, caused higher IL-10 production. Activated microglia were the main source of IL-10 in brain. IL-10 injected systemically reduced disease and spirochetemia. The results suggest IL-10 plays a protective role as a down-regulator of inflammation and pathogen load during infection with relapsing fever spirochetes.
Relapsing fever (RF)1 is a multisystemic spirochetosis caused by infection with different Borrelia spp.2 Several organs are involved including the skin, the heart, and the brain.3 The hallmark of RF is febrile periods concurrent with high-level spirochetemia alternating with periods of well being with low spirochetemia. RF borrelias evade the host’s serotype-specific antibody response through variation of immunodominant outer membrane lipoproteins known as variable major proteins (VMPs). VMPs are the serotypic determinants and group in two sizes, variable small proteins (Vsp) and variable large proteins (Vlp). VMP variation occurs by switching the vmp gene at the expression locus, which spontaneously occurs at a frequency of 1 in 1000 per generation.4 VMP-specific IgM antibodies from B1b cells are responsible for serotype clearance,5,6 but newly emerging serotypes spontaneously arise and cause relapses.
We have been studying the possibility that another role of VMP variation may be modulation of disease severity and tissue tropism. This requires the use of B-cell-deficient mice to avoid serotype clearance. In severe combined immunodeficiency (SCID) mice, which are deficient in B and T cells, we isolated two isogenic serotypes of the North American RF agent Borrelia turicatae (Bt) that differ only in the expression of their VMP, yet show significant differences in disease severity and tissue tropism: serotype 2 (Bt2, formerly known as BtB), defined by expression of Vsp2 (formerly VspB), is more virulent as evidenced by lethality in infant mice, higher spirochetemia, and more severe arthritis. In contrast, serotype 1 (Bt1, formerly known as BtA), defined by expression of Vsp1 (formerly VspA), is more neurotropic: it enters the central nervous system earlier and in higher numbers despite 10-fold lower spirochetemia than Bt2.3,7,8
During RF, there is a significant inflammatory response to the infection characterized by production of several cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, and IL-10.9 The levels of cytokines further increase after treatment with antibiotics resulting in sudden fever, rigors, and persistent hypotension known as the Jarisch-Herxheimer reaction.10 It is possible that differences in cytokine production explain the differences in disease severity observed during persistent infection with Bt1 or Bt2.
The original goal of the present study was to compare the disease during persistent infection with Bt1 or Bt2 in mice deficient in B or B and T cells. The results not only confirmed significant differences in disease severity between these mice but, more importantly, suggest that IL-10 may play an important protective role during persistent borrelial infection.
Materials and Methods
Strains and Culture Conditions
Isogenic serotypes 1 (Bt1) and 2 (Bt2) have been previously characterized.3,7,11 Spirochetes were cultured as described.12 Before infection, borrelia viability was assessed by microscopy and serotype identity was confirmed by Western blot with monoclonal antibodies.7,11
Mouse Infections
Female C57BL/6-Igh6−/− (B6.129S2-Igh-6tm1Cgn) and Rag1−/− (B6.129S7-Rag1tm1Mom) mice (4 to 5 weeks old) (Jackson Laboratories, Bar Harbor, ME) were inoculated intraperitoneally with 103 Bt1 or Bt2 in 200 μl of phosphate-buffered saline (PBS) or with PBS alone and kept for 2 or 4 weeks. Groups of four to six mice each were used for all experiments. Mice were maintained in a germ-free environment. Housing and care was in accordance with the Animal Welfare Act in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Borrelias were counted using a Petroff-Hauser chamber. To confirm infection, necropsy plasma was added to BSK II culture media. Positive spirochetal cultures were pelleted, washed, and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis.4 Mice were euthanized as described.12 One half of brain and heart was snap-frozen for RNA extraction, whereas the other half was fixed in 4% paraformaldehyde for 6 hours at room temperature and paraffin-embedded.
IL-10 Treatment
To assess the effect of IL-10 on disease, groups of four 4- to 5-week-old female Igh6−/− mice persistently infected with either Bt1 or Bt2 were treated intraperitoneally with either 1) daily doses of 50 μg/kg murine rIL-10 (Pierce, Rockford, IL) starting 3 hours before inoculation; 2) three daily doses of 100 μg/kg rIL-10 on days 11 to 13; 3) a single dose of 150 μg/kg rIL-10 on day 13 only; or 4) PBS alone as a control. Mice treated with a single dose of rIL-10 on day 13 received injections of PBS on days 11 and 12 to preserve masking of the examiner. Dosing of rIL-10 was estimated according to a previously published regimen.13
Clinical Examination
Severity of signs was assessed by an investigator masked to mouse genotype and infection status using a clinical score: A) spinning (held off by tail; vestibular dysfunction): none = 0, unsustained = 1, sustained = 2; B) ruffled fur (skin infection): normal = 0, ruffled = 1, ruffled and dry = 2; C) conjunctival secretion (eye infection, conjunctivitis): none = 0, mucous = 1, purulent = 2; and D) joint swelling (arthritis): the most swollen tibiotarsal joint was scored as none = 0, mild = 1, moderate to severe = 2, and measured with a caliper. For all clinical measures, the sum score was calculated.
Immunohistochemistry
Five-μm sagittal brain sections were deparaffinized, rehydrated, and stained for spirochetes as described.8 Primary antibodies were rabbit anti-Vsp1 or anti-Vsp2 (1/5000). IgG of the same species (Sigma, St. Louis, MO) or nonrelevant primary antibodies matched for concentration and isotype were used as negative controls. For antigen retrieval, sections were heated in a microwave at 30% power for 10 minutes. Incubation time for primary antibodies was 30 minutes. Primary antibodies were followed by a biotinylated secondary antibody and peroxidase-streptavidin (all Biogenex, San Ramon, CA). Slides were counterstained with Mayer’s hematoxylin for 1 minute. Leptomeningeal borrelias were counted in ×200 microscopic fields of sagittal brain sections.
Immunofluorescence
To identify microglial/macrophages, the primary antibodies were rat anti-mouse F4/80, diluted 1/1000 (clone A3-1; Serotec, Oxford, UK) and rabit anti-Iba1 polyclonal diluted 1/600 (no. 019-1971; Wako Chemicals, Osaka, Japan). To identify IL-10-producing cells fluorescent immunohistochemistry was performed similar to a previous protocol14 using goat anti-mouse/rat IL-10 (1/20; R&D Systems, Minneapolis, MN) or rat anti-mouse IL-10 (1/20; clone JES5-2A5; BD Biosciences, San Jose, CA) antibodies incubated for 24 hours at 4°C. Both anti-IL-10 antibodies gave comparable results and staining specificity was demonstrated by reduction of antibody binding after preadsorption with an excess amount of rIL-10 (Serotec) for 2 hours at 37°C. Secondary antibodies were Alexa-488 or -594-conjugated goat or chicken anti-rat, -goat, or -rabbit IgG (Molecular Probes, Eugene, OR). Nuclei were counterstained for 1 minute with 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI, 1/50,000; Molecular Probes) and slides were mounted in Fluoromount G (EMS, Hatfield, PA).
RNA Extraction
Perfused brain or heart tissues were placed into green-top FastRNA tubes (BIO-101, Irvine, CA). One ml of Trizol (Invitrogen, Carlsbad, CA) was added to each tube followed by homogenization using a Fast Prep FP120 tissue homogenizer (Qbiogene, Morgan, Irvine, CA) for 20 seconds. Samples were then stored at −80°C. After thawing, 200 μl of chloroform was added and tubes were agitated for 15 seconds, incubated at room temperature for 3 minutes, and centrifuged at 12,000 rpm and 4°C for 15 minutes. The upper aqueous layer was transferred to a fresh tube, gently mixed with an equal volume of 70% ethanol, and added onto RNeasy columns (Qiagen, Valencia, CA). RNA was extracted per the manufacturer’s instructions and quantified by UV spectroscopy.
Quantitative Real-Time Reverse Transcriptase (RT)-Polymerase Chain Reaction (PCR)
RT was performed using TaqMan RT reagents (Applied Biosystems, Foster City, CA). A total of 34.4 ng of RNA per 55-μl reaction volume was reverse-transcribed to cDNA with final concentrations of 1× TaqMan RT buffer, 5.5 mmol/L MgCl2, 500 μmol/L of each deoxyNTP, 2.5 μmol/L random hexamers, 0.4 U/μl RNase inhibitor, and 1.25 U/μl of Multiscribe reverse transcriptase in a GeneAmp real-time PCR System 9700 for 10 minutes at 25°C, 30 minutes at 48°C, and 5 minutes at 95°C. Quantitative real-time PCR was performed on an ABI Prism 7700 sequence detection system (Applied Biosystems) for 40 cycles. Ten μl of cDNA equivalent to 6.25 ng of RNA was added to a 40-μl PCR mixture containing 1× TaqMan Universal master mix and final concentrations of borrelial 16S rRNA primer (0.2 μmol/L) and probe (0.1 μmol/L), corresponding to 16S rRNA of B. burgdorferi B31 (GenBank accession number U03396), sequence numbering 739 to 760 for the upstream (5′-GGTCAAGACTGACGCTGAGTCA-3′), 874 to 853 (5′-GGCGGCACACTTAACACGTTAG-3′) for the downstream primer, and 801 to 829 [(6FAM) 5′-TCTACGCTGTAAACGATGCACATTGGTG-3′(TAMRA)] for the probe; 18S rRNA (Mm4308329), IL-10 (Mm0043961), IL-10Rα (Mm00434151), and TNF-α (Mm00443258; all Applied Biosystems) primer/probes according to manufacturer’s instructions. Samples/standards were plated in duplicates (triplicates: calibrators and quantification of 16S rRNA in Igh6−/− brains) and submitted to amplification (95°C for 15 seconds, 60°C for 1 minute, 40 cycles). Analysis was performed using AB sequence detection system software (version 1.9.1; Foster City, CA). The spirochetal load was expressed as borrelias/10 ng of total RNA. For this purpose and to control for RNA integrity/RT efficiency, two standard curves were generated for every assay using 10-fold dilutions of RNA extracted from a known number of spirochetes (borrelial 16S rRNA) and twofold dilutions of a known amount of total brain RNA (18S rRNA). For relative quantification of IL-10, IL-10Rα, and TNF-α mRNA expression the ΔΔCT method was applied15 using a single calibrator from an uninfected mouse brain for all assays. For every primer/probe set PCR efficiencies were evaluated. The induction of mRNA was expressed as 2−ΔΔCt.
Enzyme-Linked Immunosorbent Assay
Concentrations of TNF-α, IL-2, IL-5, IL-6, IL-9, IL-10, IL-12, IL-13, GM-CSF, interferon-γ, RANTES, KC, MIP-1α, and MCP-1 were quantified in necropsy plasma in both infected and uninfected control mice using the Luminex 100 system (Luminex, Austin, TX) per the manufacturer’s directions.
Statistical Analysis
Results were expressed as median and range. Two-sided nonparametric tests were used to determine differences between medians (Mann-Whitney U-test) and correlations (Spearmans rank test). P < 0.05 was considered significant (*), P < 0.01 was considered highly significant (**).
Results
Spirochetemia
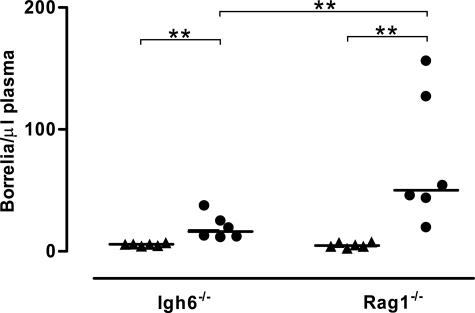
We began by studying whether there were any differences in spirochetemia between Bt1 and Bt2 in Igh6−/− and Rag1−/− mice infected for 4 weeks. We also examined a group of Igh6−/− mice earlier, 2 weeks after inoculation, to investigate whether spirochetemia with Bt1 or Bt2 increases throughout time. Assessment of necropsy blood cultures by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot confirmed that all Igh6−/− and Rag1−/− mice were persistently infected with the serotype they were originally inoculated with (not shown). As expected, we found Igh6−/− and Rag1−/− mice were unable to clear Bt1 or Bt2 from the circulation. Similar to SCID mice, we found the spirochetemia was significantly higher with Bt2 than with Bt1 in both Igh6−/− and Rag1−/− mice (Figure 1). The spirochetemia of Igh6−/− mice examined 2 or 4 weeks after inoculation was similar, an indication that once spirochetemia peaks it does not continue increasing throughout time in antibody-deficient mice (not shown). Interestingly, Bt2 spirochetemia 4 weeks after inoculation was significantly higher in Rag1−/− than in Igh6−/− mice, reaching more than 100 spirochetes per μl plasma in some Rag1−/− mice (Figure 1). This did not occur with Bt1. We concluded that Bt2 causes higher spirochetemia than Bt1 not only in SCID mice but also in Igh6−/− and Rag1−/− mice and that Igh6−/− mice control Bt2 spirochetemia better than Rag1−/− mice.
Figure 1.
Spirochetemia with Bt1 and Bt2 in Rag1−/− and Igh6−/− mice. Blood was harvested by cardiac puncture from groups of six Igh6−/− or Rag1−/− mice each infected throughout 4 weeks. Spirochetes were counted by phase-contrast microscopy and shown as median/μl plasma (linear scale). The spirochetemia was significantly higher with Bt2 (circles) than Bt1 (triangles) in both Igh6−/− and Rag1−/− mice and with Bt2 in Rag1−/− compared with Igh6−/− mice. **P < 0.01 (Mann-Whitney U-test).
Disease Severity
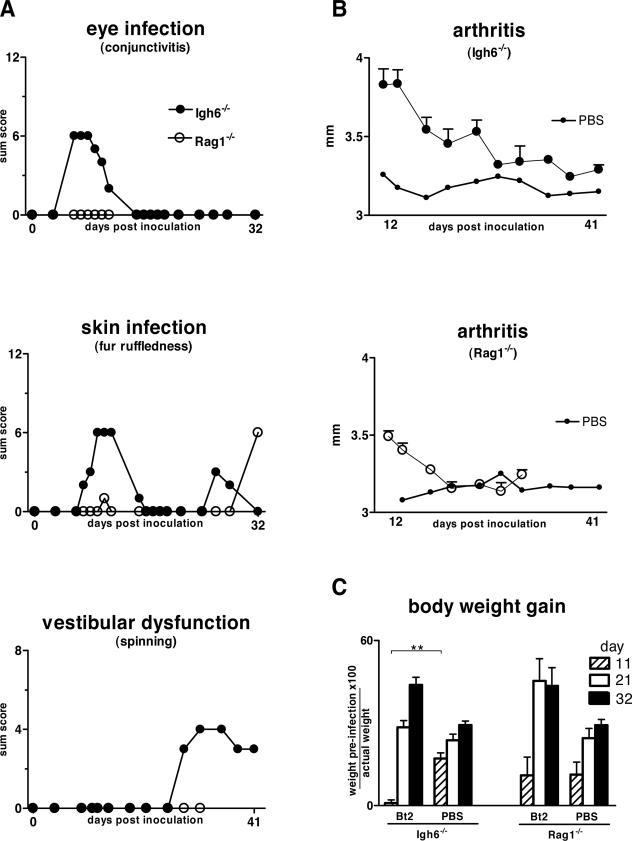
Next we studied whether there are differences in clinical disease between Bt1- or Bt2-infected Rag1−/− or Igh6−/− mice. A masked examiner (H.G.) scored infected and uninfected control mice of both genotypes for signs of ocular, skin, joint, and vestibular disease previously found to characterize infection of SCID mice.3 Unexpectedly, the results showed that Rag1−/− mice despite their higher spirochetemia had significantly less clinical disease than Igh6−/− mice (Figure 2). Clinical disease started with purulent conjunctival discharge and ruffled fur by week 1, followed by tibiotarsal joint swelling by week 2, and signs of vestibular dysfunction by week 3, resembling the pattern seen in SCID mice. Bt2-infected Igh6−/− mice also failed to gain weight during the first 11 days of infection compared with Bt2-infected Rag1−/− mice and uninfected controls (Figure 2C). Later, Bt2-infected mice of both genotypes gained weight compared with uninfected controls (P < 0.05 for days 21 and 31), corresponding to development of hepato-splenomegaly. Although this weight gain started earlier in the Rag1−/− mice (Figure 2C), by 4 weeks of infection the splenomegaly was higher in Igh6−/− than in Rag1−/− mice (15- versus 11-fold increase compared with uninfected controls, respectively; P < 0.01). We concluded that Igh6−/− mice, despite a lower spirochetemia, had a significantly more severe disease course than Rag1−/− mice.
Figure 2.
Disease severity in Bt2-infected Igh6−/− and Rag1−/− mice. The severity of disease associated with persistent infection with Bt2 compared with uninfected controls was assessed throughout 32 days by an investigator masked to genotype and infectious status. A: Disease severity in the eyes, skin, and vestibular system was scored as described in Materials and Methods. For each graph, the highest value on the y axis equals the maximum possible sum score per group. Note higher disease scores in Igh6−/− compared with Rag1−/− mice in all organs examined. B: Severity of arthritis was assessed by measuring the most swollen tibiotarsal joint with a metric caliper. Results are shown for each group of infected Igh6−/− or Rag1−/− mice as mean size (mm) ± SE. For clarity, only the average size is shown for PBS-inoculated control animals. C: Body weight gain: change in total body weight was assessed by comparing the weight before inoculation and 11, 21, and 31 days thereafter. Results are expressed as percent weight gain throughout the course of infection. **P < 0.01 (Mann-Whitney U-test).
Cytokines in Plasma
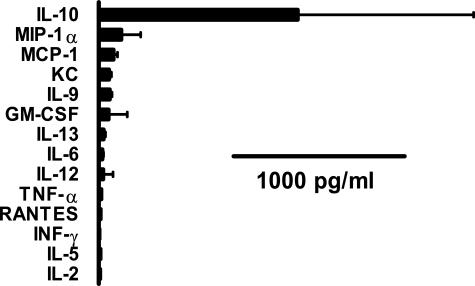
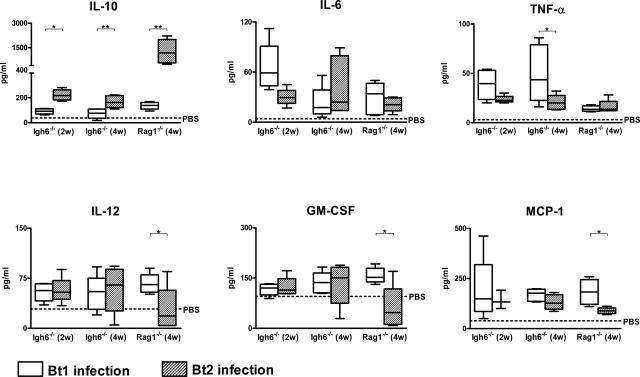
It was possible that the negative correlation between spirochetemia and clinical disease was explained by differences in the cytokine response to the infection between Igh6−/− and Rag1−/− mice. To investigate this, we measured the levels of several cytokines and chemokines in necropsy blood. First, 14 different cytokines and chemokines were measured in the blood of the Bt2-infected Rag1−/− mice necropsied after 4 weeks. The results showed that by far the most abundant of all of the cytokines and chemokines examined was IL-10 (Figure 3). To examine this further we compared the levels of IL-10 in plasma with that of the proinflammatory cytokines TNF-α, IL-6, and IL-12 and the chemokines GM-CSF and MCP-1 in all six groups of mice (Figure 4). The results showed that Bt2-infected Rag1−/− mice had the highest IL-10 plasma concentrations of all of the groups examined, whereas none of the other cytokines or chemokines were significantly elevated. In fact, Bt2-infected Rag1−/− mice had significant negative correlations between IL-10 and IL-12 (r = −0.71), GM-CSF (r = −0.74), and MCP-1 (r = −0.75) (all P < 0.01) (Figure 4). Bt2-infected Rag1−/− mice had lower levels of IL-12, GM-CSF, and MCP-1 than Bt2-infected Igh6−/− mice (Figure 4). IL-10 correlated significantly with spirochetemia in all infected groups including Igh6−/− mice infected for 2 weeks (r = 0.92; P < 0.01) and 4 weeks (r = 0.68; P < 0.05) and Rag1−/− mice infected for 4 weeks (r = 0.82; P < 0.01). In Igh6−/− mice TNF-α was higher with Bt1 than with Bt2 with a significant negative correlation with spirochetemia (r = −0.8; P < 0.01) (Figure 4). These results revealed that IL-10 is the main cytokine produced in the blood during persistent infection with B. turicatae with significant positive correlations with spirochetemia and negative correlations with proinflammatory cytokines and chemokines.
Figure 3.
Concentrations of cytokines and chemokines in plasma of Bt2-infected Rag1−/− mice 4 weeks after inoculation as determined by enzyme-linked immunosorbent assay. IL-10 in Bt2-infected Rag1−/− mice was by far the most abundant of all of the cytokines and chemokines measured in both Igh6−/− and Rag1−/− mice during persistent infection with B. turicatae serotype 2.
Figure 4.
IL-10 compared with proinflammatory cytokines and chemokines in plasma of Bt1- and Bt2-infected Igh6−/− and Rag1−/− mice 2 and 4 weeks after inoculation. Average cytokine values in the plasma of PBS-inoculated control mice (n = 6 infected and 4 uninfected controls) did not differ between genotypes and are indicated by the dotted line. Group data sets are graphed as box plots (linear scale) where open boxes represent Bt1-infected and shaded boxes Bt2-infected groups. The box plots show the maximum and minimum values, median, upper, and lower quartiles. Note that the increase in IL-10 is paralleled by a decrease in all of the other proinflammatory cytokines and chemokines measured. *P < 0.05; **P < 0.01 (Mann-Whitney U-test).
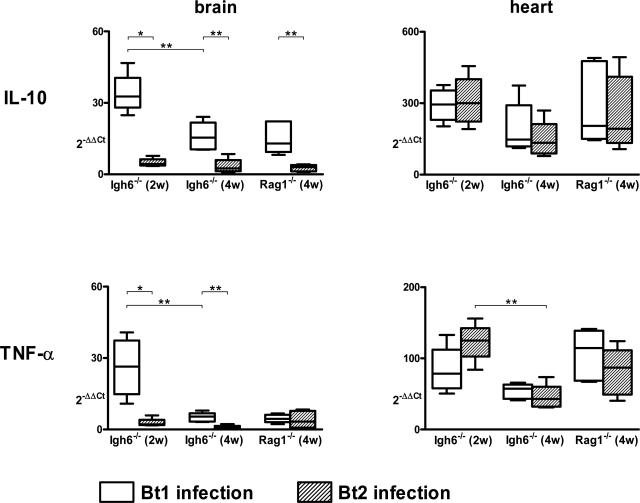
Up-Regulation of IL-10 in Tissues
The previous results revealed that IL-10 was the main cytokine produced in the blood during persistent infection with B. turicatae. To investigate whether infection also resulted in up-regulation of IL-10 in tissues, we measured IL-10 mRNA by real-time RT-PCR in the brain and the heart of Igh6−/− and Rag1−/− mice persistently infected with Bt1 or Bt2 and in uninfected controls and compared this with TNF-α. In the brain the results showed significantly more IL-10 mRNA in Bt1-infected compared with Bt2-infected mice of both genotypes 2 and 4 weeks after inoculation (Figure 5). Although TNF-α mRNA was also increased in the brain of Bt1-infected Igh6−/− mice at 2 and 4 weeks, it decreased significantly between weeks 2 and 4 (P < 0.05). Because Bt1 is more neurotropic than Bt2, we investigated whether there was a correlation between IL-10 and the pathogen load in the brain. For this, we measured the number of spirochetes in the brain by immunohistochemistry as before.8 Spirochetes were found within the subarachnoid space and along the leptomeninges (Figure 6A) but not in the brain parenchyma. No spirochetes were observed free in the cerebral vasculature, as expected after effective intracardiac perfusion with buffer at necropsy. The median (range) number of spirochetes per ×200 leptomeningeal microscopic field in Igh6−/− mice examined 4 weeks after inoculation was 0.85 (0.72/1.4) and 0.15 (0.05/0.34) for Bt1 and Bt2, respectively (P < 0.001). We found a significant positive correlation between the pathogen load and IL-10 mRNA in the brain of Bt1-infected Igh6−/− mice examined 2 (r = 0.76, P < 0.05) and 4 (r = 0.8, P < 0.05) weeks after inoculation. Expressing the leptomeningeal borrelia counts as a function of spirochetemia (borrelias per μl of necropsy plasma) revealed that Bt1 disseminated significantly better from blood to brain than Bt2: the median (range) ratios of leptomeningeal spirochetes to spirochetes per μl of plasma were 0.14 (0.12/0.32) and 0.01 (0/0.02) for Bt1 and Bt2, respectively (P < 0.001). Unlike in the brain, we found similar production of IL-10 mRNA in the heart of Bt1- or Bt2-infected mice (Figure 5), corresponding to their similar pathogen loads (not shown). The production of TNF-α in the heart was lower than that of IL-10, and as in the brain of Bt1-infected mice, it significantly decreased between weeks 2 and 4 of infection. These results revealed strong positive correlations between the pathogen load and production of IL-10 in tissues, with concomitant reductions of TNF-α.
Figure 5.
IL-10 and TNF-α expression in the brain and the heart during persistent infection with Bt1 or Bt2. RNA was extracted from half of the brain and heart of infected and control mice. mRNA was quantified by real-time RT-PCR in relation to calibrator RNA extracted from the brain of a noninfected mouse applying the 2−ΔΔCT method. For every infected mouse, the average 2−ΔΔCT value derived from PBS-inoculated control mice of the same genotype was subtracted. Note the serotype-specific IL-10 expression in the brain (compared with plasma, Figure 4). Group data sets are graphed as box plots (linear scale) where open boxes represent Bt1-infected and shaded boxes Bt2-infected groups. *P < 0.05; **P < 0.01 (Mann-Whitney U-test).
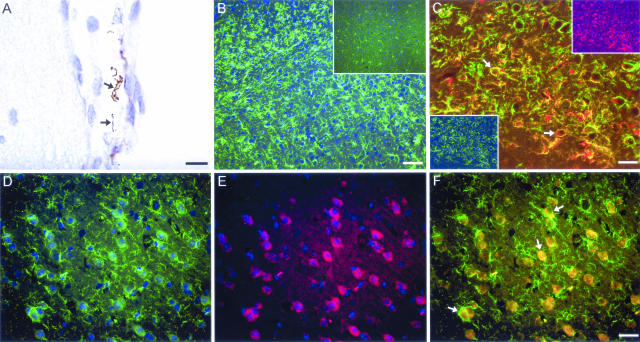
Figure 6.
The majority of IL-10-producing cells in the brain of B-cell-deficient mice persistently infected with B. turicatae are F4/80 and Iba1 immunoreactive. Light or fluorescent immunohistochemistry in 5-μm paraffin sections from B-cell-deficient mice 4 weeks after inoculation with B. turicatae serotype 1 (Bt1) or PBS. A: Staining of borrelia (DAB, brown) with anti-Vsp1. Arrows point to borrelia in the leptomeninges and subarachnoid space of a Bt1-infected Igh6−/− mouse. B: Fluorescent detection of microglia/brain macrophages with the leptin Iba1 as single staining in a Bt1-infected compared with a PBS-injected control mouse (inset). C: Double-labeling of microglia/brain macrophages in a Bt1-infected Igh6−/− mouse stained with Iba1 (red, top inset) and F4/80 (green, bottom inset). Arrows point to double-positive microglial cells that are activated and less ramified (overlay, yellow). D–F: Fluorescent double labeling for Iba1 (green in D) and IL-10 (red in E) in the brain of a Bt1-infected Igh6 −/− mouse: note that the majority of IL-10-producing cells are also positive for Iba1 (overlay in F, yellow) and display an amoeboid structure, characteristic of a fully activated microglia. Scale bars = 10 μm (A); 60 μm (B); 30 μm (C–F).
We also investigated whether the up-regulation of IL-10 was paralleled by up-regulation of its receptor. For this purpose, we measured IL-10Rα by real-time RT-PCR and found up-regulation in Bt1- but not in Bt2-infected brains: The median (range) of (2−ΔΔCT − average 2−ΔΔCT of uninfected controls) for Bt1-infected mice was 4.2 (2.8 to 6.6) at 2 weeks and 1.7 (1.3 to 2.5) at 4 weeks for Igh6−/− mice and 1 (0.9 to 1.9) for Rag1−/− mice at 4 weeks. In contrast, similar up-regulation of IL-10Rα was found in the heart with both serotypes (not shown). We concluded that the up-regulation of IL-10 in response to the pathogen load occurs not only in the blood but also in the brain and the heart, strongly correlates with the pathogen load and is mirrored by up-regulation of its receptor.
Activated Microglia Are the Main Source of IL-10 in Borrelia-Infected Brains
To study the up-regulation of IL-10 in infected brains at the protein level and to determine its cellular source, we performed immunohistochemistry with anti IL-10 antibodies. For this we focused on Igh6−/− mice infected with Bt1 for 4 weeks. The results showed more IL-10-producing cells in the brain of infected compared with uninfected controls (not shown). The majority of IL-10-positive cells had ameboid appearance of the soma and some ramifications, characteristic of fully activated microglia (Figure 6). They were diffusely distributed throughout the whole brain but more abundant in the deep white matter. By fluorescent double-label immunohistochemistry, IL-10-producing cells were positive for both F4/80 (activated microglia/macrophages) and Iba1 (resting and activated microglia/macrophages). Cells positive only for Iba1 rarely appeared with the morphological features of macrophages such as a large cell body. These ramified Iba1-positive cells stained for IL-10 to a much lesser extent, an indication that resting microglia produced little IL-10. Consistent with the typical behavior of microglia, F4/80-Iba1 double-positive cells were frequently surrounded by ramified, Iba1 single-positive microglia. Astrocytes (GFAP) and oligodendrocytes (CNPase) were only rarely positive for IL-10. We concluded that the up-regulation of IL-10 in the brain as a result of the infection was also seen at the protein level and that activated microglia were the main source of IL-10.
Effect of Exogenous IL-10 on Clinical Disease and Pathogen Load
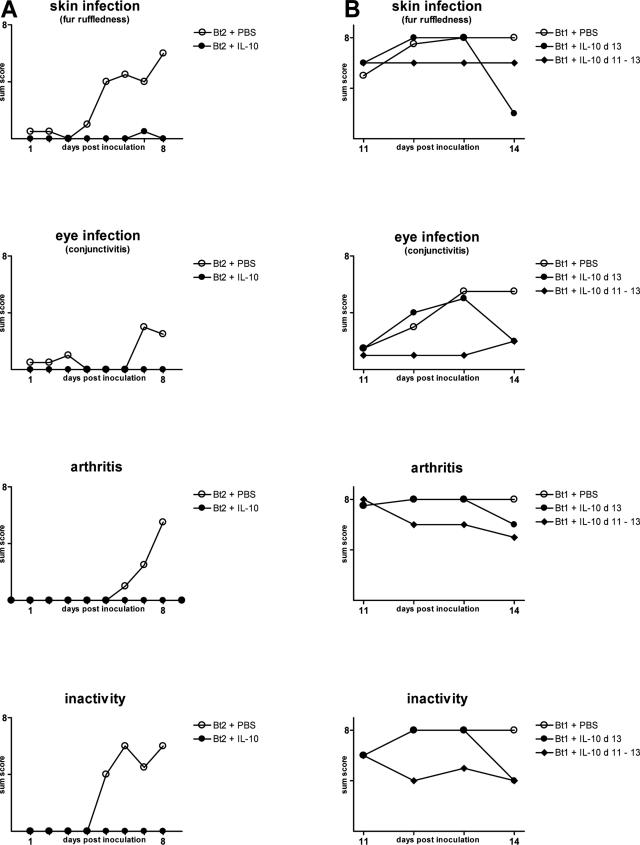
The previous experiments pointed to IL-10 as a potent suppressor of the inflammatory response in persistent borrelia infection. To confirm this, we compared clinical disease in Igh6−/− mice infected with Bt1 or Bt2 and treated by intraperitoneal injections of various doses of recombinant IL-10 (rIL-10) or PBS as a control. The doses of IL-10 were arbitrarily chosen based on what others have found to be effective in murine models of microbial inflammation.13 First, we studied whether daily injections of 50 μg/kg/day of rIL-10 starting before inoculation of Bt2 could prevent the onset of clinical manifestations. The results showed that, in contrast to Bt2-infected mice that were given PBS, treatment with 50 μg/kg/day rIL-10 prevented development of purulent eye discharge, joint swelling and redness, ruffled fur, and inactivity (Figure 7A). Next, we studied whether administration of higher doses of rIL-10 to mice already infected could ameliorate established clinical manifestations of persistent borrelia infection. For this, groups of four Igh6−/− mice infected with Bt1 were treated with either 1) three daily injections of 100 μg/kg/day of rIL-10 given on days 11 to 13 after inoculation, 2) a single injection of 150 μg/kg/day given on day 13 only, or 3) PBS as a control given on days 11 to 13. Clinical examination by an observer (D.C.) masked to the respective treatment and infection revealed that, compared with Bt1-infected mice treated with PBS, clinical disease was significantly reduced by treatment with rIL-10 daily for 3 days and even after a single dose (Figure 7B). Examination of spirochetemia at necropsy revealed that a single injection of the highest dose we used (150 μg/kg) resulted in a significant drop in spirochetemia (Table 1). We concluded that IL-10 both prevented and ameliorated clinical disease and at high doses even reduced the spirochetemia of persistent borrelia infection in the absence of B cells.
Figure 7.
IL-10 prevents and lessens disease severity in Igh6−/− mice infected with B. turicatae serotypes 1 or 2. A: IL-10 prevents development of clinical disease. Fifty μg/kg/day of rIL-10 in 300 μl of PBS or PBS alone as a control were injected intraperitoneally into 5-week-old Igh6−/− mice (n = 4/group) for 9 consecutive days starting 3 hours before inoculation of 104 Bt2 spirochetes intraperitoneally. Clinical scores were obtained daily by an examiner masked to treatment and infection status until necropsy. Results are given as sum score for clinical signs of involvement of skin, eyes, joints, and for reduced activity as described in Materials and Methods. B: IL-10 reverses established clinical disease in RF borreliosis. One hundred μg/kg/day of rIL-10 in 300 μl of PBS was injected intraperitoneally on days 11 to 13 or 150 μg/kg as a single dose on day 13 into 5-week-old Igh6−/− mice (n = 4) persistently infected with Bt1. The control group (n = 4) received 300 μl of PBS only. For each graph, the highest value on the y axis equals the maximum possible sum score per group.
Table 1.
Spirochetemia at Necropsy in Groups of Four Igh6−/− Mice Each Uninfected (PBS) or Infected with B. turicatae Serotypes 1 (Bt1) or 2 (Bt2) and Treated with Either rIL-10 in Different Schedules or PBS as a Control
| Group | Infection | Treatment | Spirochetes/ml plasma, median (range) |
|---|---|---|---|
| I | PBS | PBS | 0 |
| II | Bt2 | PBS | 8 × 107 (6.5 × 107 to 1.7 × 108) |
| III | Bt2 | rIL-10 at 50 μg/kg on days 0 to 8 | 1.5 × 108 (7.5 × 107 to 1.5 × 108) |
| IV | Bt1 | PBS | 2 × 107 (1.5 × 107 to 2.5 × 107)* |
| V | Bt1 | rIL-10 at 150 μg/kg on day 13 | 2 × 106 (1 × 106 to 5.5 × 106)* |
| VI | Bt1 | rIL-10 at 100 μg/kg on days 11 to 13 | 1 × 107 (1 × 107 to 2 × 107) |
P < 0.05 for the difference between groups IV and V and V and VI (Mann-Whitney U-test).
Discussion
The pathogenesis of RF borreliosis remains incompletely understood. The fever pattern and recurrent peaks of high spirochetemia are the consequence of antigenic variation of the spirochetes, specifically of the outer membrane proteins that confer serotype identity referred to as VMPs.16 During the febrile periods with high spirochetemia patients are more symptomatic. Studies of louse-borne RF have found elevated plasma levels of several cytokines and chemokines, including TNF-α, IL-6, IL-10, and IL-8.9,17,18 After treatment with penicillin, there are further elevations of TNF-α, which coincide with the onset of the Jarisch-Herxheimer reaction.17 Treatment with anti-TNF-α Fab antibody fragments reduces the frequency of the Jarisch-Herxheimer reaction from 90 to 50%.10 Anti-TNF-α-treated patients had significantly lower increases in temperature, pulse rate, and systolic blood pressure and lower plasma concentrations of IL-6 and IL-8 after treatment with penicillin.10 However, treatment with rIL-10 had no effect on the Jarisch-Herxheimer reaction.9
Outer membrane lipoproteins such as Vsp1 and Vsp2 in Bt1 and Bt2, respectively, are mainly responsible for the inflammatory response to the infection.19 However, little is known about the identity of the host’s regulatory mechanisms that may counteract this potent inflammatory response. A study by Cooper and colleagues9 found extremely high levels of IL-10 in the blood of patients with louse-borne RF. The patients examined by Cooper and colleagues9 seemed clinically stable despite the acute infection suggesting IL-10 is protective in RF borreliosis. Our study in mice persistently infected with B. turicatae provides novel experimental evidence that this may indeed be the case. The main findings from our study are as follows: 1) Bt2 caused higher spirochetemia than Bt1 in both Igh6−/− and Rag1−/− mice; 2) the clinical manifestations of persistent infection were more severe in Igh6−/− than in Rag1−/− mice despite lower pathogen loads; 3) Igh6−/− mice controlled Bt2 spirochetemia significantly better than Rag1−/− mice; 4) the higher spirochetemia of Bt2-infected Rag1−/− mice was associated with very high plasma levels of IL-10 and down-regulation of proinflammatory cytokines and chemokines; 5) Bt1, which was more neurotropic than Bt2, caused higher production of IL-10 in the brain that was produced mainly by activated microglia; and 6) exogenous recombinant IL-10 reduced the clinical manifestations of the infection and, at high doses, lowered the pathogen load.
In murine RF borreliosis the absence of B cells results in persistent infection with a high pathogen load that causes severe multisystemic complications, as has been documented in SCID mice infected with B. turicatae.3 IgM produced by B1b cells independently of T cells are responsible for resolution of infection with RF borrelias,5,6 which can occur independently of complement.20 Unlike B cells, little is known about the role of T cells in RF borreliosis. To investigate this we began the current study by comparing disease during persistent infection of mice deficient only in B cells (Igh6−/− mice) with mice deficient in both B and T cells (Rag1−/−). The results revealed that Igh6−/− mice developed significantly more severe clinical disease than Rag1−/− mice. In this sense, Igh6−/− mice were more similar to SCID mice.3 These results suggest that the presence of T cells in Igh6−/− mice either directly or indirectly worsened the inflammatory response to persistent borrelia infection. One mechanism by which T cells may have exacerbated disease is by dampening the surge in IL-10 production by the innate immune system in response to rising pathogen loads, as seen in the Rag1−/− mice. In the B. burgdorferi murine model of Lyme disease, McKisic and colleagues21 found evidence that the presence of γδ T cells worsens carditis but not arthritis. Cell transfer experiments by these investigators showed that CD4+ T cells were responsible for the aggravated carditis.21 However, one can also speculate that the greater inflammation in Igh6−/− mice was attributable to the loss of some regulatory effect of B cells on T cells. Unlike the Lyme disease model, we observed worsening of the disease across all of the clinical measures, including arthritis, conjunctivitis, ruffled fur, vestibular dysfunction, and weight loss (Figure 2). T- and B-cell transfer experiments with further characterization of subpopulations of cells will be needed to clarify this further.
Another important observation was that Igh6−/− mice controlled Bt2 spirochetemia significantly better than Rag1−/− mice (Figure 1). Interestingly, this was not observed for Bt1. Much higher Bt2 than Bt1 spirochetemia suggests that the presence of T cells in Igh6−/− mice may have helped to control further rises in pathogen load beyond what occurs during Bt1 infection. Previous studies have failed to find evidence that T cells are needed to control the pathogen load in RF borreliosis.22,23 However, it is possible that this function becomes apparent only at very high pathogen load as seen with Bt2 infection.
Because IL-10 strongly correlated with the pathogen load in both blood and tissues, it is likely that IL-10 is produced as a direct response to the presence of the pathogen. This IL-10, in turn, was probably responsible for the observed suppression of proinflammatory cytokines and chemokines and the amelioration of clinical disease in Rag1−/− mice. The mice with the highest spirochetemia, Bt2-infected Rag1−/− mice, had ∼10 times more plasma IL-10 than any of the other cytokines/chemokines, reaching levels similar to wild-type C57BL/6J mice injected with a lethal dose of lipopolysaccharide.24 Bt2-infected Rag1−/− mice became moribund after several weeks of persistent infection, an indication that the ability of IL-10 to protect the host from the infection is temporary. This is similar to infection with Yersinia pestis, in which IL-10 induced via toll-like receptor 2 is protective but the mice eventually reach lethal pathogen numbers.25 In addition to systemic production of IL-10, we also found strong IL-10 production in infected brains, mainly by activated microglia. As in the blood, IL-10 up-regulation in the brain also significantly correlated with the pathogen load. Production of IL-10 may explain the absence of detectable injury in the brain of these mice (D.C. and H.G., submitted for publication).26
The finding that treatment of Igh6−/− mice with high doses of exogenous IL-10 (150 μg/kg) decreased spirochetemia was unexpected (Table 1). Because this was not observed at lower doses (50 to 100 μg/kg), this seems to be dose-dependent. In most animal models of bacterial, fungal, and parasitic infections, administration of exogenous IL-10 results in increased, not decreased, pathogen load.27 For example, in the mouse model of Lyme borreliosis, the absence of IL-10 reduced the pathogen load.28 In our model it is possible that IL-10 improved the ability of the innate immune system to remove spirochetes from the circulation independently of B cells, probably by improving phagocytosis.29 In support of this is the finding that IL-10 is needed for efficient elimination of some bacteria.30
IL-10 plays a central role in infectious diseases, particularly in balancing between destruction and protection, which is the most essential of its many functions.27 There have been few studies of the role of IL-10 in spirochetal infections. A crucial observation was the finding by Cooper and colleagues9 of extraordinarily high levels of IL-10, in plasma from patients with louse-borne RF 5 to 60 times greater than those described in septic shock. Because these patients were clinically stable despite high spirochetemia, this suggests a protective role of IL-10. However, an injection of 25 μg/kg of recombinant human IL-10 5 minutes before treatment with intramuscular penicillin did not prevent the Jarisch-Herxheimer reaction nor did it affect plasma cytokine levels or pathogen clearance.9 In this respect, IL-10 was similar to steroids, which are not effective against the Jarisch-Herxheimer reaction.17 We therefore hypothesize that IL-10 has a protective role during conditions of high but not low pathogen loads. This protective role is likely important not only systemically but also in tissues, as shown by the recent finding that IL-10 was one of only a handful of cytokines/chemokines significantly increased in cerebrospinal fluid of patients with Lyme neuroborreliosis.31
In vitro, Philipp and colleagues32 has shown that blood monocytes produce large amounts of IL-10 in response to B. burgdorferi lipoproteins. In mice, cultured monocytes from C3H/HeJ mice, which are more susceptible to Lyme arthritis, produced lower amounts of IL-10 than monocytes from the more resistant strain C57BL/6J.33 Furthermore, IL-10 deficiency in C57BL/6J mice resulted in more severe arthritis.28 It is possible that reduced IL-10 production by C3H/HeJ mice explains their increased susceptibility to arthritis during B. burgdorferi infection. In syphilis there is also evidence that IL-10 is induced by the spirochete Treponema palidum in both humans34 and experimental animals.35
Although one may be tempted to assign the differences in disease severity between Bt1 and Bt2 infected mice to differences in the proinflammatory ability of Vsp1 and Vsp2, several lines of evidence indicate this is not the case: 1) Vsp1 and Vsp2, like all members of the Vsp/OspC family of borrelia lipoproteins, have the same common lipid modification7,11,36; 2) our measures of inflammation in the heart and the brain of mice infected with Bt1 and Bt2 show strong correlations with the pathogen load rather than with the Vsp that is expressed (H.G. and D.C., submitted for publication)37; and 3) we found no evidence of macrophage activation by nonlipidated rVsp1 or Vsp2 (D.C., unpublished results).
The finding of significant suppression of clinical disease and proinflammatory cytokines and chemokines by endogenous IL-10 in Rag1−/− mice and by exogenous rIL-10 in Igh6−/− mice is consistent with the concept that IL-10 is an important down-regulator of the inflammatory response in RF borreliosis. In this context, IL-10-mediated suppression of the host’s proinflammatory response could be advantageous to RF spirochetes as it would improve the host’s tolerance to high pathogen load with reduced morbidity and delayed mortality. In nature, this could increase the likelihood of transmission to the tick or lice vectors.
Footnotes
Address reprint requests to Dr. Diego Cadavid, UMDNJ-New Jersey Medical School, 185 South Orange Ave., MSB H506, Newark, NJ 07103. E-mail: cadavidi@umdnj.edu.
Supported by the Foundation of University of Medicine and Dentistry New Jersey (to D.C.); the Heritage Affiliate of the American Heart Association (Scientist Development grant 0235464T to D.C.); the L’Hommedieu MS Fund at University of Medicine and Dentistry New Jersey, National Institute of Neurological Disorders and Stroke, and National Institute of Allergy and Infectious Diseases (to H.G.); the Deutsche Forschungsgemeinschaft (Schm 1669/1-1 to J.S.); the National Institute of Neurological Disorders and Stroke (to J.S.); and the National Cancer Institute, National Institutes of Health (contract no. NO1-CO-12400).
H.G. and J.S. contributed equally to this article.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade name, commercial products, or organization imply endorsements by the U.S. Government.
References
- Southern P, Sanford J. Relapsing fever. Medicine. 1969;48:129–149. [Google Scholar]
- Barbour AG, Hayes SF. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadavid D, Thomas DD, Crawley R, Barbour AG. Variability of a bacterial surface protein and disease expression in a possible mouse model of systemic Lyme borreliosis. J Exp Med. 1994;179:631–642. doi: 10.1084/jem.179.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Tessier SL, Stoenner HG. Variable major proteins of Borrellia hermsii. J Exp Med. 1982;156:1312–1324. doi: 10.1084/jem.156.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SE, Benach JL. Cutting edge: the spirochetemia of murine relapsing fever is cleared by complement-independent bactericidal antibodies. J Immunol. 2001;167:3029–3032. doi: 10.4049/jimmunol.167.6.3029. [DOI] [PubMed] [Google Scholar]
- Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT, Leong JM. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol. 2003;170:3819–3827. doi: 10.4049/jimmunol.170.7.3819. [DOI] [PubMed] [Google Scholar]
- Cadavid D, Pennington PM, Kerentseva TA, Bergstrom S, Barbour AG. Immunologic and genetic analyses of VmpA of a neurotropic strain of Borrelia turicatae. Infect Immun. 1997;65:3352–3360. doi: 10.1128/iai.65.8.3352-3360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadavid D, Pachner AR, Estanislao L, Patalapati R, Barbour AG. Isogenic serotypes of Borrelia turicatae show different localization in the brain and skin of mice. Infect Immun. 2001;69:3389–3397. doi: 10.1128/IAI.69.5.3389-3397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PJ, Fekade D, Remick DG, Grint P, Wherry J, Griffin GE. Recombinant human interleukin-10 fails to alter proinflammatory cytokine production or physiologic changes associated with the Jarisch-Herxheimer reaction. J Infect Dis. 2000;181:203–209. doi: 10.1086/315183. [DOI] [PubMed] [Google Scholar]
- Fekade D, Knox K, Hussein K, Melka A, Lalloo DG, Coxon RE, Warrell DA. Prevention of Jarisch-Herxheimer reactions by treatment with antibodies against tumor necrosis factor alpha. N Engl J Med. 1996;335:311–315. doi: 10.1056/NEJM199608013350503. [DOI] [PubMed] [Google Scholar]
- Pennington PM, Cadavid D, Barbour AG. Characterization of VspB of Borrelia turicatae, a major outer membrane protein expressed in blood and tissues of mice. Infect Immun. 1999;67:4637–4645. doi: 10.1128/iai.67.9.4637-4645.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadavid D, Bundoc V, Barbour AG. Experimental infection of the mouse brain by a relapsing fever Borrelia species: a molecular analysis. J Infect Dis. 1993;168:143–151. doi: 10.1093/infdis/168.1.143. [DOI] [PubMed] [Google Scholar]
- Nishio R, Matsumori A, Shioi T, Ishida H, Sasayama S. Treatment of experimental viral myocarditis with interleukin-10. Circulation. 1999;100:1102–1108. doi: 10.1161/01.cir.100.10.1102. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Metselaar JM, Gold R. Intravenous liposomal prednisolone downregulates in situ TNF-alpha production by T-cells in experimental autoimmune encephalomyelitis. J Histochem Cytochem. 2003;51:1241–1244. doi: 10.1177/002215540305100915. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Barbour AG, Burman N, Carter CJ, Kitten T, Bergstrom S. Variable antigen genes of the relapsing fever agent Borrelia hermsii are activated by promoter addition. Mol Microbiol. 1991;5:489–493. doi: 10.1111/j.1365-2958.1991.tb02132.x. [DOI] [PubMed] [Google Scholar]
- Negussie Y, Remick DG, DeForge LE, Kunkel SL, Eynon A, Griffin GE. Detection of plasma tumor necrosis factor, interleukins 6, and 8 during the Jarisch-Herxheimer reaction of relapsing fever. J Exp Med. 1992;175:1207–1212. doi: 10.1084/jem.175.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas LE, Borgnolo G, Hailu B, Smith G, Almaviva M, Hart CA. Tumour necrosis factor, interleukin-6 and C-reactive protein in patients with louse-borne relapsing fever in Ethiopia. Ann Trop Med Parasitol. 1995;89:49–54. doi: 10.1080/00034983.1995.11812928. [DOI] [PubMed] [Google Scholar]
- Vidal V, Scragg IG, Cutler SJ, Rockett KA, Fekade D, Warrell DA, Wright DJ, Kwiatkowski D. Variable major lipoprotein is a principal TNF-inducing factor of louse-borne relapsing fever. Nat Med. 1998;4:1416–1420. doi: 10.1038/4007. [DOI] [PubMed] [Google Scholar]
- Newman K, Jr, Johnson RC. In vivo evidence that an intact lytic complement pathway is not essential for successful removal of circulating Borrelia turicatae from mouse blood. Infect Immun. 1981;31:465–469. doi: 10.1128/iai.31.1.465-469.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKisic MD, Redmond WL, Barthold SW. Cutting edge: T cell-mediated pathology in murine Lyme borreliosis. J Immunol. 2000;164:6096–6099. doi: 10.4049/jimmunol.164.12.6096. [DOI] [PubMed] [Google Scholar]
- Newman K, Jr, Johnson RC. T-cell-independent elimination of Borrelia turicatae. Infect Immun. 1984;45:572–576. doi: 10.1128/iai.45.3.572-576.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SE, Thanassi DG, Benach JL. Generation of a complement-independent bactericidal IgM against a relapsing fever Borrelia. J Immunol. 2004;172:1191–1197. doi: 10.4049/jimmunol.172.2.1191. [DOI] [PubMed] [Google Scholar]
- Purswani MU, Eckert SJ, Arora HK, Noel GJ. Effect of ciprofloxacin on lethal and sublethal challenge with endotoxin and on early cytokine responses in a murine in vivo model. J Antimicrob Chemother. 2002;50:51–58. doi: 10.1093/jac/dkf091. [DOI] [PubMed] [Google Scholar]
- Brubaker RR. Interleukin-10 and inhibition of innate immunity to Yersiniae: roles of Yops and LcrV (V antigen). Infect Immun. 2003;71:3673–3681. doi: 10.1128/IAI.71.7.3673-3681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasley A, Tranguch SL, Rati DM, Marriott I. Murine glia express the immunosuppressive cytokine, interleukin-10, following exposure to Borrelia burgdorferi or Neisseria meningitidis. Glia. 2006;53:583–592. doi: 10.1002/glia.20314. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Brown JP, Zachary JF, Teuscher C, Weis JJ, Wooten RM. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect Immun. 1999;67:5142–5150. doi: 10.1128/iai.67.10.5142-5150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlow CJ, Garcia-Monco JC, Coleman JL, Benach JL. Murine microglia are effective phagocytes for Borrelia burgdorferi. J Neuroimmunol. 2005;168:183–187. doi: 10.1016/j.jneuroim.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Gjertsson I, Hultgren OH, Tarkowski A. Interleukin-10 ameliorates the outcome of Staphylococcus aureus arthritis by promoting bacterial clearance. Clin Exp Immunol. 2002;130:409–414. doi: 10.1046/j.1365-2249.2002.01999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht T, Pfister HW, Angele B, Kastenbauer S, Wilske B, Koedel U. The chemokine CXCL13 (BLC): a putative diagnostic marker for neuroborreliosis. Neurology. 2005;65:448–450. doi: 10.1212/01.wnl.0000171349.06645.79. [DOI] [PubMed] [Google Scholar]
- Giambartolomei GH, Dennis VA, Lasater BL, Philipp MT. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect Immun. 1999;67:140–147. doi: 10.1128/iai.67.1.140-147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giambartolomei GH, Dennis VA, Lasater BL, Murthy PK, Philipp MT. Autocrine and exocrine regulation of interleukin-10 production in THP-1 cells stimulated with Borrelia burgdorferi lipoproteins. Infect Immun. 2002;70:1881–1888. doi: 10.1128/IAI.70.4.1881-1888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis WC, Barrett LK, Koelle DM, Nasio JM, Plummer FA, Lukehart SA. Primary and secondary syphilis lesions contain mRNA for Th1 cytokines. J Infect Dis. 1996;173:491–495. doi: 10.1093/infdis/173.2.491. [DOI] [PubMed] [Google Scholar]
- Wicher V, Scarozza AM, Ramsingh AI, Wicher K. Cytokine gene expression in skin of susceptible guinea-pig infected with Treponema pallidum. Immunology. 1998;95:242–247. doi: 10.1046/j.1365-2567.1998.00596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis JJ, Ma Y, Erdile LF. Biological activities of native and recombinant Borrelia burgdorferi outer surface protein A: dependence on lipid modification. Infect Immun. 1994;62:4632–4636. doi: 10.1128/iai.62.10.4632-4636.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londoño D, Bai Y, Zuckert WR, Gelderblom H, Cadavid D. Cardiac apoptosis in severe relapsing fever borreliosis. Infect Immun. 2005;73:7669–7676. doi: 10.1128/IAI.73.11.7669-7676.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]