Abstract
Although cancer cells are not generally controlled by normal regulatory mechanisms, tumor growth is highly dependent on the supply of oxygen, nutrients, and host-derived regulators. It is now established that tumor vasculature is not necessarily derived from endothelial cell sprouting; instead, cancer tissue can acquire its vasculature by co-option of pre-existing vessels, intussusceptive microvascular growth, postnatal vasculogenesis, glomeruloid angiogenesis, or vasculogenic mimicry. The best-known molecular pathway driving tumor vascularization is the hypoxia-adaptation mechanism. However, a broad and diverse spectrum of genetic aberrations is associated with the development of the “angiogenic phenotype.” Based on this knowledge, novel forms of antivascular modalities have been developed in the past decade. When applying these targeted therapies, the stage of tumor progression, the type of vascularization of the given cancer tissue, and the molecular machinery behind the vascularization process all need to be considered. A further challenge is finding the most appropriate combinations of antivascular therapies and standard radio- and chemotherapies. This review intends to integrate our recent knowledge in this field into a rational strategy that could be the basis for developing effective clinical modalities using antivascular therapy for cancer.
Until recently, vascularization of malignant tumors was considered the exclusive result of directed capillary ingrowth (endothelial sprouting). However, recent advances have been made in identifying the processes involved in angiogenesis and vascular remodeling. Consequently, the simplistic model of an invading capillary sprout has been deemed insufficient to describe the entire spectrum of morphogenic and molecular events required to form a neovascular network. Before discussing the different ways a tumor is vascularized, we should emphasize that these mechanisms are not mutually exclusive; in fact, in most cases they are interlinked, participating concurrently in physiological as well as in pathological angiogenesis. Although the various types of cancer vascularization share some molecular features and may be controlled in part by similar sets of regulatory factors, a considerable variety of differences also exists. Although the molecular regulation of endothelial sprouting has been extensively studied and reviewed in the literature, the morphogenic and molecular events associated with alternative cancer vascularization mechanisms are less understood. Therefore, this review focuses on the pathogenesis of the different forms of “nonsprouting angiogenesis” and, more specifically, on the possibilities and the potential use of novel antiangiogenic and vascular targeting strategies against alternative tumor vascularization mechanisms.
Vascularization Mechanisms in Cancer
Endothelial Sprouting
The best-known mechanism by which tumors promote their own vascularization is inducing new capillary buds from pre-existing host tissue capillaries. The first description of this process dates back to the 1970s, when Ausprunk and Folkman1 suggested the following sequence for tumor-induced capillary sprouting (Figure 1, Alt. 1). 1) The basement membrane is locally degraded on the side of the dilated peritumoral postcapillary venule situated closest to the angiogenic stimulus, interendothelial contacts are weakened, and endothelial cells (ECs) emigrate into the connective tissue, toward the angiogenic stimuli. 2) There is formation of a solid cord by ECs succeeding one another in a bipolar fashion. 3) Lumen formation occurs by cell-body curving of a single EC or by participation of more ECs in parallel with the synthesis of the new basement membrane and the recruitment of pericytes/mural cells. The main disadvantages of this model are its inability to identify the nature and origin of the stimulus necessary for lumen formation and the assumption that dedifferentiation and redifferentiation take place during the same process, manifest in the loss and regaining of luminal-basal EC polarity. Furthermore, although it has been well established that the stimulus necessary for lumen formation comes from the developing basement membrane, according to this model, basement membrane deposition occurs after lumen formation. In the early 1990s, a different sprouting model was described2 (Figure 1, Alt. 2). This model suggests a three-stage sequence to explain ultrastructural changes during tumor-induced endothelial sprouting. 1) There is structural alteration of the basement membrane characterized by the loss of electron density (gel-sol transition) over the entire circumference of the dilated “mother vessel” (although basement membrane components such as laminin and collagen IV can still be detected by immunohistochemistry). Partial and regulated degradation of the altered basement membrane occur only at places where EC processes (connected by intercellular junctions) are projecting into the connecting tissue. 2) Further migration of ECs, which are arranged in parallel, maintaining their basal-luminal polarity and forming a slit-like lumen, takes place continuously with the lumen of the mother vessel and sealed by intact interendothelial junctions. Basement membrane of low electron density is deposited continuously by the polarized ECs while only the very tip of the growing capillary bud is free of basement membrane material. 3) Proliferating pericytes of the mother vessel migrate along the basement membrane of the capillary bud, resulting in complete pericyte coverage of the new vessel. In parallel, the appearance of electron-dense basement membrane around the maturing capillary buds (sol-gel transition) can be observed. According to the above model, no stimulus is necessary for the induction of lumen formation, because ECs do not lose their polarity during the process.
Figure 1.
Endothelial sprouting. Schematic representation of the EC sprouting models suggested by Ausprunk and Folkman (Alt. 11) and by Paku and Paweletz (Alt. 22). Red cells represent endothelial cells; brown cells are pericytes. Yellow cells are mural cells of other origin (fibroblasts or bone marrow-derived cells). See Vascularization Mechanisms in Cancer for details.
The molecular background of capillary sprouting has been extensively studied and reviewed in the literature.3 During the process, vessels initially dilate and become leaky in response to vascular permeability factor/vascular endothelial growth factor (VPF/VEGF).4 This is mediated by the up-regulation of nitric oxide, the development of fenestrations and vesiculo-vacuolar organelles, and by the redistribution of CD31/PECAM-1 and vascular endothelial (VE)-cadherin. The so-called gel-sol transition of the basement membrane, probably mediated by matrix metalloproteases (MMPs), gelatinases, and the urokinase plasminogen activator system, could be partly responsible for the initiation of EC proliferation and migration. Ang-2 (Angiopoetin-2, a mediator of Tie-2 signaling) is involved in the detachment of pericytes and loosening of the matrix. A vast number of molecules stimulate endothelial proliferation and migration, including transforming growth factor (TGF)-β1, tumor necrosis factor (TNF)-α, members of the chemokine system and the VEGF, fibroblast growth factor, and platelet-derived growth factor (PDGF) families.3 It could be argued that integrins represent the most important adhesion receptors in migrating ECs.5 A wide variety of integrins have been shown to be expressed during sprouting, including α1β1, α2β1, α3β1, α5β1, αvβ5, and αvβ3. Perhaps the most important among them is αvβ3, which mediates the migration of ECs in the fibrin-containing cancer stroma and maintains the sol state of the basement membrane because of its ability to bind to MMP-2. During maturation of nascent vessels, PDGF-BB recruits pericytes and smooth muscle cells, whereas TGF-β1 and Ang-1/Tie-2 stabilize the interaction between endothelial and mural cells.3 All in all, sprouting is controlled by a tightly regulated balance of proangiogenic factors and inhibitors: an angiogenic cytokine promotes EC proliferation, migration, or lumen formation, whereas an inhibitor interferes with these steps and modulates the proliferation or migration activity of ECs. However, individual tumor types use various combinations of proangiogenic and inhibitory cytokines.3
Vessel Co-Option
When tumors arise in or metastasize to a pre-existing, usually well-vascularized, tissue, their growth not only depends on expansion, like a balloon, more typical of slow-growing benign tumors, but also on the invasion of host tissue, allowing the cancer cells close contact with the surface of blood vessels. Therefore, malignant cells may initially associate with and grow preferentially along pre-existing microvessels. Until recently, however, no studies have focused on the role played by the host vasculature in the process of tumor vascularization. Although in 1987 Thompson6 had already proposed that tumors acquire their vasculature by incorporation of host tissue capillaries, the first study suggesting the existence of vessel co-option was not published until 1999 by Holash et al.7 In their model, Holash and colleagues found that co-option is limited to the initial phases of tumorigenesis.7 However, additional morphological evidence in human malignancies suggests that co-option of pre-existing blood vessels might persist during the entire period of primary or metastatic tumor growth. In cutaneous melanoma, we found that during tumor growth, there are no signs of directed vessel ingrowth; instead, these tumors appear to grow by co-opting the massive vascular plexus present in the peritumoral connective tissue.8 In non-small cell lung cancer, a putatively nonangiogenic growth pattern was observed.9 In this “alveolar type” of growth, cancer cells filled the alveoli, entrapping but not destroying the co-opted alveolar capillaries. In liver metastases of human colorectal carcinomas, different growth patterns (replacement, pushing, and desmoplastic) were observed, depending on the degree of differentiation. In replacement growth type, the architecture of the liver was preserved, and the ECs of sinusoids showed low mitotic activity. However, pushing and desmoplastic tumor types destroyed the liver architecture.10 According to our previous results in experimental hepatic metastases, during growth of sinusoidal-type metastases, invading cancer cells advance between the basement membrane and the endothelial lining of the sinusoids and evoke proliferation of ECs. This process resulted in the development of large tortuous vessels without basement membrane inside the tumor nodules. Conversely, sprouting-type angiogenesis was observed in portal-type metastases. The replacement growth pattern corresponded to sinusoidal-type metastases of undifferentiated tumors, whereas desmoplastic tumors showed similarities to portal-type metastases.11 In the pushing-type growth pattern, we recently described a mechanism for the development of blood supply and supportive connective tissue12 (Figure 2). This process includes the proliferation of smooth muscle actin-positive stellate, but not endothelial, cells on the surface of the tumor spheroid accompanied by capillarization of the sinusoids in this region. Because of the pressure of the tumor and the proliferating stellate cells, the hepatocytes disappear from the closest vicinity of the tumor, leading to the fusion of the sinusoids and the appearance of vascular lakes at the surface of the tumor. Together with the collagen-producing cells, these vascular lakes are incorporated into the tumor, resulting in the development of vessel-containing connective tissue columns that traverse the tumor. These columns represent the main structural and functional unit, providing blood supply for the inner part of the growing metastasis. Thus, the presence of the above mechanisms further supports earlier observations that vascularization of metastases in the liver is a heterogeneous process, depending on the degree of tumor differentiation or localization of the metastases within the liver.13
Figure 2.
Examples for vessel co-option. A–D: Pushing-type angiogenesis in liver metastases of colorectal cancer. A: Cross-section of a compressed invagination. SMA-expressing cells (blue fluorescence) facing the tumor tissue, hepatocytes are crowded in the middle of the invagination (pan-cytokeratin, green fluorescence). Continuous CD31 staining (red fluorescence), representing fused sinusoids (arrows), is visible in contact with the SMA-positive cells. Note the paucity of sinusoids between the hepatocytes. B: Laminin (blue fluorescence) co-localizes with α6 integrin within the columns. The column tightly packed with SMA-positive cells (red fluorescence). C: α6 integrin (green fluorescence) is present at the periphery of the column and around the central vessel. D: Schematic representation of the development of vasculature in pushing-type liver metastases. For better visibility of the vessels, hepatocytes are depicted only in the upper part of the drawings. At the early stage of the tumor development, the tumor faces normal liver architecture. As the compression of the tumor grows, the hepatocytes “step back,” and fusion of the sinusoids takes place. The fused vessel, together with the newly synthesized connective tissue, is incorporated into the tumor. The pressure of the tumor results in the separation of the vessel from the liver parenchyma. The vessel in the direction of the axis of the column remains connected to the sinusoidal system of the liver. Column formation is finished by the back-to-back fusion of the basement membranes of the tumor bulges. Green, tumor; brown, hepatocytes; red, sinusoids and central vessel.
Although sprouting capillaries are more vulnerable to apoptosis than their quiescent counterparts,14 maintenance of incorporated mature microvessels depends on the survival of ECs as well. The continued survival of co-opted ECs is intimately tied to their local microenvironment and, in particular, to the presence of pericytes, survival-promoting cytokines, and extracellular matrix proteins. Thus, the molecular repertoire that ECs may use to survive during vessel co-option is diverse and may vary for a given tumor type or host environment. The major players that control this process are angiopoetins and VEGF.7 Based on the model of vessel co-option described by Holash et al7 and in other recent studies,15 Ang-1 activates Tie-2 and induces subsequent signal transduction pathways favoring EC survival, endothelial quiescence, and tumor-vessel maintenance. Conversely, Ang-2 is thought to act as a nonsignaling Tie-2 ligand that binds to endothelial Tie-2 and thereby negatively interferes with agonistic Ang-1/Tie-2 signals. In co-opted blood vessels, the up-regulation of Ang-2 disrupts the interaction between Tie-2 and Ang-1, which in turn causes the destabilization of capillary walls (ie, the detachment of pericytes from the endothelial tube).16 Once ECs are separated from pericytes, they become particularly vulnerable. In the presence of VEGF, EC survival and new vessel growth are promoted; however, the lack of stimulatory factors results in the regression of destabilized vessels.17
VEGF was first described as a survival factor for retinal ECs and has now been shown to promote survival in different EC models. This antiapoptotic and survival function of VEGF seems to depend on an interaction between vascular endothelial growth factor receptor (VEGFR)-2, β-catenin, and VE-cadherin.18 However, targeting of VEGF has been shown to result in apoptosis only in newly formed tumor vessels and in the developing vasculature of the neonatal mouse but not that of adult mice or of quiescent tumor vascular networks.17 In summary, although cytokines responsible for EC survival could be the key molecules, their precise role in initiation and maintenance of vessel co-option still requires investigation.
Intussusceptive Microvascular Growth (IMG)
IMG refers to vessel network formation by insertion of connective tissue columns, called tissue pillars, into the vessel lumen and to subsequent growth of these pillars, resulting in partitioning of the vessel lumen (Figure 3). This type of angiogenesis, which has been observed in a wide variety of normal and malignant tissues, is faster and more economical than sprouting, occurs within hours or even minutes and does not primarily depend on EC proliferation, basement membrane degradation, and invasion of the connective tissue.19 However, in contrast to sprouting, IMG can work only on existing vessel networks. The most important feature of IMG, therefore, seems to be its ability to increase the complexity and density of the tumor microvessel network already built by sprouting, independent of EC proliferation. In addition, IMG can provide more surface area for further sprouting. Its molecular regulation, however, is poorly understood since IMG was first described only a few years ago. Nevertheless, the role of some players is gradually becoming clearer. We know that local stimuli, such as intravascular shear stress, might induce a cascade of physiological or pathological reactions in ECs, and new capillary development by tissue pillar formation could be one of them.20 Furthermore, intussusception is certainly synchronized by several cytokines. Major candidates are those capable of mediating information between ECs or from ECs to mural cells, such as PDGF-BB, angiopoietins, and their Tie receptors, TGF-β, monocyte chemotactic protein-1, and ephrins and Eph-B receptors.19
Figure 3.
Intussusceptive microvascular growth. Schematic representation of intussusceptive microvessel growth. The first step of the process is the development of the transluminal endothelial bridge. This is followed by the reorganization of the endothelial lining, a process that is largely unknown. The division of the vessel is completed by the development of a connective tissue pillar through the vessel lumen. Red cells are endothelial cells; brown cells are pericytes. Gray, basement membrane.
After the initial stage of immature capillary network formation by sprouting, additional vascular growth and development of complex vascular beds, including their continuous remodeling and adaptation, may occur by intussusception in cancers. The absence of intense EC proliferation in IMG implies that neovascularization by this mechanism would be resistant to angiosuppressive treatment in itself.
Glomeruloid Angiogenesis
Glomeruloid bodies (GBs) are best known in high-grade glial malignancies, where they are one of the diagnostic histopathological features of glioblastoma multiforme. However, these complex vascular aggregates have also been described in a wide variety of other malignancies.21 They are composed of several closely associated microvessels surrounded by a variably thickened basement membrane within which a limited number of pericytes are embedded. In recent studies, the presence of GBs was associated with markers of aggressive tumor behavior and significantly reduced survival in cancer patients.22 In the first animal model,23 GBs developed in mother vessels from recruitment and proliferation of ECs and pericytes (in the absence of tumor cells), and VEGF was essential for their induction and maintenance. In contrast to this model and based on our previous results in the first experimental tumor model of glomeruloid angiogenesis,24 we believe that GB formation starts immediately after tumor cell extravasation, much earlier than necrosis appears within the metastases. We found that the proliferating and migrating tumor cells are able to pull the capillaries and the adjacent capillary branching points into the tumor cell nests. This process leads to the appearance of simple coiled vascular structures that later develop into GBs with multiple narrowed afferent and efferent capillaries (Figure 4). Despite the absence of sprouting angiogenesis, necrosis was scarce in these lesions, suggesting that the blood supply from the pre-existent vascular bed is sufficient to provide the tumor cells with oxygen and nutrients. This type of GB formation cannot be termed as true angiogenesis; it rather represents a remodeling of the existing vasculature of the host tissue. Whether GBs represent an accelerated form of angiogenesis or a dysfunctional, possibly abortive, form remains an open question. However, it cannot be excluded that “active” and “passive” types of glomeruloid angiogenesis can operate concurrently in various cancer types.
Figure 4.
Glomeruloid angiogenesis. A: Experimental brain metastases stained for laminin (green fluorescence) and CD31 (blue fluorescence), 28 days following intracarotid inoculation of the A2058 human melanoma cell line. Glomeruloid bodies are connected to each other by a capillary that is very small in diameter (arrows). The outlines of the metastases are clearly visible because of the strong laminin positivity of the tumor cells (arrowheads). B: Schematic representation of glomeruloid body formation. Following extravasation, the tumor cells (green) adhere firmly to the abluminal surface of the capillary basement membrane (gray). In the first step, because of the contractile force of the tumor cell a loop develops on the capillary. Proliferating tumor cells pull the capillary inward, resulting in the development of further loops and reduction of the diameter of the capillary segment lying outside the glomeruloid body. The last drawing shows the cross-section of a fully developed glomeruloid body built by ECs (red), pericytes (brown), and tumor cells (green). Extreme large cytoplasmic projections of the tumor cells adhere to different segments of the capillary.
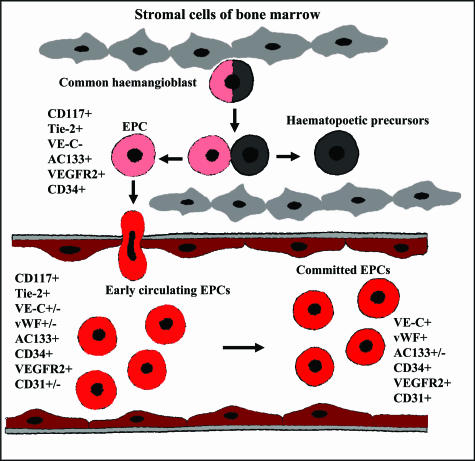
Postnatal Vasculogenesis: The Role of Endothelial Progenitor Cells
Vasculogenesis (defined as the in situ differentiation of vascular ECs from primitive precursor cells) has long been thought to occur only in the early phases of vascular development. Recent studies, however, have demonstrated that circulating bone marrow-derived endothelial progenitor cells (EPCs) home to sites of physiological and pathological neovascularization and differentiate into ECs (Figure 5). EPCs may be mobilized by tumor tissue-derived cytokines from the bone marrow by a mechanism recently described by Asahara et al.25 Best characterized among these cytokines is VEGF. During tumor progression, the level of circulating VEGF has been shown to rise, and this level was found to correlate with the number of EPCs in the circulation. Furthermore, PDGF-CC promoted vascularization in part by stimulating outgrowth of EPCs. In contrast, Ang-1 was shown to reduce EPC mobilization from bone marrow (reviewed in Ref. 26).
Figure 5.
Endothelial progenitor cells. Schematic representation of postnatal vasculogenesis. The term “EPC” encompasses a group of cells existing in a variety of stages ranging from common hemangioblasts to fully differentiated ECs. Although their putative precursors and the exact differentiation lineage of EPCs remain to be determined, to date it is widely accepted that early EPCs (localized in the bone marrow or immediately after migration into the circulation) are AC133+/CD34+/VEGFR-2+ cells, whereas circulating EPCs are positive for CD34 and VEGFR-2, lose AC133, and begin to express cell surface markers typical of mature ECs such as CD31, VE-cadherin, and von Willebrand Factor (vWF).
After homing, ie, after adhesion and insertion of EPCs into the monolayer of surrounding mature vascular ECs, additional local stimuli may promote the activation of local endothelium to express adhesion molecules to recruit EPCs. This process may be completed by mechanisms not yet elucidated. In addition to the physical contribution of EPCs to newly formed microvessels, the angiogenic cytokine release of EPCs may be a supportive mechanism to improve neovascularization as well.27 It is also important to note that Lyden et al recently identified VEGFR-1+ hematopoietic progenitor cells that multiply in the bone marrow, mobilize to the peripheral blood along with VEGFR-2+ EPCs, and incorporate into pericapillary connective tissue, thus stabilizing tumor vasculature.28 More interestingly, these cells seem to home in before the tumor cells arrive, promoting metastatic growth by forming niches where cancer cells can locate and proliferate.29
Although EPCs obviously participate in the vascularization process of malignant tumors, it is still unclear whether they are essential for these processes or what the relative contribution of EPCs is compared with that of in situ proliferating ECs. Moreover, it has yet to be determined whether EPCs can be targeted to treat certain types of malignancies, or alternatively—as they are endowed with the capacity to home to the tumor vasculature—can be used to deliver toxins or vascular-targeting agents.
Vasculogenic Mimicry
“Vasculogenic mimicry” is defined by the unique ability of aggressive melanoma cells to express an EC phenotype and to form vessel-like networks in three-dimensional culture, “mimicking” the pattern of embryonic vascular networks and recapitulating the patterned networks seen in patients’ aggressive tumors correlating with a poor prognosis.30 Comparative global gene analyses of aggressive and poorly aggressive human cutaneous and uveal melanoma cell lines unexpectedly revealed the ability of aggressive tumor cells to express genes (and proteins) associated with multiple cellular phenotypes and their respective precursor stem cells, including endothelial, epithelial, pericyte, fibroblast, and several other cell types.31–33 These new and intriguing findings support the premise that aggressive melanoma cells acquire a multipotent, plastic phenotypea concept that challenges our current thinking on how to target tumor cells that can possibly masquerade as other cell types, particularly with embryonic stem cell-like properties. The etiology of the melanoma vasculogenic phenotype remains unclear; however, it seems to involve dysregulation of the lineage-specific phenotype and the concomitant transdifferentiation of aggressive cancer cells into other cell types—such as endothelial-like cells. Vasculogenic mimicry has been confirmed in breast, prostate, ovarian, chorio-, and lung carcinomas; synovial-, rhabdomyo-, and Ewing sarcomas; and phaeochromocytoma.34 Expression profiling studies revealed that the most significantly up-regulated genes by aggressive melanoma cells include those that are involved in angiogenesis and vasculogenesis, such as the genes encoding VE-cadherin, erythropoietin-producing hepatocellular carcinoma-A2 (EphA2), MMPs, and laminin 5γ2 chain (LAMC2). These molecules, with their binding partners, are a few of the factors required for the formation and maintenance of blood vessels and also for vasculogenic mimicry in melanomas. Perhaps equally significant is the down-regulation of the gene MART-1 (melanoma antigen recognized by T cell 1, also called Melan-A), a classic marker for melanocytes and melanoma, by aggressive melanoma cells. The concept of vasculogenic mimicry was developed further to include the existence of a fluid-conducting, laminin-containing extracellular matrix meshwork, providing a site for nutritional exchange for aggressive tumors, and therefore possibly preventing necrosis (Figure 6).34,35 Functional studies revealed the close association of tumor-cell-lined networks with angiogenic mouse vessels at the human-mouse interface and the cooperation between the two systems.36,37 The molecular dissection of the physiological mechanisms critical to the function of the fluid-conducting meshwork revealed the biological relevance of the up-regulated expression of tissue factor pathway-associated genes—essential for the anticoagulation properties of the intratumoral, extracellular matrix-rich extravascular fluid-conducting pathway. Gene profiling, protein detection, and immunohistochemistry validation demonstrated up-regulation of tissue factor (TF), TF pathway inhibitor 1 (TFPI-1), and TFPI-2—critical genes that initiate and regulate the coagulation pathways—in aggressive, as opposed to poorly aggressive, melanoma. It was found that TFPI-2 contributes to vasculogenic mimicry and endothelial transdifferentiation by melanoma cells, whereas TFPI-1 has anticoagulant functions for perfusion of fluid-conduction meshworks formed by TF-expressing melanoma cells. Additional studies have focused on the signal transduction pathways that regulate blood vessel formation and stabilization during vasculogenesis and angiogenesis, addressing critical signaling events that regulate melanoma vasculogenic mimicry and their endothelia-like phenotype.38–40 It was demonstrated that VE-cadherin and EphA2 were co-localized in cell-cell junctions and VE-cadherin can regulate the expression of EphA2 at the cell membrane by mediating its ability to become phosphorylated through interactions with its membrane-bound ligand, ephrin-A1. These studies illuminate a novel signaling pathway that could be potentially exploited for therapeutic intervention. Additional investigation uncovered the role of phosphoinositide 3-kinase (PI3K) as a critical regulator of vasculogenic mimicry, specifically affecting membrane type-1 MMP (MT1-MMP) and MMP-2 activity. Both MMPs are essential for the process of vasculogenic network formation by aggressive melanoma tumor cells, and the downstream effect on the cleavage of laminin 5γ2 chain into the γ2′ and γ2x promigratory fragments.38,39 Furthermore, these results showed that blocking PI3K resulted in abrogation of vasculogenic mimicry. Most recent studies have identified focal adhesion kinase (FAK)-mediated signal transduction pathways to promote not only the aggressive phenotype but also vasculogenic mimicry of melanoma cells as well.40 In addition, expression of a negative regulator of FAK signaling, the FAK-related non-kinase in aggressive melanoma cells, resulted in an inhibition of melanoma vasculogenic mimicry concomitant with a decrease in melanoma cell invasion and migration. This biological effect was mediated in part through an extracellular signal-regulated kinase 1/2 signaling pathway that resulted in a down-regulation of urokinase and MMP-2/MT1-MMP activity.40 These results suggest that FAK may serve as a new target for therapeutic intervention in treating aggressive melanomas with capabilities for vasculogenic mimicry.
Figure 6.
Vasculogenic mimicry. This diagram represents the current interpretation of data generated from several studies involving the use of tracers and perfusion analyses of mice containing aggressive melanoma cells (green) during tumor development. The endothelial-lined vasculature is closely apposed to the tumor cell-formed fluid conducting meshwork, and hypothetically, it is presumed that as the tumor remodels, the vasculature becomes leaky, resulting in the extravascular conduction of plasma. There is also evidence of a physiological connection between the endothelial-lined vasculature and the extravascular melanoma meshwork.
Antivascular Therapy of Cancer
It has been over 30 years since Judah Folkman hypothesized that tumor growth is angiogenesis dependent.41 Subsequent research has led to the identification of several regulators of angiogenesis, some of which represent therapeutic targets. However, although antivascular agents are often highly active in preclinical studies, recent clinical trials including these agents have been both encouraging and disappointing. Because of the predominant role of capillary sprouting and its main molecular mediator VEGF in tumor vascularization, inhibition of VEGF seems to be necessary but is probably insufficient to halt tumor progression permanently in many cancer types. Due to the existence of multiple vascularization mechanisms and angiogenic signaling pathways, inhibition of just a single pathway will presumably trigger alternative vascularization mechanisms and additional growth factor pathways. Consequently, application of antivascular therapy in cancer patients requires the identification of the individual vascularization profile and the molecular machinery behind the vascularization process and, furthermore, the individualization of antivascular therapy to realize any potential benefits.42,43 In the second part of this review, we will briefly summarize the antivascular therapies that are currently being tested in the clinic. Subsequently, we will give an overview of how these classes of agents can be incorporated in the current multimodality of anticancer strategies. Finally, we will discuss potential novel approaches that enforce tumor regression by exploiting the emerging basic knowledge of tumor vascularization.
Antivascular Strategies in Cancer Therapy: Current Status of the Clinical Development
Any classification of antivascular strategies is difficult, with overlap in several features. However, the main categories of these approaches that have been developed are angiosuppressive (anti-angiogenic agents) and vascular-targeting therapies (vascular-disrupting agents).44 Although metronomic chemotherapy (MCT) uses conventional cytotoxic drugs, the main targets of this strategy are the tumor ECs. This is the reason that Browder et al45 coined the term “anti-angiogenic chemotherapy” to describe this treatment and why MCT is discussed here.
It is beyond the scope of this review to discuss all drugs that affect tumor capillaries. Therefore, we concentrate here on the agents that are at a more advanced stage of clinical development.
Angiosuppressive Therapy (Antiangiogenic Agents)
This approach is motivated by the fact that neoangiogenesis in cancer requires the induction of EC proliferation by specific or nonspecific mitogens. These agents target the production of endothelial mitogens, the mitogens themselves, their endothelial receptors, the associated signaling pathways, the endothelial integrins and the MMPs46 (Table 1). Consequently, it is most probable that angiosuppressive therapy can only be applied when cancer vascularization involves EC sprouting and/or postnatal vasculogenesis (Table 2).
Table 1.
Examples of Antivascular Agents in Clinical Development
| Agent | Target/mechanism of action | Phase |
|---|---|---|
| Angiosuppressive and anti-HIF-1 agents | ||
| Anti-VEGF agents | ||
| Bevacizumab | mAb against VEGF-A | III; FDA-approved in colorectal cancer |
| VEGF-trap | VEGF-A, PlGF, VEGF-B binding | I |
| VEGF-AS | VEGF-A, VEGF-C, VEGF-D | I |
| VEGFR and other TK receptor targeting agents | ||
| IMC-1C11 | mAb against VEGFR2 | I |
| ZD6474 | VEGFR-2, EGFR, RET | I/II |
| PTK787/ZK222584 (vatalanib) | VEGFR-1, -2, -3; PDGFR-β, c-Kit | II/III |
| BAY 43–9006 (sorafenib) | VEGFR-2, -3; PDGFR, RET, c-Kit, Raf | III; FDA-approved in renal cancer |
| SU11248 (sunitinib) | VEGFR-1, -2; PDGFR, FLT3, c-Kit | III; FDA-approved in renal cancer |
| AG-013736 | VEGFR-1, -2, -3; PDGFR-β, c-Kit | I/II |
| Angiozyme | VEGFR-1 mRNA-destroying ribozyme | I/II |
| Integrin signaling | ||
| EMD 121974 (Cilengitide) | Mimicking the RGD ligand recognition peptidic domain common to αv integrin ligands | I/II |
| MEDI-522 (Vitaxin) | mAb against αvβ3 | I/II |
| Miscellaneous | ||
| Thalidomide | Multiple inhibitory effect on bFGF, VEGF, and TNF-α-induced EC sprouting | I/II |
| AE-941 | Inhibitor of MMP-2, -9, -12, and VEGFR-2 | II/III |
| Marimastat | MMP2/9 | III |
| Bay-12-9566 | MMP2/9 | III |
| AG3340 | MMP2/9 | III |
| Endostatin | Integrin α5β1 | II/III |
| ABT-627 | Endothelin receptor | II/III |
| Nonselective inhibitors of HIF-1 | ||
| Topotecan and other camptothecin analogues, DX-2-1, GL331 | Topoisomerase I/II | Preclinical; phase I |
| 2-Methoxyestradiol | Microtubules | I |
| YC-1 | Soluble guanyl cyclase | Preclinical |
| PX-478 | Translation/deubiquitination | Preclinical |
| 17-AAG, geldanamycin, radicicol, KF58333 | HSP-90 | Preclinical |
| VDAs | ||
| Ligand-directed VDAs | ||
| L19 single chain Fv | ED-B domain of fibronectin | Preclinical |
| mAb against endoglin linked to ricin-A | Endoglin | Preclinical |
| Anti-VCAM-1 AB linked to coagulation inducing protein TF | VCAM-1 | Preclinical |
| Anti-TES-23 linked to neocarzinostatin | CD44-related EC marker | Preclinical |
| Naked AB against phosphatidylserine | Phosphatidylserine | Preclinical |
| αvβ3 targeting ligand delivering EC apoptosis inducing ATPμ-Raf | Targeted ATPμ-Raf gene | Preclinical |
| Small molecule VDAs | ||
| CA4-prodrug; AVE8062 and Oxi4503 (synthetic analogues of combretastatin) | Actin polymerization, filament stabilization via Rho signaling pathway | I/II |
| ZD6126 | Inhibition of EC microtubule polymerization | I |
| ABT-751 | β-Tubulin | I |
| DMXAA (analogue of flavone acetic acid) | TNF-α release, induction of nuclear factor-κβ | I/II |
mAb, monoclonal antibody; PlGF, placenta growth factor; bFGF, basic fibroblast growth factor; 17-AAG, 17-N-allylamino-17-demethoxygeldanamycin; VCAM-1, vascular cell adhesion molecule-1; CA4, combrestatin-A4.
Table 2.
Theoretical Strategy of Antivascular Therapy of Cancer According to the Stage of Tumor Progression and to the Mechanisms of Vascularization
| Type of vascularization | Individual cancer cells in host tissue | Microscopic tumor pre-angiogenic phase | Microscopic tumor angiogenic phase | Macroscopic tumor |
|---|---|---|---|---|
| Sprouting | Antiangiogenic agents; metronomic chemotherapy | Antiangiogenic agents; metronomic chemotherapy | Antiangiogenic agents; metronomic chemotherapy | Antiangiogenic agents; “vascular targeting” therapy; metronomic chemotherapy |
| Intussusceptive microvascular growth | N.A. | N.A. | Vascular targeting therapy; metronomic chemotherapy | Vascular targeting therapy; metronomic chemotherapy |
| Vessel co-option | N.A. | N.A. | Vascular targeting therapy | Vascular targeting therapy |
| Glomeruloid angiogenesis | N.A. | N.A. | Vascular targeting therapy | Vascular targeting therapy |
| Vasculogenic mimicry | N.A. | N.A. | Vascular targeting therapy; metronomic chemotherapy | Vascular targeting therapy; metronomic chemotherapy |
| Postnatal vasculogenesis (endothelial progenitors) | N.A. | N.A. | Antiangiogenic agents; vascular targeting therapy; metronomic chemotherapy | Antiangiogenic agents; vascular targeting therapy; metronomic chemotherapy |
N.A., not applicable.
Despite the promising preclinical results with these agents, in the early clinical trials positive responses in patients were rarely seen. The clinical breakthrough for angiosuppressive therapy came from a phase III trial demonstrating a significantly prolonged survival when bevacizumab, an anti-VEGF antibody, was used with chemotherapy in metastatic colorectal cancer patients.47 Based on these results, bevacizumab became the first antiangiogenic agent to be approved by the United States Food and Drug Administration (FDA) for cancer treatment. In subsequent phase III trials, bevacizumab in combination with standard chemotherapy improved overall survival in lung cancer patients and progression-free survival in breast cancer patients.42 In addition, it has been reported to be active in patients with metastatic renal-cell cancer as monotherapy (benefit in progression-free survival but not in overall survival).48
Further clinical success was obtained recently with broad-spectrum multitargeted agents that target VEGF receptors and other tyrosine kinases present in endothelial and cancer cells (Table 1). Phase III trials have demonstrated the efficacy of SU11248/sunitinib [targeting VEGFR-1, -2; platelet-derived growth factor receptor (PDGFR), FLT3, and c-Kit] and BAY-43-9006/sorafenib (targeting VEGFR-2, -3; PDGFR, RET, c-Kit, and Raf) in the treatment of patients with renal cancer.42 Based on these results, sunitinib and sorafenib are now approved by the FDA as monotherapies for kidney cancer. Promising results have also been found with the combination of ZD6474 [targeting VEGFR2, epidermal growth factor receptor (EGFR), and RET] and chemotherapy in nonsmall cell lung cancer patients. Interestingly, replacing bevacizumab with similar tyrosine kinase (TK) inhibitors, such as PTK787/ZK 222584/vatalanib (targeting VEGFR-1, -2, -3; PDGFR-β, and c-Kit), in the combined regimen did not result in similar efficacy in chemotherapy-naive or previously treated colorectal cancer patients.49 However, the clinical success of bevacizumab, sunitinib, and sorafenib as novel medicines for the treatment of cancer patients has confirmed the relevance of angiogenesis research and has stimulated the search for novel and more effective antiangiogenic approaches. Accordingly, various angiosuppressive strategies are being actively investigated, most of which are registered with the clinical trials database of the National Cancer Institute (http://www.nci.nih.gov/clinicaltrials).
Vascular Targeting Therapy (Vascular Disrupting Agents; VDAs)
Vascular targeting therapy (including anti-EC antibodies and ligand based and small molecule VDAs; Table 1) recognizes the fact that clinical diagnosis of cancer frequently occurs when the tumor tissue has already established its vasculature.44,46 This strategy relies on ability of VDAs to distinguish the ECs of tumor capillaries from normal ones based on their different phenotype, increased proliferative potential and permeability, and inherent dependence on the tubulin cytoskeleton. VDAs cause selective and rapid shutdown of the established tumor capillaries, resulting in extensive cancer cell death in the central areas of tumors, although they leave the perfusion in peripheral tumor regions relatively intact.44,50 It is evident from the mechanism of VDAs that the effects of these drugs do not depend on the type of vascularization occurring in a given cancer. Based on promising preclinical developments, several VDAs have entered clinical development.51
MCT and Its Antivascular Effects
Among the different antivascular strategies, MCT merits particular mention. MCT refers to the close, even daily, administration of chemotherapeutic drugs in doses below the maximum tolerated dose, over prolonged periods, and with no extended drug-free breaks. Phase II trials of MCT, sometimes applied in combination with antiangiogenic drugs, have yielded promising results in adult patients with advanced cancer.52,53 Furthermore, pediatric oncologists successfully use a metronomic-like modality of chemotherapies called “maintenance chemotherapy” to treat various pediatric malignancies such as acute lymphoblastic leukemia, neuroblastoma, or Wilms’ tumor; however, the anti-angiogenic background of maintenance chemotherapy is poorly described.54
Although cytotoxic effects of MCT in the tumor parenchyma could still contribute to the observed efficacy of metronomic regimens, preclinical studies suggest that the primary targets of MCT are the tumoral ECs. Low-dose chemotherapy affects tumor capillaries directly (growth arrest and apoptosis of activated ECs) but also induces the production of an angiogenesis inhibitor thrombospondin-1 and suppresses the mobilization of EPCs.52
As mentioned above, several phase I and II studies were performed involving low, continuous doses of cytotoxic drugs, with encouraging results.53 However, the clinical benefits of MCT remain to be validated in randomized prospective phase III trials. There is also a need for surrogate markers to help define the optimal dose of this approach. Circulating ECs55 and EPCs56 have been used successfully as markers in preclinical and early clinical studies but have not yet been validated clinically. Further challenges are the definition of valid clinical endpoints, the confirmation of long-term safety of MCT, and the identification of suitable antiangiogenic agents and VDAs to be combined with MCT. Finally, it will be important to determine the types of vascularization that might be the most responsive to this therapy. MCT is probably more effective in EC sprouting, postnatal vasculogenesis, IMG, and vasculogenic mimicry (Table 2). However, detailed clinicopathologic analysis is needed to confirm this hypothesis.
Considerations for Combination Treatment Strategies
Because antivascular agents and traditional anticancer strategies have distinctive target cells and mechanisms of action, it should be possible to achieve an increase in therapeutic efficacy with little or no increase in toxicity. In fact, although some antivascular agents have demonstrated activity as monotherapies, most human trials to date indicate that they are most effective when combined with conventional antitumor strategies, especially chemotherapy.42,43
Combination of Angiosuppressive and Chemo- and/or Radiation Therapy
Angiosuppressive therapy reduces cancer growth by suspending the blood supply, resulting in hypoxia. Because hypoxia itself is a major cause of ineffective chemo-irradiation therapy,57 one would expect that a further decrease in intratumoral oxygen levels would deteriorate the efficacy of a cytotoxic regime, but experimental and clinical data do not support this scenario. In several preclinical models, a combination of cytotoxic drugs (taxanes, cisplatin, or 5-fluorouracil) with angiogenesis inhibitors (TNP470, endostatin, SU11248) produced at least additive but in certain cases synergistic antitumoral effects.46 Thalidomide, a still ill-defined angiogenesis inhibitor, has also been shown successful preclinically in combination with standard anticancer regimes in solid tumors.58 In addition to experimental data, there are now clinical examples of the improved efficacy of chemotherapy in combination with an angiosuppressive agent. As mentioned above, bevacizumab in combination with chemotherapy improved overall survival in colorectal and lung cancer patients and progression-free survival in breast cancer patients (see review42). In addition, the combination of bevacizumab and chemotherapy was found to be active in pancreatic59 and ovarian60 cancer patients.
There are several explanations for the improved efficacy. An obvious effect of angiogenesis inhibitors is the decrease in interstitial pressure in cancer tissue improving the delivery of cytotoxic agents. Furthermore, a hypothesis called “normalization of tumor vasculature” was put forth by Jain and colleagues recently to explain the clinical effects of antiangiogenic agents.42 According to this theory, tumor vasculature is structurally insufficient to provide maximal blood supply for cancer cells as a result of capillary leakiness and tortuosity. Because the key regulator cytokine family of tumoral vessels is the VEGF/VEGFR system, targeting it could potentially help in the “normalization” of tumor vasculature and in the improvement of the delivery of chemotherapeutic agents.42 Accordingly, recent experimental data indicate that anti-VEGF therapy induces rapid alterations in tumor vasculature. Within a few hours, EC proliferation is halted, luminal stability vanishes, and circulation ceases in tumor capillaries. Some ECs undergo apoptosis and disappear. Remaining capillaries lack endothelial fenestrations and have reduced VEGFR-2 and VEGFR-3 expression.61 Thus, inhibition of VEGF signaling devastates some tumor capillaries and transforms others into a more normal phenotype.42
Further mechanisms for the additional benefits experienced for combined chemo- and angiosuppressive therapy might be the direct killing of proliferating ECs and/or the inhibition of the mobilization/viability of EPCs by cytotoxic drugs. Results of preclinical studies support this hypothesis. On the other hand, VEGF inhibition might have direct cytotoxic effects on tumor cells that aberrantly express VEGF receptors and depend to some extent on VEGF for their survival. Finally, it has also been suggested recently that antiangiogenic agents prevent rapid cancer cell repopulation during the break periods between courses of chemotherapy (see review43).
Experimental studies indicate that antiangiogenic therapy in combination with irradiation is an encouraging concept for the improvement of the radiation response of tumors.62 In addition, recent discoveries show that the EC layer of the tumor vessels is one key target of radiotherapy.63 In fact, the antivascular effect of radiotherapy predicts its anti-cancer effect.64 Thus far, although early phase human trials have also yielded promising results, there are no large phase III trials known in which such combinations were successfully applied. Nevertheless, the discovery of the “normalization window” of angiosuppressive agents when combined with radiotherapy in preclinical models65 suggests that it would be as difficult to design a successful combination strategy with radiation as with chemotherapy.
In this normalization window (the time period during which the vasculature normalizes and hypoxia decreases), the antiangiogenic drugs improve the efficacy of chemoradiotherapy.42 Although these studies were performed in experimental tumor systems, one may expect a similar effect on the human tumor vasculature and oxygenation. However, intratumoral hypoxia, responsible for chemo- and radiotherapy resistance and triggering molecular pathways that promote cancer progression, is due not only to the inefficient blood supply by the abnormal tumor vessels but to the systemic anemia of the host as well.66 Unfortunately, although the oxygen tension of experimental tumors tends to rise with increasing Hb levels67 and treatment with recombinant human erythropoietin (rHuEpo) significantly reduces the risk for red blood cell transfusions in cancer patients, correction of anemia with rHuEpo does not necessarily improve survival of cancer patients.66 The issue of Epo/EpoR co-expression in tumor cells and EpoR expression in ECs is critical in this perspective. The expression of EpoR in tumor cells has raised the possibility that exogenous rHuEPO may directly influence cancer cell proliferation, apoptosis, or sensitivity to chemoradiation therapy. In addition, the EpoR expression in ECs has suggested potential effects of Epo on the tumor capillaries, such as the stimulation of angiogenesis.68 However, as it has been suggested by experimental studies, the overall direct effect of Epo-EpoR signaling on tumor progression and therapy is not a straightforward one. For instance, rHuEpo administration has recently been shown to be associated with decreased intratumoral VEGF expression, remodeling of tumor capillaries, and increased chemosensitivity to 5-fluorouracil treatment of human tumor xenografts.69 In a preclinical myeloma model, rHuEpo induced tumor regression and antitumor immune responses.70 In addition, human kidney carcinoma and myelomonocytic leukemia cell lines treated with rHuEpo exhibited an increase in apoptosis in response to chemotherapy.71 Overall, these findings warrant additional experimental and clinical research of rHuEpo to clarify further the risks of its use as well as optimize its known or potential benefits.
Combination of VDAs and Chemo- and/or Radiation Therapy
VDAs work best in the poorly perfused hypoxic central tumor areas, leaving a viable rim of well-perfused cancer tissue at the periphery, which rapidly regrows.50 Consequently, responses of tumors to VDAs given as single agents have been poor; however, combination therapy with chemoradiotherapy, which targets cancer cells at the tumor periphery, has produced promising responses in preclinical models. Nevertheless, the timing and sequencing of VDAs and chemo-irradiation therapies are important in such treatments. By far the greatest enhancement was observed when the VDA was administered within a few hours after chemo- and/or irradiation therapy. Based on these experimental results, the VDA compounds 5,6-dimethylxantlenone-4-acetic acid (DMXAA) and combretastatin A4 phosphate (CA4P) are being evaluated in human phase II trials in combination with conventional anticancer therapies.51
Combination of Angiosuppressive and Vascular Disrupting Agents
Because both angiogenesis and the integrity of the existing vasculature are critical to tumor progression and survival, dual targeting of the tumor vasculature would seem to have considerable promise. Preclinical results demonstrated that this strategy could significantly enhance therapeutic response beyond that achieved with either antivascular agent alone.51 One example of this strategy is the combination of the inhibitor of VEGFR2-associated TK ZD6474 with the microtubulin-disrupting VDA ZD6126.72 Further combinations that are under preclinical testing include the combination of OXi-4503, CA4P, and DMXAA with bevacizumab. Clinical testing of combined antivascular therapy has started with the recent initiation of a phase I human trial combining CA4P with bevacizumab.51
Theoretical Considerations for Designing Antivascular Therapy of Cancer
From the discussion above it is clear that the combination of either angiosuppressive or the vascular disrupting therapies with conventional chemoradiotherapy of cancer is highly problematic and must be carefully designed in cases where the sequence of the multiple types of agents might be critical. The molecular machinery behind the vascularization process and type of tumor vascularization are further issues that have to be taken into account. Thus, an efficient antivascular cancer therapy could be designed based on the identification of the molecular targets of the angiogenic geno-/phenotype (molecular pathway-based approach) or on the vascularization mechanism (vascular mechanism-based approach). However, it is most probable that the two approaches would have to be combined. We propose below a rationale for the design of antivascular strategies with the aim that such consideration may help to improve the clinical efficacy of these novel therapies.
Molecular Pathway-Based Antivascular Therapy of Cancer
Because of its pivotal role in neovascularization, the VEGF/VEGFR axis has been a major target of basic and clinical research. It is, therefore, not surprising that most of the antivascular strategies currently in clinical development focus on inhibition of VEGF signaling.46,73 However, the development of the angiogenic phenotype of cancer is characterized by several interconnected pathways. One of the major triggers of this phenotype is tissue hypoxia, which is responsible for the activation of gene expression of angiogenic cytokines through up-regulation of the transcription factor hypoxia inducible factor-1α (HIF1-α). Nevertheless, HIF-1 may already be active in particular cancers due to hyperactive growth factor signaling or genetic alteration of the HIF1α gene itself or its regulators [ie, von Hippel-Lindau (VHL) and p53].74 Because HIF-1 plays such a central role in triggering numerous pathways responsible for cancer progression, disruption of the HIF-1-mediated pathways is expected to cause cancer cell death due to a combination of metabolic dysregulation and reduced microvessel growth. The aim of anti-HIF-1 therapy (used as an antivascular modality) therefore might be to cause the angiogenic phenotype of cancer to revert to a less angiogenic one, thereby preventing the production of the major angiogenic cytokines.75 HIF1α can be inhibited by guanyl cyclase or HSP90 inhibitors and even by the targeting of topoisomerase-1, and several of such agents are in clinical trials (Table 1). However, none of the currently available inhibitors seems to disrupt the HIF-1 pathway as their exclusive target.75 If the additional targets of nonselective HIF-1 inhibitors are also involved in cancer progression, these agents could be therapeutically beneficial, but inhibition of the pathways involved in normal cellular homeostasis could result in an unacceptable toxicity profile. Therefore, the design of more specific HIF-1 targeting agents is the focus of current research efforts. However, it is also important to note that HIF-1 targeting alone may not be enough to halt angiogenesis and tumor progression, as HIF-independent pathways may bypass or overcome HIF inhibition. Consequently, a combination of anti-HIF agents with conventional anticancer modalities or other molecular-targeted drugs may be required.
VEGF expression is not only associated with hypoxia or VHL mutations but also is influenced by a broad spectrum of onco- and tumor suppressor genes. A growing body of evidence suggests that inactivation of tumor suppressor genes such as p53 and PTEN and activation of oncogenes such as Ras, c-Src, EGFR, human epidermal growth factor receptor 2 (HER-2), FBJ murine osteosarcoma viral oncogene homolog (FOS), neurotrophic receptor tyrosine kinase B (trkB), V-p3K, and Bcl-2 are connected to the up-regulation of VEGF. Consequently, molecular targeting of these regulators is also a potential strategy for indirectly modulating the VEGF/VEGFR axis.73 For example, based on the results of recent clinical trials, cetuximab (a monoclonal antibody that binds to EGFR with high specificity) induces a significant decrease in circulating VEGF levels in colon cancer patients,76 or likewise, imatinib mesylate (a specific inhibitor of Bcr/Abl protein TK activity) reduces VEGF plasma concentration77 and bone marrow microvessel densities78 in patients with chronic myeloid leukemia. However, preclinical and early phase clinical data demonstrate that the addition of anti-VEGF therapy to anti-EGFR therapies generates further beneficial effects on angiogenesis inhibition and tumor reduction.42 This suggests that inhibiting upstream signaling of VEGF does not necessarily provide the same benefit as the direct targeting of it and, more importantly, that the dual targeting of cancer and endothelial cells might become a successful practice in clinical oncology.
Mechanism-Based Antivascular Therapy of Cancer
A proposal for the application of antivascular therapies according to the alternative vascularization mechanisms in cancer is summarized in Table 2. Probably the most important aspect of mechanism-based antivascular therapy is its strict dependence on the stage of tumor progression. Interestingly, antivascular therapy may have an effect at the very early stages of tumor growth. This idea was put forward by Li et al,79 who analyzed the earliest events that take place during the onset of tumor neovascularization and found that individual tumor cells exhibited a chemotaxis-like growth pattern toward the host vasculature. When the tumor cell population reached approximately 60 to 80 cells, clear evidence of perivascular tumor cell migration (ie, vessel co-option), and host vessel dilation was observed. Moreover, in a mouse model of glomeruloid angiogenesis, our group found that even single tumor cells can induce radical changes in the host tissue vasculature24 (Figure 4). These observations are important in two ways. First, they suggest that anti-invasive agents (which are not yet available clinically) may have a therapeutic effect on the interaction between cancer and endothelial cells and, consequently, on the processes of vessel co-option and glomeruloid angiogenesis. Second, the finding that single tumor cells can induce increased capillary permeability/tortuosity highlights the need for application of angiosuppressive/antiangiogenic therapy at the very early stages of cancer progression. These considerations may be true for the next step of tumorigenesis (pre-angiogenic phase) as well.
After the onset of “angiogenic switch,” elevated serum levels of angiogenic growth factors in cancer patients may activate and mobilize EPCs to support local microvessel growth.26 If we accept this assumption, then, in addition to angiogenesis inhibitors and metronomic chemotherapy,52 ligand-based, EPC-specific VDAs may also be useful in eliminating circulating EPCs throughout the further stages of tumor progression (Table 2). Furthermore, because IMG can be effective only in tumor capillary networks already built by other vascularization mechanisms (mainly sprouting and vessel co-option), steps should be taken to impede the additional increase in the density of the tumor tissue capillary bed following the angiogenic switch. This could be achieved by the use of VDAs and/or “metronomic chemotherapy,” which both target the cytoskeleton of ECs responsible for the remodeling of capillary walls.
Because ischemic milieu is what forces aggressive tumor cells to express endothelial genes and form vascular channels,33,80 the initiation of this mechanism is most likely simultaneous with the angiogenic switch. Therefore, when vasculogenic mimicry plays a role in the nutrient supply in cancer, besides the use of ligand-based VDAs against cancer cells with endothelial phenotype, targeting those pathways responsible for the development of this mechanism such as Eph2A, PI3K, or FAK seems to be an appropriate strategy. On the other hand, metronomic scheduling of chemotherapy52 may also effectively target cancer cells with vasculogenic geno/phenotype when both physiological angiogenesis inhibitors and angiosuppressive drugs are unable to modify this vascularization mechanism.80
The next stage of malignant progression is when tumor tissue reaches macroscopic size detectable by simple or sophisticated imaging techniques. As we know, for cancer survival “the edge is the future and the center is history,”81 because active tumor vascularization processes, resulting in vascular networks built by defective new capillaries, occur mainly, though not exclusively, at the tumor periphery. Consequently, at this stage the main target of antivascular therapies is the invading front of the cancer tissue. However, since in addition to causing chemo- and radiotherapy resistance, reduction of vascularity in the center of tumors can lead to the appearance of more aggressive/highly metastatic hypoxia-resistant cancer cells and to the induction of vasculogenic mimicry, when designing antivascular strategies central tumor areas cannot be neglected. We should emphasize, therefore, that in the case of clinically detectable tumors the whole range of antivascular weapons should be used theoretically. Although antiangiogenic agents targeting proliferating ECs could possibly be the key drugs at the tumor boundary, established tumor vasculature might well be attacked by VDAs and/or metronomic chemotherapy in the central tumor areas. Altogether, it seems feasible that antivascular therapy in tumors can only be successful if the entire vascular network and all of the possible vascularization mechanisms are targeted and, furthermore, if the phenotypic analysis of tumor capillaries/vascular channels is adequately performed.
Conclusion
Although tumors, as other tissues, require a vessel network supplying them with blood, tumor vasculature is not necessarily derived by EC proliferation and sprouting of new capillaries. In addition to alternative vascularization mechanisms, the novel antivascular strategies must be harmonized with the stage of tumor progression and with the molecular mechanism responsible for the angiogenic phenotype. A further challenge is to combine antivascular strategies with the existing therapeutic regimes in at least an additive manner. We have provided here proposals for a rational application of antivascular agents with the notion that these therapies have to be individually tailored in a given cancer type. Better understanding of the different vascularization mechanisms of the various cancer types will certainly help to fine-tune these novel anti-cancer strategies.
Acknowledgments
We are grateful to Sirpa Jalkanen (Medicity, University of Turku, Turku, Finland) and to Sándor Eckhardt (National Institute of Oncology, Budapest, Hungary) for critique and suggestions.
Footnotes
Address reprint requests to József Tímár, M.D., Ph.D., Department of Tumor Progression, National Institute of Oncology, Rath Gy.7-9, Buda-pest, H-1122 Hungary. E-mail: jtimar@oncol.hu.
Supported by grants from the Ministry of Economy GVOP-KMA-0040-2004 (J.Tí.) and KFIIF-00063/2005 (B.D.); Ministry of Health ETT-410/2006 (B.D.), ETT-383/2006 (S.P.); National Science TS49887, D048519, F046501 (J.Tó.); Ministry of Education NKFP1a-0024-05 (J.Tí.); and National Institutes of Health/National Cancer Institute grants CA59702 and CA80318 (M.J.C.H.).
The authors indicate no potential conflicts of interest.
References
- Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977;14:53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- Paku S, Paweletz N. First steps of tumor-related angiogenesis. Lab Invest. 1991;65:334–346. [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: a sticky business. Exp Cell Res. 2006;312:651–658. doi: 10.1016/j.yexcr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Thompson WD, Shiach KJ, Fraser RA, McIntosh LC, Simpson JG. Tumours acquire their vasculature by vessel incorporation, not vessel ingrowth. J Pathol. 1987;151:323–332. doi: 10.1002/path.1711510413. [DOI] [PubMed] [Google Scholar]
- Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- Döme B, Paku S, Somlai B, Timar J. Vascularization of cutaneous melanoma involves vessel co-option and has clinical significance. J Pathol. 2002;197:355–362. doi: 10.1002/path.1124. [DOI] [PubMed] [Google Scholar]
- Pezzella F, Pastorino U, Tagliabue E, Andreola S, Sozzi G, Gasparini G, Menard S, Gatter KC, Harris AL, Fox S, Buyse M, Pilotti S, Pierotti M, Rilke F. Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am J Pathol. 1997;151:1417–1423. [PMC free article] [PubMed] [Google Scholar]
- Vermeulen PB, Colpaert C, Salgado R, Royers R, Hellemans H, Van Den Heuvel E, Goovaerts G, Dirix LY, Van Marck E. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J Pathol. 2001;195:336–342. doi: 10.1002/path.966. [DOI] [PubMed] [Google Scholar]
- Paku S, Lapis K. Morphological aspects of angiogenesis in experimental liver metastases. Am J Pathol. 1993;143:926–936. [PMC free article] [PubMed] [Google Scholar]
- Paku S, Kopper L, Nagy P. Development of the vasculature in “pushing-type” liver metastases of an experimental colorectal cancer. Int J Cancer. 2005;115:893–902. doi: 10.1002/ijc.20886. [DOI] [PubMed] [Google Scholar]
- Paku S, Bodoky G, Kupcsulik P, Timar J. Blood supply of metastatic hepatic tumors: suggestions for improved delivery of chemotherapeutic agents. J Natl Cancer Inst. 1998;90:936–937. doi: 10.1093/jnci/90.12.936. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118:771–780. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18:5356–5362. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Kurz H, Burri PH, Djonov VG. Angiogenesis and vascular remodeling by intussusception: from form to function. News Physiol Sci. 2003;18:65–70. doi: 10.1152/nips.01417.2002. [DOI] [PubMed] [Google Scholar]
- Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol. 2002;158:773–785. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brat DJ, Van Meir EG. Glomeruloid microvascular proliferation orchestrated by VPF/VEGF: a new world of angiogenesis research. Am J Pathol. 2001;158:789–796. doi: 10.1016/S0002-9440(10)64025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straume O, Chappuis PO, Salvesen HB, Halvorsen OJ, Haukaas SA, Goffin JR, Begin LR, Foulkes WD, Akslen LA. Prognostic Importance of Glomeruloid Microvascular Proliferation Indicates an Aggressive Angiogenic Phenotype in Human Cancers. Cancer Res. 2002;62:6808–6811. [PubMed] [Google Scholar]
- Sundberg C, Nagy JA, Brown LF, Feng D, Eckelhoefer IA, Manseau EJ, Dvorak AM, Dvorak HF. Glomeruloid microvascular proliferation follows adenoviral vascular permeability factor/vascular endothelial growth factor-164 gene delivery. Am J Pathol. 2001;158:1145–1160. doi: 10.1016/S0002-9440(10)64062-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döme B, Timar J, Paku S. A novel concept of glomeruloid body formation in experimental cerebral metastases. J Neuropathol Exp Neurol. 2003;62:655–661. doi: 10.1093/jnen/62.6.655. [DOI] [PubMed] [Google Scholar]
- Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol. 2004;287:C572–C579. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- Garmy-Susini B, Varner JA. Circulating endothelial progenitor cells. Br J Cancer. 2005;93:855–858. doi: 10.1038/sj.bjc.6602808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniotis AJ, Folberg R, Hess AR, Seftor EA, Gardner LMG, Pe’er J, Trent JM, Meltzer PS, Hendrix MJC. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic Mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seftor EA, Meltzer PS, Kirschmann DA, Pe’er J, Maniotis AJ, Trent JM, Folberg R, Hendrix MJC. Molecular determinants of human uveal melanoma invasion and metastasis. Clin Exp Metast. 2002;19:233–246. doi: 10.1023/a:1015591624171. [DOI] [PubMed] [Google Scholar]
- Seftor EA, Meltzer PS, Schatteman GC, Gardner LMG, Hess AR, Kirschmann DA, Seftor REB, Hendrix MJC. Expression of multiple molecular phenotypes by aggressive melanoma tumor cells: role of vasculogenic mimicry. Crit Rev Oncol Hematol. 2002;44:17–27. doi: 10.1016/s1040-8428(01)00199-8. [DOI] [PubMed] [Google Scholar]
- Hendrix MJC, Seftor EA, Hess AR, Seftor REB. The molecular plasticity of human melanoma cells. Oncogene. 2003;22:3070–3075. doi: 10.1038/sj.onc.1206447. [DOI] [PubMed] [Google Scholar]
- Hendrix MJC, Seftor EA, Hess AR, Seftor REB. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nature Rev Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Chen X, Garcia C, DeChristopher PJ, Wu D, Pe’er J, Folberg R. Control of melanoma morphogenesis, endothelial survival, and perfusion by extracellular matrix. Lab Invest. 2002;82:1031–1043. doi: 10.1097/01.lab.0000024362.12721.67. [DOI] [PubMed] [Google Scholar]
- Hendrix MJC, Seftor EA, Meltzer PS, Hess AR, Gruman LM, Nickoloff BJ, Miele L, Sheriff DD, Schatteman GC, Bourdon MA, Seftor REB. The stem cell plasticity of aggressive melanoma tumor cells. Sell ES, editor. Totowa, NJ,: Humana Press, Inc.,; Germinal Stem Cells. 2004:pp 297–306. [Google Scholar]
- Ruf W, Seftor EA, Prtrovan R, Weiss RM, Gruman LM, Margaryan NV, Seftor REB, Miyagi Y, Hendrix MJC. Differential role of tissue factor pathway inhibitor-1 and 2 (TFPI-1 and 2) in melanoma vasculogenic mimicry. Cancer Res. 2003;63:5381–5389. [PubMed] [Google Scholar]
- Walker-Daniels J, Hess AR, Hendrix MJ, Kinch MS. Differential regulation of EphA2 in normal and malignant cells. Am J Pathol. 2003;162:1037–1042. doi: 10.1016/S0002-9440(10)63899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess AR, Seftor EA, Seftor REB, Hendrix MJC. Phosphoinositide 3-kinase regulates MT1-MMP and MMP-2 activity during melanoma cell vasculogenic mimicry. Cancer Res. 2003;63:4757–4762. [PubMed] [Google Scholar]
- Hess AR, Postovit LM, Margaryan NV, Seftor EA, Schneider GB, Seftor REB, Nickoloff BJ, Hendrix MJC. Focal adhesion kinase promotes aggressive melanoma phenotype. Cancer Res. 2005;65:9851–9860. doi: 10.1158/0008-5472.CAN-05-2172. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science. 2006;312:1171–1175. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]
- Siemann DW, Bibby MC, Dark GG, Dicker AP, Eskens FA, Horsman MR, Marme D, Lorusso PM. Differentiation and definition of vascular-targeted therapies. Clin Cancer Res. 2005;11:416–420. [PubMed] [Google Scholar]
- Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- Gasparini G, Longo R, Fanelli M, Teicher BA. Combination of antiangiogenic therapy with other anticancer therapies: results, challenges, and open questions. J Clin Oncol. 2005;23:1295–1311. doi: 10.1200/JCO.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito A, De Maio E, Di Maio M, Normanno N, Perrone F. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions. Oncologist. 2006;11:753–764. doi: 10.1634/theoncologist.11-7-753. [DOI] [PubMed] [Google Scholar]
- Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5:423–435. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- Chaplin DJ, Horsman MR, Siemann DW. Current development status of small-molecule vascular disrupting agents. Curr Opin Investig Drugs. 2006;7:522–528. [PubMed] [Google Scholar]
- Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- Baruchel S, Stempak D. Low-dose metronomic chemotherapy: myth or truth? Onkologie. 2006;29:305–307. doi: 10.1159/000093971. [DOI] [PubMed] [Google Scholar]
- Kamen BA, Glod J, Cole PD. Metronomic therapy from a pharmacologist’s view. J Pediatr Hematol Oncol. 2006;28:325–327. doi: 10.1097/00043426-200606000-00001. [DOI] [PubMed] [Google Scholar]
- Mancuso P, Colleoni M, Calleri A, Orlando L, Maisonneuve P, Pruneri G, Agliano A, Goldhirsch A, Shaked Y, Kerbel RS, Bertolini F. Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood. 2006;108:452–459. doi: 10.1182/blood-2005-11-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaked Y, Emmenegger U, Man S, Cervi D, Bertolini F, Ben-David Y, Kerbel RS. Optimal biologic dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood. 2005;106:3058–3061. doi: 10.1182/blood-2005-04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P, Kelleher DK, Hockel M. Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol. 2001;28(Suppl 8):29–35. doi: 10.1016/s0093-7754(01)90210-6. [DOI] [PubMed] [Google Scholar]
- Sleijfer S, Kruit WH, Stoter G. Thalidomide in solid tumours: the resurrection of an old drug. Eur J Cancer. 2004;40:2377–2382. doi: 10.1016/j.ejca.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Kindler HL, Friberg G, Singh DA, Locker G, Nattam S, Kozloff M, Taber DA, Karrison T, Dachman A, Stadler WM, Vokes EE. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033–8040. doi: 10.1200/JCO.2005.01.9661. [DOI] [PubMed] [Google Scholar]
- Cohn DE, Valmadre S, Resnick KE, Eaton LA, Copeland LJ, Fowler JM. Bevacizumab and weekly taxane chemotherapy demonstrates activity in refractory ovarian cancer. Gynecol Oncol. 2006;102:134–139. doi: 10.1016/j.ygyno.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15:102–111. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nieder C, Wiedenmann N, Andratschke N, Molls M. Current status of angiogenesis inhibitors combined with radiation therapy. Cancer Treat Rev. 2006;32:348–364. doi: 10.1016/j.ctrv.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- Lövey J, Lukits J, Remenar E, Koronczay K, Kasler M, Nemeth G, Timar J. Antiangiogenic effects of radiotherapy but not initial microvessel density predict survival in inoperable oropharyngeal squamous cell carcinoma. Strahlenther Onkol. 2006;182:149–156. doi: 10.1007/s00066-006-1447-6. [DOI] [PubMed] [Google Scholar]
- Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Bohlius J, Weingart O, Trelle S, Engert A. Cancer-related anemia and recombinant human erythropoietin–an updated overview. Nat Clin Pract Oncol. 2006;3:152–164. doi: 10.1038/ncponc0451. [DOI] [PubMed] [Google Scholar]
- Boogaerts M, Mittelman M, Vaupel P. Beyond anaemia management: evolving role of erythropoietin therapy in neurological disorders, multiple myeloma and tumour hypoxia models. Oncology. 2005;69(Suppl 2):22–30. doi: 10.1159/000088285. [DOI] [PubMed] [Google Scholar]
- Hardee ME, Arcasoy MO, Blackwell KL, Kirkpatrick JP, Dewhirst MW. Erythropoietin biology in cancer. Clin Cancer Res. 2006;12:332–339. doi: 10.1158/1078-0432.CCR-05-1771. [DOI] [PubMed] [Google Scholar]
- Tóvári J, Gilly R, Raso E, Paku S, Bereczky B, Varga N, Vago A, Timar J. Recombinant human erythropoietin alpha targets intratumoral blood vessels, improving chemotherapy in human xenograft models. Cancer Res. 2005;65:7186–7193. doi: 10.1158/0008-5472.CAN-04-2498. [DOI] [PubMed] [Google Scholar]
- Mittelman M, Neumann D, Peled A, Kanter P, Haran-Ghera N. Erythropoietin induces tumor regression and antitumor immune responses in murine myeloma models. Proc Natl Acad Sci USA. 2001;98:5181–5186. doi: 10.1073/pnas.081275298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho G, Lefaucheur C, Cherbonnier C, Metivier D, Chapel A, Pallardy M, Bourgeade MF, Charpentier B, Hirsch F, Kroemer G. Chemosensitization by erythropoietin through inhibition of the NF-kappaB rescue pathway. Oncogene. 2005;24:737–745. doi: 10.1038/sj.onc.1208205. [DOI] [PubMed] [Google Scholar]
- Shi W, Siemann DW. Targeting the tumor vasculature: enhancing antitumor efficacy through combination treatment with ZD6126 and ZD6474. In Vivo. 2005;19:1045–1050. [PubMed] [Google Scholar]
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–84. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- Welsh SJ, Koh MY, Powis G. The hypoxic inducible stress response as a target for cancer drug discovery. Semin Oncol. 2006;33:486–497. doi: 10.1053/j.seminoncol.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Vincenzi B, Santini D, Russo A, Silletta M, Gavasci M, Battistoni F, Di Cuonzo G, Rocci L, Gebbia N, Tonini G. Angiogenesis modifications related with cetuximab plus irinotecan as anticancer treatment in advanced colorectal cancer patients. Ann Oncol. 2006;17:835–841. doi: 10.1093/annonc/mdl031. [DOI] [PubMed] [Google Scholar]
- Legros L, Bourcier C, Jacquel A, Mahon FX, Cassuto JP, Auberger P, Pages G. Imatinib mesylate (STI571) decreases the vascular endothelial growth factor plasma concentration in patients with chronic myeloid leukemia. Blood. 2004;104:495–501. doi: 10.1182/blood-2003-08-2695. [DOI] [PubMed] [Google Scholar]
- Kvasnicka HM, Thiele J, Staib P, Schmitt-Graeff A, Griesshammer M, Klose J, Engels K, Kriener S. Reversal of bone marrow angiogenesis in chronic myeloid leukemia following imatinib mesylate (STI571) therapy. Blood. 2004;103:3549–3551. doi: 10.1182/blood-2003-08-2734. [DOI] [PubMed] [Google Scholar]
- Li CY, Shan S, Huang Q, Braun RD, Lanzen J, Hu K, Lin P, Dewhirst MW. Initial stages of tumor cell-induced angiogenesis: evaluation via skin window chambers in rodent models. J Natl Cancer Inst. 2000;92:143–147. doi: 10.1093/jnci/92.2.143. [DOI] [PubMed] [Google Scholar]
- van der Schaft DWJ, Seftor EA, Hess AR, Gruman LM, Kirschmann DA, Yokoyama Y, Seftor REB, Griffioen AW, Hendrix MJC. Differential effects of angiogenesis inhibitors on vascular network formation by endothelial and melanoma cells. J Natl Cancer. 2004;96:1473–1477. doi: 10.1093/jnci/djh267. [DOI] [PubMed] [Google Scholar]
- Thompson WD. Tumour versus patient: vascular and tumour survival versus prognosis. J Pathol. 2001;193:425–426. doi: 10.1002/1096-9896(200104)193:4<425::AID-PATH830>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]