Abstract
Vascular aging is associated with dysregulation of tumor necrosis factor (TNF)-α expression. TNF-α is a master regulator of vascular proatherogenic phenotypic changes, and it has been linked to endothelial dysfunction and apoptosis. To test the hypothesis that anti-TNF-α treatment exerts vasculoprotective effects in aging, aged (29 months old) F344 rats were treated with etanercept (1 mg/kg/week for 4 weeks), which binds and inactivates TNF-α. In aged carotid arteries, relaxations to acetylcholine were decreased, and endothelial O2⨪ production was increased (as shown by dihydroethidine fluorescence measurements). Etanercept treatment significantly improved responses to acetylcholine and decreased vascular NAD(P)H oxidase activity and expression. In aged carotid and coronary arteries, there were increases in DNA fragmentation rate and caspase 3/7 activity (indicating an increased rate of apoptotic cell death), which were attenuated by etanercept treatment. In aged vessels, there was an up-regulation of inflammatory markers, including inducible nitric-oxide synthase and intercellular adhesion molecule-1, which was decreased by etanercept treatment. In carotid arteries of young animals, recombinant TNF-α elicited endothelial dysfunction, oxidative stress, and increased apoptosis and proinflammatory gene expression, mimicking many of the symptoms of vascular aging. Thus, we propose that anti-TNF-α treatment exerts anti-aging vasculoprotective effects.
Epidemiological studies have shown that even healthy aging is an independent risk factor for cardiovascular disease, suggesting that a large percentage of the US population will die as a consequence of aging-related vascular diseases (ischemic heart disease, stroke, aortic aneurysm; reviewed recently by Lakatta and Levy1). Previous studies by this and other laboratories have shown that the aging vascular system undergoes characteristic changes that include endothelial dysfunction,2–5 oxidative stress,2,4,6,7 and enhanced endothelial apoptosis.3,8 However, the underlying mechanisms responsible for these proatherogenic changes in vascular phenotype in aging remain unclear. In addition, there are no treatments available to reverse or delay aging-induced decline of vascular function.
Recent studies revealed that plasma levels of the proinflammatory cytokine tumor necrosis factor (TNF)-α significantly increase in aging.9–12 We have demonstrated that in aged coronary arteries there is an up-regulation of TNF-α3,13 associated with a gene ex-pression profile, suggestive of an inflammatory re-sponse.2–4,12–14 An increased TNF-α production has also been demonstrated in the carotid arteries, aortic wall,15 and heart16 of aged rodents. Importantly, recent clinical and experimental studies have linked TNF-α to endothelial impairment, atherosclerosis, and heart failure.17,18 Previous studies by us and others have demonstrated that administration of exogenous TNF-α can induce oxidative stress by up-regulating/activating NAD(P)H oxidase,19 endothelial dysfunction,20 endothelial apoptosis,3 and up-regulation of proatherogenic inflammatory mediators, such as inducible nitric-oxide synthase (iNOS) and adhesion molecules. All these effects closely mimic aging-induced functional alterations of the vascular endothelium.2,3,6,13,21 However, the link between TNF-α and age-related functional and phenotypic vascular alterations has not been explored.
Recent studies demonstrated that anti-TNF-α therapies (eg, etanercept or infliximab), may improve inflammation-related endothelial dysfunction in various pathophysiological conditions,22,23 including heart failure.24 In addition, perfusion of rat hearts25 and coronary arteries19 with a TNF-α neutralizing antibody was shown to exert cardiovascular protective effects against oxidative stress. Although advanced age is associated with an increased presence of TNF-α, there are no studies extant investigating the potential beneficial vascular effects of TNF-α inhibition in aging.
On the basis of the aforementioned data, we hypothesized that up-regulated TNF-α contributes to aging-induced endothelial alterations. To test the hypothesis that anti-TNF-α treatment exerts anti-aging vasculoprotective effects, aged F344 rats were treated with etanercept, which binds and inactivates TNF-α.18,26
Materials and Methods
Animal Models
All animal use protocols were approved by the Institutional Animal Care and Use Committee of the New York Medical College, Valhalla, NY. Male Fisher 344 rats [ages: from 3 months old (young) to 29 months old (aged), n = 70, purchased from the National Institute of Aging, Bethesda, MD; kept under pathogen-free conditions] were used as described.3,13 In three 29-month old rats, subcutaneous adenoid tumor, hypophyseal tumor, and splenomegaly of unknown origin, respectively, were diagnosed on autopsy, and thus the vessels isolated from these animals were discarded. All other aged rats were disease-free with no signs of systemic inflammation and/or neoplastic alterations. Inflammatory markers in the plasma were measured by an immunoassay method (Charles River Laboratories, Austin, TX). To evaluate the role of chronic in vivo TNF-α inhibition, aged rats (n = 20) were treated with either etanercept (a TNF-α inhibitor; Immunex Corp., Thousand Oaks, CA), subcutaneously administered at 1 mg/kg/week, or vehicle for 4 weeks before experimentation. In other experiments, etanercept treatment of rats was discontinued for 2 weeks. Etanercept (Enbrel) is a Food and Drug Administration-approved drug that is composed of the extracellular ligand-binding portion of the human 75-kd (p75) TNF receptor 2, which binds and inactivates circulating TNF-α with well-characterized pharmacodynamics. The etanercept dose for chronic studies was chosen based on effective TNF-α inhibition from previous studies in humans (RENESSAINCE and RECOVER trials27) and rats.28 We have not attempted to determine plasma TNF-α levels in etanercept-treated animals in the present study because of the known dissociation between TNF-α immunoreactivity and TNF-α bioactivity after etanercept treatment.29 In other experiments, aged TNF-α knockout (n = 5, 14 to 16 months old; Jackson Laboratories, Bar Harbor, ME) and age-matched wild-type control mice were used. All animals were sacrificed by an overdose of sodium pentobarbital as described.3,13
Functional Studies
Endothelial function was assessed as previously described.30 In brief, carotid arteries of etanercept-treated and control rats were cut into ring segments 2 mm in length and mounted on 40-μm stainless steel wires in the myograph chambers (Danish Myo Technology A/S, Inc., Aarhus, Denmark) for measurement of isometric tension. The vessels were superfused with Krebs’ buffer solution (118 mmol/L NaCl, 4.7 mmol/L KCl, 1.5 mmol/L CaCl2, 25 mmol/L NaHCO3, 1.1 mmol/L MgSO4, 1.2 mmol/L KH2PO4, and 5.6 mmol/L glucose, at 37°C and gassed with 95% air and 5% CO2). Relaxations of precontracted (10−6 mol/L phenylephrine) vessels to acetylcholine (ACh; from 10−9 to 10−4 mol/L) and the NO donor S-nitrosopenicillamine (from 10−9 to 3 × 10−5 mol/L) were obtained. The effects of the NAD(P)H oxidase inhibitor apocynin (3 × 10−4 mol/L) and the O2⨪ scavenger Tiron (10 mmol/L) on ACh-induced responses of aged arteries were also tested.
Measurement of Vascular Superoxide Levels
Production of O2⨪ in segments of the same carotid arteries that were used for functional studies was determined in the absence and presence (preincubation, 1 hour) of diphenyleneiodonium [10−5 mol/L, an inhibitor of flavoprotein-containing oxidases, including NAD(P)H oxidases] or apocynin using the lucigenin (10 μmol/L) chemiluminescence method, as described.2,19,31,32 NAD(P)H oxidase activity was measured in vessel homogenates after the addition of 10−4 mol/L NAD(P)H as reported.2
Hydroethidine, an oxidative fluorescent dye, was used to localize superoxide production in situ as we previously reported.2,31,32 This method provides sensitive detection of O2⨪ levels in situ.2,31–33 In brief, cells are permeable to hydroethidine, which in the presence of O2⨪ is oxidized to fluorescent ethidium bromide (EB), which is trapped by intercalation with DNA. Isolated, living vessels were incubated with hydroethidine (10−6 mol/L, at 37°C for 60 minutes). Then the arteries were washed three times, embedded in OCT medium, and cryosectioned. Vascular sections were imaged using a Zeiss AxioCam Mrm camera mounted on a Zeiss Axiovert 200 fluorescence microscope (Carl Zeiss, Gottingen, Germany). In other experiments, optical sections of en face vascular preparations were obtained using the Zeiss Apotome technology. Images were captured at ×20 magnification and analyzed using the Zeiss Axionvision imaging software. Ten to 15 entire fields per group were analyzed with one image per field. The mean fluorescence intensities of EB-stained nuclei in the endothelium and medial layer were measured in each view field.
Detection of Apoptotic Cell Death by Enzyme-Linked Immunosorbent Assay
Vessels were lysed and cytoplasmic histone-associated DNA fragments, which indicate apoptotic cell death, were quantified by the Cell Death Detection ELISAPlus kit (Roche Diagnostics Corp., Indianapolis, IN) as described.3 Results are reported as arbitrary optical density units normalized to protein concentration.
Caspase Activity Assay
Arteries were homogenized in lysis buffer, and caspase activities were measured using the Caspase-Glo 3/7 assay kit according to the manufacturer’s instruction (Promega, Madison, WI). In 96-well plates, the 50-μl sample was mixed gently for 30 seconds with 50 μl of Caspase-Glo 3/7 reagent and incubated for 2 hours at room temperature. The lysis buffer with the reagent served as blank. Luminescence of the samples was measured using an Infinite M200 plate reader (Tecan, Research Triangle Park, NC). Luminescent intensity values were normalized to the sample protein concentration.
Quantitative Real-Time Polymerase Chain Reaction (PCR)
Total RNA from the arterial samples was isolated with Mini RNA isolation kit (Zymo Research, Orange, CA) and was reverse-transcribed using Superscript II RT (Invitrogen, Carlsbad, CA) as described previously.2,13 Real-time RT-PCR technique was used to analyze mRNA expression using the Strategen MX3000, as reported.2,3,13,19 The housekeeping gene β-actin was used for internal normalization. Oligonucleotides used for real-time QRT-PCR are listed in Table 1. Fidelity of the PCR reaction was determined by melting temperature analysis and visualization of product on a 2% agarose gel.
Table 1.
Oligonucleotides for Real-Time RT-PCR
| mRNA targets | Sense | Anti-sense |
|---|---|---|
| TNF-α | 5′-TCGTAGCAAACCACCAAG-3′ | 5′-CTGACGGTGTGGGTGA-3′ |
| iNOS | 5′-TCCCGAAACGCTACACT-3′ | 5′-CAATCCACAACTCGCT-3′ |
| ICAM-1 | 5′-CACAGCCTGGAGTCTC-3′ | 5′-CCCTTCTAAGTGGTTGGAA-3′ |
| gp91phox | 5′-GGATGAATCTCAGGCCAA-3′ | 5′-TTAGCCAAGGCTTCGG-3′ |
| p22phox | 5′-TGCCAGTGTGATCTACC-3′ | 5′-AGCTATTAACCATGTTTATTACAGT-3′ |
| p47 | 5′-AGCACCAAGAGGAAACT-3′ | 5′-CCTAGCAATACCCGTGGA-3′ |
| nox1 | 5′-TGAATCTTGCTGGTTGACACTTGC-3′ | 5′-GAGGGACAGGTGGGAGGGAAG-3′ |
| nox4 | 5′-TGCCTCCATCAAGCCAAG-3′ | 5′-TTCCAGTCATCCAGTAGAGTG-3′ |
| β-Actin | 5′-GAAGTGTGACGTTGACAT-3′ | 5′-ACATCTGCTGGAAGGTG-3′ |
Vessel Culture Studies
Arteries of young rats were isolated and maintained in organoid culture (for 24 hours) under sterile conditions in F12 medium (Life Technologies, Inc., Grand Island, NY) containing antibiotics (100 UI/l penicillin, 100 mg/l streptomycin, and 10 μg/l Fungizone) and supplemented with 5% fetal calf serum (Boehringer-Mannheim, Indianapolis, IN), as previously described.3,19,31,32 Arteries were treated with recombinant TNF-α (from 0.1 to 100 ng/ml). After the culture period vessels were subjected to subsequent functional and molecular studies.
Cell Culture Studies
Primary rat coronary arterial endothelial cells (Celprogen, San Pedro, CA) and aortic smooth muscle cells (Cell Applications Inc., San Diego, CA) were treated with recombinant TNF-α (from 0.1 to 100 ng/ml) as described.34,35 Effect of TNF-α on nuclear factor (NF)-κB activity in coronary arterial endothelial cells was tested by a reporter gene assay as described.35 We used a NF-κB reporter comprised of a NF-κB response element upstream of firefly luciferase (NF-κB-Luc; Stratagene, La Jolla, CA) and a Renilla luciferase plasmid under the control of the CMV promoter (as an internal control). All transfections were performed with Novafector (Venn Nova LLC, Pompano Beach, FL) following the manufacturer’s protocols. Firefly and Renilla luciferase activities were assessed after 42 hours using a dual luciferase reporter assay kit (Promega) and a luminometer.
Data Analysis
Data were normalized to the respective control mean values and expressed as means ± SEM. Statistical analyses of data were performed by Student’s t-test or by two-way analysis of variance followed by the Tukey post hoc test, as appropriate. P < 0.05 was considered statistically significant.
Results
Effect of Anti-TNF-α Treatment on Endothelial Vasodilator Function and Reactive Oxygen Species Production
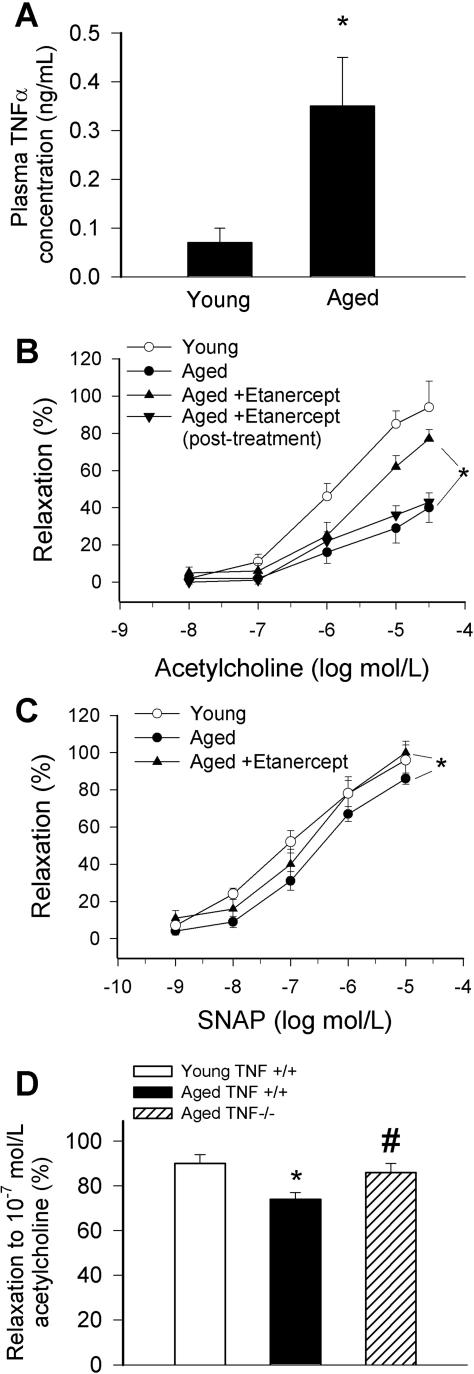
Aged F344 rats exhibited significantly higher plasma TNF-α levels than young ones (Figure 1A). Advancing age resulted in a significant decline in acetylcholine-induced relaxation of rat carotid arteries, which was significantly improved by chronic etanercept treatment (Figure 1B). The beneficial effect of etanercept on acetylcholine-induced relaxation was not evident 2 weeks after the last etanercept injection (Figure 1B). Neutralization of TNF-α had no effect on acetylcholine-induced relaxation of vessels from young rats (at 10−6 mol/L: 87 ± 8%, n.s.). Aging also tended to decrease S-nitrosopenicillamine-induced relaxation in rat carotid arteries, which was also normalized by etanercept treatment (Figure 1C). Acetylcholine-induced relaxation of carotid arteries from aged mice with genetic lack of TNF-α was greater compared with responses of vessels from aged wild-type mice (Figure 1D). S-Nitrosopenicillamine-induced responses were similar in carotid arteries of young and aged wild-type mice and in vessels of aged TNF-α−/− mice (not shown).
Figure 1.
A: Plasma levels of TNF-α in 3-month-old and 29-month-old F344 rats. *P < 0.05 (n = 3 to 4). B and C: Relaxations to acetylcholine (B) and the NO donor S-nitrosopenicillamine (C) in carotid arteries of aged (29 months old) F344 rats treated with etanercept (1 mg/kg/week, for 4 weeks). Responses of vessels from untreated aged and young rats and rats 2 weeks after discontinuation of etanercept treatment are shown for comparison. Data are mean ± SEM (n = 5 to 10). *P < 0.05. D: Relaxations to acetylcholine in carotid arteries of young and aged TNF-α+/+ and TNF-α−/− mice. Data ± SEM are normalized to the young mean values (n = 5 to 10). *P < 0.05.
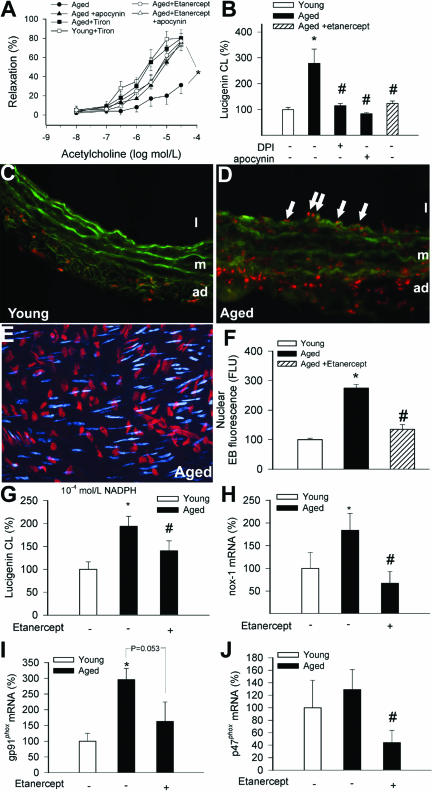
Both apocynin and Tiron improved acetylcholine-induced relaxation of aged carotid arteries (Figure 2A), consistent with the view that up-regulation of NAD(P)H oxidase activity is responsible, at least in part, for aging-induced endothelial dysfunction.2 Lucigenin chemiluminescence measurements showed that in carotid arteries of aged rats there was an increased O2⨪ production, which could be inhibited by apocynin and diphenyleneiodonium (Figure 2B). Vascular O2⨪ generation was also significantly reduced in etanercept-treated aged rats (Figure 2B). In carotid arteries of aged rats (n = 6), the relative number of EB-positive nuclei was significantly increased both in the endothelium (arrows) and media compared with vessels from young rats (Figure 2, C–E), showing that in aged vessels there is a significantly increased O2⨪ generation both in the endothelial and smooth muscle cells. Nuclear EB fluorescence was significantly reduced in etanercept-treated aged rats (Figure 2F). Neutralization of TNF-α had no significant effect on O2⨪ production in vessels from young rats (not shown). Our data revealed an enhanced NAD(P)H-driven O2⨪ generation in homogenates of aged carotid arteries, which was significantly attenuated in etanercept-treated animals (Figure 2G). Etanercept treatment tended to decrease the expression of nox-1, gp91phox, and p47phox subunits of the NAD(P)H oxidase (Figure 2, H–J). Expression of the p22phox and nox-4 subunits was unaffected by etanercept treatment (not shown).
Figure 2.
A: Relaxations to acetylcholine in carotid arteries of aged F344 rats with or without etanercept treatment (1 mg/kg/week, for 4 weeks) in the absence and presence of apocynin (3 × 10−4 mol/L) or Tiron (10 mmol/L). Data are mean ± SEM (n = 5 to 7). *P < 0.05. B: Superoxide production in vessels of young, aged, and etanercept-treated aged rats, as measured by the lucigenin chemiluminescence method. The NAD(P)H oxidase inhibitors diphenyleneiodonium (10−5 mol/L) and apocynin (3 × 10−4 mol/L) significantly decreased O2⨪ generation in aged vessels. Data are mean ± SEM. *P < 0.05 versus young, #P < 0.05 versus untreated. C and D: Fluorescent photomicrographs showing that compared with young vessels (C), there was a significantly increased O2⨪ production in the endothelial (arrows) and smooth muscle cells of aged arteries (D), as indicated by the intensive red fluorescent staining of the nuclei by EB. Green autofluorescence is shown for orientation purposes (L, lumen; m, media; ad, adventitia). Images are representative of six independent experiments. E: Representative image of en face preparation of EB-stained nuclei of endothelial cells (red) and smooth muscle cells (pseudocolored blue) in an aged artery. Optical sections were obtained using the Zeiss Apotome technology. F: Bar graphs are summary data for nuclear EB fluorescence intensities in endothelial cells in arteries of young, aged, and etanercept-treated aged rats. Data are mean ± SEM. *P < 0.05 versus young, #P < 0.05 versus untreated. G: NAD(P)H-driven O2⨪ generation (assessed by the lucigenin chemiluminescence method) in homogenates of carotid arteries of young, aged, and etenercept-treated aged rats. Data are mean ± SEM. *P < 0.05 versus young, #P < 0.05 versus untreated. H–J: Vascular expression of the NAD(P)H oxidase subunits nox-1 (H), gp91phox (I), and p47phox (J) in young, aged, and etenercept-treated aged rats. Data are mean ± SEM. *P < 0.05 versus young, #P < 0.05 versus untreated.
Effect of Anti-TNF-α Treatment on Vascular Apoptotic Cell Death
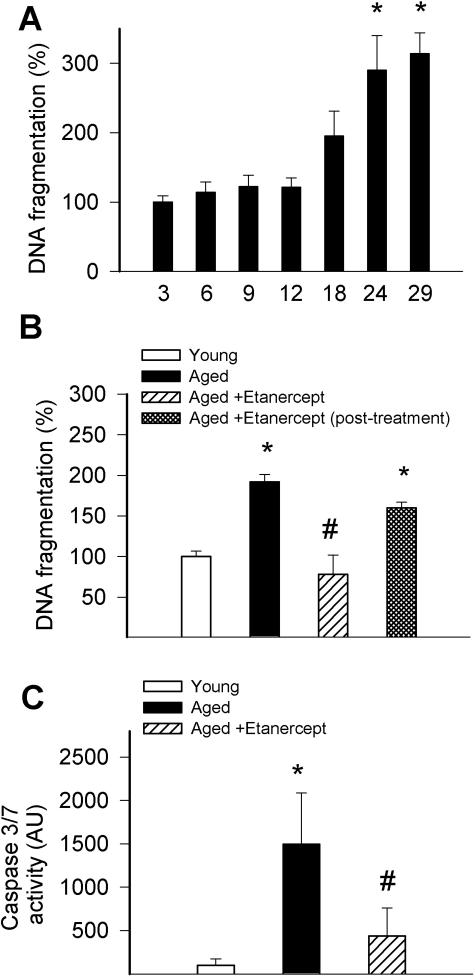
Using vessels from various age groups we demonstrated that DNA fragmentation rate starts to increase from 18months of age and reaches high levels in rats aged ≥2 years (Figure 3A). There was also an increased caspase 3/7 activity in aged vessels (Figure 3C). Chronic etanercept treatment significantly reduced DNA fragmentation rate and caspase 3/7 activity in the aged arteries (Figure 3, B and C). The anti-apoptotic effect of etanercept was diminished 2 weeks after the last etanercept injection (Figure 3B).
Figure 3.
A: Age-dependent increases in DNA fragmentation in vessels of F344 rats. B: DNA fragmentation in carotid arteries of young, aged, and etenercept-treated aged F344 rats. Data obtained in vessels from rats 2 weeks after discontinuation of etanercept treatment are also shown. C: Caspase 3/7 activity in vessels of young, aged, and etenercept-treated aged F344 rats. Data ± SEM are normalized to the young mean values (n = 4 to 5 for each group). *P < 0.05 versus young, #P < 0.05 versus untreated.
Effect of Anti-TNF-α Treatments on Vascular Expression of Inflammatory Markers
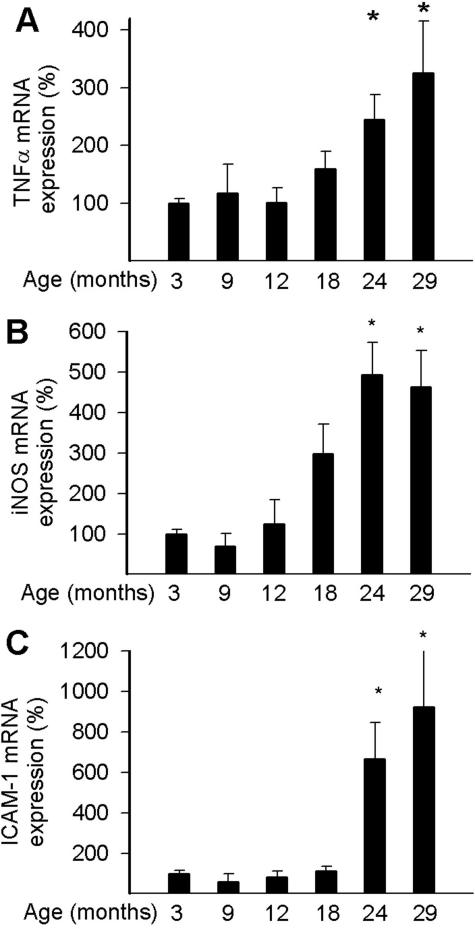
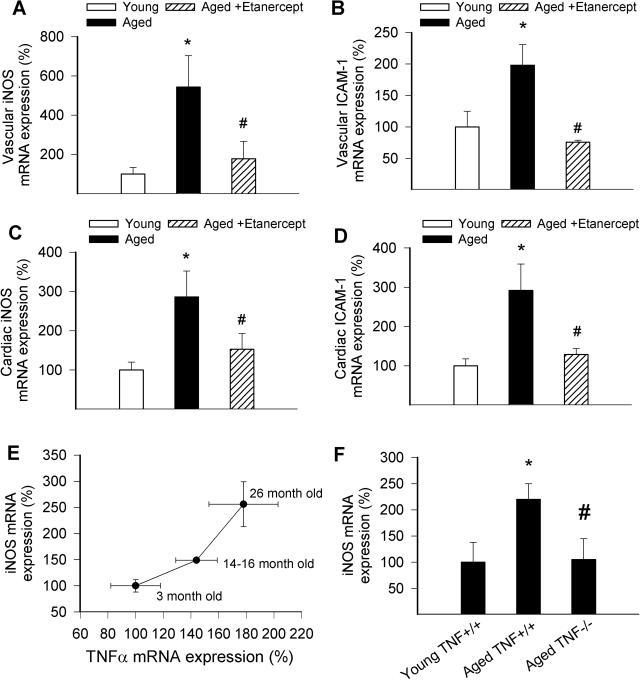
We found that vascular expression of TNF-α and the inflammatory markers iNOS and intercellular adhesion molecule (ICAM)-1 increases as a function of age after mid-life (Figure 4, A–C), extending our previous findings.2,3,13 Chronic etanercept treatment significantly decreased the expression of both inflammatory markers in aged vessels (Figure 5, A and B). Similar results were obtained in the heart (Figure 5, C and D) of etanercept-treated aged rats. Expression of iNOS increased with age in carotid arteries of wild-type mice and showed a positive correlation with up-regulation of TNF-α (Figure 5G). Our recent microarray analysis also showed that age-related phenotypic changes in carotid arteries are already present in 14- to 16-month-old mice (Z. Ungvari and G. Kaley, unpublished results). We found that genetic lack of TNF-α prevented age-related up-regulation of iNOS in the carotid arteries of mice in this age group (Figure 5H).
Figure 4.
Expression of TNF-α (A), iNOS (B), and ICAM-1 (C) in vessels of 3-, 9-, 12-, 18-, 24-, and 29-month-old F344 rats. Analysis of mRNA expression was performed by real-time QRT-PCR. Data ± SEM are normalized to the 3-month-old mean values (n = 4 to 5 for each group). *P < 0.05 versus 3 months old.
Figure 5.
Vascular (A, B) and cardiac (C, D) expression of iNOS (A, C) and ICAM-1 (B, D) mRNA in aged (29 months old) rats with or without etanercept treatment (4 weeks, 1 mg/kg/week). Analysis of mRNA expression was performed by real-time QRT-PCR. Data ± SEM are normalized to the young (3 months old) mean values (n = 5 for each group). *P < 0.05 versus young, #P < 0.05 versus untreated. E: Correlation between age-related increases in TNF-α and iNOS mRNA expression in carotid arteries of wild-type mice. F: Expression of iNOS mRNA in carotid arteries of young and aged TNF+/+ mice and TNF−/− mice. Data ± SEM are normalized to the 3-month-old mean values (n = 5 to 6 for each group). *P < 0.05 versus young, #P < 0.05 versus wild type.
Exogenous TNF-α Elicits Oxidatives Stress, Endothelial Dysfunction, and Apoptotic Cell Death and Promotes Endothelial Inflammatory Phenotypic Changes
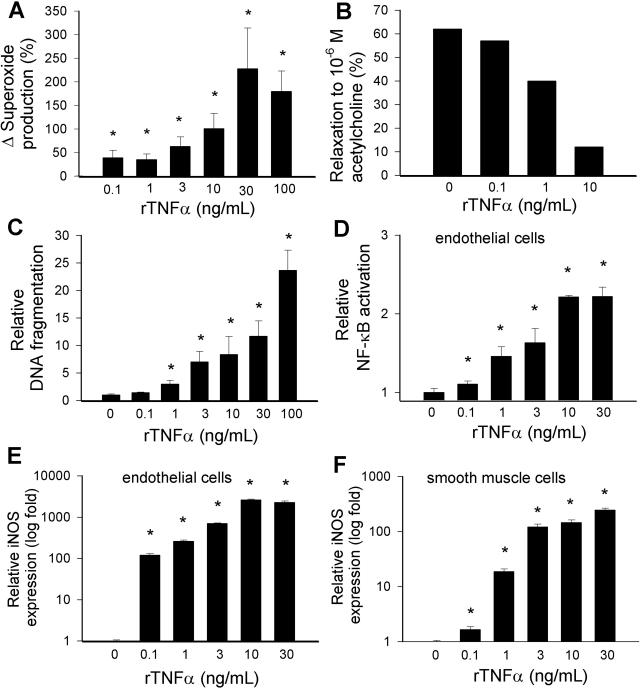
In isolated carotid arteries from young rats, recombinant TNF-α significantly increased O2⨪ generation and impaired ACh-induced relaxations (Figure 6, A and B). DNA fragmentation, a marker of apoptotic cell death, was significantly increased by TNF-α in these vessel preparations (Figure 6C). We also analyzed the effects of rTNF-α on cultured coronary arterial endothelial cells and smooth muscle cells. TNF-α elicited substantial endothelial NF-κB induction and up-regulation of iNOS (both in endothelial and smooth muscle cells; Figure 6, D–F). Similar results were obtained in TNF-α-treated vessels as well (not shown). Interestingly, cultured endothelial cells appeared to be more sensitive toward TNF-α than smooth muscle cells (Figure 6, compare E with F).
Figure 6.
Concentration dependence of the vascular effects of TNF-α. A and B: Superoxide production (A; measured by the lucigenin chemiluminescence method) and relaxations to acetylcholine (B) and in ring preparations of carotid arteries of young F344 rats maintained in vessel culture (for 24 hours) in the absence and presence of TNF-α. Data are mean ± SEM (n = 4 to 6 in each group) *P < 0.05. C: DNA fragmentation in arteries of young F344 rats maintained in vessel culture (for 24 hours) in the absence and presence of TNF-α. Data are mean ± SEM (n = 4 to 6 in each group) *P < 0.05. D: Reporter gene assay showing the effects of TNF-α on NF-κΒ reporter activity in coronary arterial endothelial cells. Endothelial cells were transiently co-transfected with NF-κΒ-driven firefly luciferase and CMV-driven Renilla luciferase constructs followed by TNF-α stimulation. Cells were then lysed and subjected to luciferase activity assay. After normalization, relative luciferase activity was obtained from four independent transfections (data are mean ± SEM, *P < 0.05 versus control). E and F: Effect of TNF-α treatment (24 hours) on the expression of iNOS in coronary arterial endothelial cells (E) and smooth muscle cells (F). Analysis of mRNA expression was performed by real-time QRT-PCR. Data are mean of four independent experiments.
Discussion
There are four salient findings in this study. First, we found that in advanced aging increased TNF-α levels9–12 (Figure 1A) were associated with significant impairment of endothelium-dependent and NO-mediated (Figure 1, B and C) relaxations extending previous findings by this and other laboratories.6,7,14 Our present data and findings from previous studies suggest that aging increases vascular O2⨪ production, at least in part, by increasing the activity of NAD(P)H oxidase (Figure 2), thereby increasing NO breakdown and decreasing its bioavailability.4,6,7,14
We found that neutralization of TNF-α by chronic treatment with etanercept improved endothelial function (Figure 1B) and NO mediation in aged arteries (Figure 1C) and reduced vascular NAD(P)H oxidase activity and expression (Figure 2). Genetic lack of TNF-α also improved endothelial function in vessels of aged mice (Figure 1D). Previously, we found that TNF-α inhibition does not significantly affect either acetylcholine-induced responses or vascular reactive oxygen species generation in young animals.19 These data suggest a link between aging, TNF-α, oxidative stress, and endothelial dysfunction.19,36,37 Recent studies also showed that in ovariectomized 12- to 15-month-old female Sprague-Dawley rats administration of etanercept (0.3 mg/kg, for 4 weeks) also improved endothelium-dependent vascular relaxations and decreased expression of the gp91phox NAD(P)H oxidase subunits.28,38 There are also studies showing that anti-TNF-α treatment with infliximab improves endothelial dysfunction in humans with vascular inflammation characterized by an up-regulation of TNF-α.23 We believe that these findings support the view that anti-TNF-α therapy, alone or in combination with standard treatments, may exert vasculoprotective effects in elderly humans. At present it is unknown how etanercept treatment affects cardiac function in aged rats or humans. It is important to note that recent clinical studies using etanercept (RENAISSANCE and RECOVER trials, the combined analysis being termed RENEWAL) failed to demonstrate significant beneficial effects on cardiac performance in patients with chronic heart failure.39
Second, our present and previous studies have demonstrated that increased DNA fragmentation rate (Figure 4) is associated with an increased number of terminal dUTP nick-end labeling-positive endothelial cells in arteries of aged rats.3 Our present data suggest increased endothelial apoptosis2,3 is a feature of advanced aging (Figure 4). The results that both chronic etanercept treatment (Figure 4, B and C) and in vitro neutralization of TNF-α3 decreased apoptotic cell death in aged vessels provide strong evidence that increased TNF-α levels in aging initiate programmed endothelial cell death and thus are likely to contribute to age-related cardiovascular pathophysiology.14 One can hypothesize that decreasing endothelial cell death also contributes to the beneficial effects of etanercept on vascular endothelial function (Figure 1). It is interesting to note that the vasculoprotective effects of etanercept diminished in the posttreatment group (Figures 1B and 3B), which is likely a consequence of the elimination of etanercept from the circulation in the 2-week period after treatment (the reported half-life of etanercept is 4 to 5 days in humans).
Third, we have demonstrated that, in addition to increased plasma TNF-α levels, advanced aging is associated with up-regulation of TNF-α expression within the vascular wall (Figure 5, A and E).2,13 Thus, both locally generated and circulating TNF-α (Figure 1A) are likely to contribute to vascular aging phenotype. Our present data (Figure 1A) and findings from previous studies suggest that plasma levels of TNF-α in elderly patients9,10 and aged experimental animals11,12 is probably in the range of ∼0.3 ng/ml. It can be assumed that endothelial cells are exposed to even higher TNF-α concentrations attributable to the local release of TNF-α from cells within the vascular wall (smooth muscle cells,2,3,13 leukocytes, and/or fibroblasts). It is likely that etanercept neutralizes TNF-α both in the plasma and within the vascular tissue. The mechanisms by which aging leads to the up-regulation of TNF-α in blood vessels13,15 and other organs16,40 are not well understood. Because TNF-α mRNA expression also tends to increase in cultured vascular smooth muscle cells with an increased number of passages,14 it is likely that up-regulation of TNF-α expression, at least in part, is intrinsic to the aging process/senescence of vascular cells. Parallel to the age-dependent increase in TNF-α levels, we found an increase in the expression of the inflammatory markers iNOS and ICAM-1 in rat arteries (Figure 4, B and C).2 The findings that chronic etanercept treatment attenuates aging-induced vascular and cardiac up-regulation of iNOS and ICAM-1 in aged rats (Figure 5, A–D) and that genetic lack of TNF-α reduces vascular expression of iNOS in aged mice (Figure 5F) suggest that TNF-α plays an important regulatory role in age-related phenotypic changes. The mechanisms by which TNF-α induces iNOS and other inflammatory genes probably involves activation of NF-κB.41 Accordingly, NF-κB binding activity seems to increase in aging of many tissues including the heart42 and carotid arteries of aged rats (A. Csiszar and Z. Ungvari, manuscript in preparation). It is significant that premature vascular aging in many pathophysiological conditions, including hyperhomocysteinemia, is also associated with an up-regulation of vascular TNF-α expression and concomitant endothelial oxidative stress and proinflammatory gene expression (including iNOS).19
Fourth, we have demonstrated that in young arteries administration of TNF-α, in a dose-dependent manner, promotes vascular oxidative stress and endothelial dysfunction (Figure 6, A and B), increases apoptotic cell death (Figure 6C), activates NF-κB (Figure 6D), and induces iNOS (Figure 6, E and F), mimicking many symptoms of vascular aging. There is increasing evidence that proinflammatory effects of TNF-α, including activation of NF-κB, are mediated by NAD(P)H oxidase activation.41 The effective concentration range of TNF-α overlaps with its plasma concentrations present in elderly patients9,10 and aged experimental animals11,12 (Figure 1A). Moreover, levels of TNF-α within the aged vascular wall are likely to far exceed the plasma levels because of the paracrine secretion of TNF-α by vascular cells.3,13,19,41
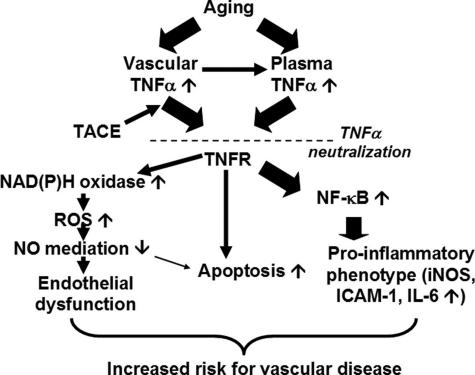
Taken together, we propose (Figure 7) that aging-induced dysregulation of TNF-α contributes (at least in part) to endothelial dysfunction and oxidative stress, promotes apoptotic cell death, and induces a proinflammatory phenotype of blood vessels. There is growing evidence that TNF-α plays a role in atherosclerosis: both genetic lack of TNF-α43 and pharmacological neutralization of TNF-α44 are anti-atherogenic in animal models (although the role of the TNF receptor p55 is still not completely understood45,46). Thus, vasculoprotective effects of anti-TNF-α treatments may be beneficial in elderly patients.
Figure 7.
Proposed scheme for the vasculoprotective effects of TNF-α neutralization in aging. In aging, plasma TNF-α levels are increased.9–12 In addition, TNF-α is also up-regulated and secreted [by the TNF-α convertase enzyme (TACE)3] by vascular cells as a paracrine mediator. The resulting increased levels of TNF-α within the vascular wall activate NAD(P)H oxidase-derived O2⨪ generation leading to endothelial dysfunction, induce apoptotic endothelial cell death, and promote vascular inflammation by inducing NF-κB activation. Etanercept is likely to neutralize both circulating and locally produced TNF-α, attenuating oxidative stress and vascular inflammation, limiting endothelial cell loss, and improving endothelial function in aging.
Footnotes
Address reprint requests to Anna Csiszar M.D., Ph.D., or Zoltan Ungvari, M.D., Ph.D., Department of Physiology, New York Medical College, Valhalla, NY 10595. E-mail: anna_csiszar@nymc.edu and zoltan_ungvari@nymc.edu.
Supported by the American Heart Association (grants 0430108N and 0435140N), the American Health Assistance Foundation, the American Federation for Aging Research, Philip Morris USA and Philip Morris International Inc., and the National Institutes of Health (HL-077256 and PO43023).
References
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Luscher TF, Volpe M, Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110:2889–2895. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Pacher P, Kaley G, Ungvari Z. Role of oxidative and nitrosative stress, longevity genes and poly(ADP-ribose) polymerase in cardiovascular dysfunction associated with aging. Curr Vasc Pharmacol. 2005;3:285–291. doi: 10.2174/1570161054368616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol. 2004;286:H2249–H2256. doi: 10.1152/ajpheart.00854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- Asai K, Kudej RK, Shen YT, Yang GP, Takagi G, Kudej AB, Geng YJ, Sato N, Nazareno JB, Vatner DE, Natividad F, Bishop SP, Vatner SF. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol. 2000;20:1493–1499. doi: 10.1161/01.atv.20.6.1493. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Skinhoj P, Pedersen AN, Schroll M, Pedersen BK. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol. 2000;121:255–260. doi: 10.1046/j.1365-2249.2000.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S, Schagdarsurengin U, Suss T, Muller-Werdan U, Werdan K, Glaser C. Relation between the tumor necrosis factor-alpha (TNF-alpha) gene and protein expression, and clinical, biochemical, and genetic markers: age, body mass index and uric acid are independent predictors for an elevated TNF-alpha plasma level in a complex risk model. Eur Cytokine Netw. 2004;15:105–111. [PubMed] [Google Scholar]
- Yamamoto K, Shimokawa T, Yi H, Isobe K, Kojima T, Loskutoff DJ, Saito H. Aging and obesity augment the stress-induced expression of tissue factor gene in the mouse. Blood. 2002;100:4011–4018. doi: 10.1182/blood-2002-03-0945. [DOI] [PubMed] [Google Scholar]
- Spaulding CC, Walford RL, Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mech Ageing Dev. 1997;93:87–94. doi: 10.1016/s0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in rat coronary arteries. FASEB J. 2003;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Kaley G. Vascular inflammation in aging. Herz. 2004;29:733–740. doi: 10.1007/s00059-004-2625-x. [DOI] [PubMed] [Google Scholar]
- Belmin J, Bernard C, Corman B, Merval R, Esposito B, Tedgui A. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. Am J Physiol. 1995;268:H2288–H2293. doi: 10.1152/ajpheart.1995.268.6.H2288. [DOI] [PubMed] [Google Scholar]
- Lee CK, Allison DB, Brand J, Weindruch R, Prolla TA. Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc Natl Acad Sci USA. 2002;99:14988–14993. doi: 10.1073/pnas.232308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt B, Kribbs SB, Clubb FJ, Jr, Michael LH, Didenko VV, Hornsby PJ, Seta Y, Oral H, Spinale FG, Mann DL. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97:1382–1391. doi: 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]
- Bozkurt B, Torre-Amione G, Warren MS, Whitmore J, Soran OZ, Feldman AM, Mann DL. Results of targeted anti-tumor necrosis factor therapy with etanercept (ENBREL) in patients with advanced heart failure. Circulation. 2001;103:1044–1047. doi: 10.1161/01.cir.103.8.1044. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, Koller A. Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2003;23:418–424. doi: 10.1161/01.ATV.0000061735.85377.40. [DOI] [PubMed] [Google Scholar]
- Vila E, Salaices M. Cytokines and vascular reactivity in resistance arteries. Am J Physiol. 2005;288:H1016–H1021. doi: 10.1152/ajpheart.00779.2004. [DOI] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Lüscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hürlimann D, Forster A, Noll G, Enseleit F, Chenevard R, Distler O, Bechir M, Spieker LE, Neidhart M, Michel BA, Gay RE, Luscher TF, Gay S, Ruschitzka F. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002;106:2184–2187. doi: 10.1161/01.cir.0000037521.71373.44. [DOI] [PubMed] [Google Scholar]
- Booth AD, Jayne DR, Kharbanda RK, McEniery CM, Mackenzie IS, Brown J, Wilkinson IB. Infliximab improves endothelial dysfunction in systemic vasculitis: a model of vascular inflammation. Circulation. 2004;109:1718–1723. doi: 10.1161/01.CIR.0000124720.18538.DD. [DOI] [PubMed] [Google Scholar]
- Fichtlscherer S, Rossig L, Breuer S, Vasa M, Dimmeler S, Zeiher AM. Tumor necrosis factor antagonism with etanercept improves systemic endothelial vasoreactivity in patients with advanced heart failure. Circulation. 2001;104:3023–3025. doi: 10.1161/hc5001.101749. [DOI] [PubMed] [Google Scholar]
- Gurevitch J, Frolkis I, Yuhas Y, Lifschitz-Mercer B, Berger E, Paz Y, Matsa M, Kramer A, Mohr R. Anti-tumor necrosis factor-alpha improves myocardial recovery after ischemia and reperfusion. J Am Coll Cardiol. 1997;30:1554–1561. doi: 10.1016/s0735-1097(97)00328-8. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Dohmen S, Adamis AP. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002;16:438–440. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- Arenas IA, Armstrong SJ, Xu Y, Davidge ST. Chronic tumor necrosis factor-alpha inhibition enhances NO modulation of vascular function in estrogen-deficient rats. Hypertension. 2005;46:76–81. doi: 10.1161/01.HYP.0000168925.98963.ef. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Coyle SM, Levi M, Jansen PM, Dentener M, Barbosa K, Buurman WA, Hack CE, ten Cate JW, Agosti JM, Lowry SF. Effect of a recombinant dimeric tumor necrosis factor receptor on inflammatory responses to intravenous endotoxin in normal humans. Blood. 1997;89:3727–3734. [PubMed] [Google Scholar]
- Gupte SA, Arshad M, Viola S, Kaminski PM, Ungvari Z, Rabbani G, Koller A, Wolin MS. Pentose phosphate pathway coordinates multiple redox-controlled relaxing mechanisms in bovine coronary arteries. Am J Physiol. 2003;285:H2316–H2326. doi: 10.1152/ajpheart.00229.2003. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation. 2003;108:1253–1258. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Kaminski PM, Wolin MS, Koller A. Chronic high pressure-induced arterial oxidative stress: involvement of protein kinase C-dependent NAD(P)H oxidase and local renin-angiotensin system. Am J Pathol. 2004;165:219–226. doi: 10.1016/S0002-9440(10)63290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labinskyy N, Csiszar A, Orosz Z, Rivera A, Smith K, Buffenstein R, Ungvari Z: Comparison of endothelial function, O2⨪ and H2O2 production and vascular oxidative stress resistance between the longest-living rodent, the naked mole-rat and mice. Am J Physiol (in press) [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ahmad M, Smith KE, Labinskyy N, Gao Q, Kaley G, Edwards JG, Wolin MS, Ungvari Z. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol. 2006;168:629–638. doi: 10.2353/ajpath.2006.050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation. 2005;111:2364–2372. doi: 10.1161/01.CIR.0000164201.40634.1D. [DOI] [PubMed] [Google Scholar]
- Frey RS, Rahman A, Kefer JC, Minshall RD, Malik AB. PKCzeta regulates TNF-alpha-induced activation of NADPH oxidase in endothelial cells. Circ Res. 2002;90:1012–1019. doi: 10.1161/01.res.0000017631.28815.8e. [DOI] [PubMed] [Google Scholar]
- De Keulenaer GW, Alexander RW, Ushio-Fukai M, Ishizaka N, Griendling KK. Tumour necrosis factor alpha activates a p22phox-based NADH oxidase in vascular smooth muscle. Biochem J. 1998;329:653–657. doi: 10.1042/bj3290653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas IA, Xu Y, Davidge ST. Age-associated impairment in vasorelaxation to fluid shear stress in the female vasculature is improved by TNF-{alpha} antagonism. Am J Physiol. 2006;290:H1259–H1263. doi: 10.1152/ajpheart.00990.2005. [DOI] [PubMed] [Google Scholar]
- Anker SD, Coats AJ. How to RECOVER from RENAISSANCE? The significance of the results of RECOVER, RENAISSANCE, RENEWAL and ATTACH. Int J Cardiol. 2002;86:123–130. doi: 10.1016/s0167-5273(02)00470-9. [DOI] [PubMed] [Google Scholar]
- Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19:668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Smith KE, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNFa-induced activation of coronary arterial endothelial cells: role of NF-kB inhibition. Am J Physiol. 2006;291:H1694–H1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- Helenius M, Hanninen M, Lehtinen SK, Salminen A. Aging-induced up-regulation of nuclear binding activities of oxidative stress responsive NF-kB transcription factor in mouse cardiac muscle. J Mol Cell Cardiol. 1996;28:487–498. doi: 10.1006/jmcc.1996.0045. [DOI] [PubMed] [Google Scholar]
- Ohta H, Wada H, Niwa T, Kirii H, Iwamoto N, Fujii H, Saito K, Sekikawa K, Seishima M. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis. 2005;180:11–17. doi: 10.1016/j.atherosclerosis.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Brånén L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- Blessing E, Bea F, Kuo CC, Campbell LA, Chesebro B, Rosenfeld ME. Lesion progression and plaque composition are not altered in older apoE−/− mice lacking tumor necrosis factor-alpha receptor p55. Atherosclerosis. 2004;176:227–232. doi: 10.1016/j.atherosclerosis.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Schreyer SA, Peschon JJ, LeBoeuf RC. Accelerated atherosclerosis in mice lacking tumor necrosis factor receptor p55. J Biol Chem. 1996;271:26174–26178. doi: 10.1074/jbc.271.42.26174. [DOI] [PubMed] [Google Scholar]