Abstract
Repeated inflammation in the heart is one of the initiation factors of dilated cardiomyopathy (DCM). In a previous study, we established a new animal model for DCM by immunization of rats with recombinant cardiac C-protein fragment 2 (CC2). The present study examined factors involved in the development of DCM. Analysis using overlapping peptides revealed that the major carditogenic epitope resides only in the residue 615–647 [CC2 peptide 12 (CC2P12)]. However, immunization with CC2P12 induced moderate inflammation without subsequent DCM. CDR3 spectratyping analysis of the T-cell repertoire demonstrated that Vβ4-positive T cells were preferentially expanded in both CC2- and CC2P12-immunized rats. Although there was no significant difference in the T-cell characteristics, examinations of the B-cell epitope revealed that marked epitope spreading occurred in CC2-immunized but not CC2P12-immunized rats from 4 weeks after immunization. Consistent with this finding, immunization with CC2P12 and simultaneous transfer of anti-peptide antisera induced significantly more severe inflammation and fibrosis than CC2P12 immunization alone. However, the transfer of the antisera without CC2P12 immunization did not induce any pathology. These findings suggest that T-cell activation and B-cell epitope spreading in the CC2 molecule is a key step for the switch from myocarditis to the development of DCM.
Dilated cardiomyopathy (DCM) is a serious and frequently fatal disorder and is a common cause of heart failure. The majority of DCM is sporadic, and mostly virus-induced immune mechanisms are suspected.1 Because the heart biopsy sometimes demonstrates the presence of inflammation, several immunosuppressive therapies have been tried to improve the status of DCM.2–4 However, significant progress has not been made, although these therapies have shown some improvements of the disease. Difficulties in finding effective therapies are mainly based on the fact that the pathogenesis of DCM is still poorly understood. The establishment of a suitable animal model that mimics human DCM and elucidation of pathogenesis of DCM will provide useful information for the development of effective therapies.
In a previous study, we demonstrated that cardiac C-protein, one of the myosin-binding proteins, induced severe experimental autoimmune carditis (EAC) and subsequent DCM in Lewis rats.5 Seventy-five percent of rats immunized with C-protein died by day 50, and all of the survivors developed DCM. Furthermore, it was revealed that cytokines and chemokines produced by T cells and macrophages were up-regulated in the heart lesions, mainly during the inflammatory phase of EAC. These findings suggest that pathogenic T cells and possibly B cells play an important role in the development of EAC and subsequent DCM.
In the present study, we first examined the carditogenic epitopes that reside in the cardiac C-protein fragment 2 (CC2) (corresponding to amino acid residues 317–647). Using overlapping peptides, we found that only peptide 12 (CC2P12) possessed the carditis-inducing ability in the CC2 molecule. Interestingly, CC2P12 induced nonfatal moderate EAC and did not develop DCM. Analysis of clonally expanded T cells in CC2- and CC2P12-immunized rats demonstrated that there was no significant difference between the two groups. In contrast, CC2-immunized rats exhibited marked B-cell epitope spreading 4 weeks after immunization and afterward, whereas CC2P12-immunized rats raised antibodies only against CC2P12 and CC2. Based on these findings, we performed transfer experiments and demonstrated that both activation of T cells and anti-peptide antibody elevation are required for the initiation and subsequent progression of the disease. The present study strongly suggests that B-cell epitope spreading is an essential step for the switch from myocarditis to DCM.
Materials and Methods
Animals and Proteins
Lewis rats were purchased from SLC Japan (Shizuoka) and bred in our animal facility. Seven- to 11-week-old male and female rats were used.
Preparation of Recombinant C-Protein Fragments and Synthetic Peptides
The preparation of recombinant C-protein was precisely described previously.5 Polymerase chain reaction (PCR) products corresponding to fragments 1, 2, 3, and 4 were inserted into a cloning vector, pCR4 Blunt-TOPO in the Zero Blunt TOPO kit (Invitrogen, Groningen, The Netherlands), and clones with correct sequences were subcloned into an expression vector, pQE30 (Qiagen, Tokyo, Japan). Then, recombinant C-protein fragments produced in transformed Escherichia coli were isolated under denaturing conditions and purified using Ni-NTA Agarose (Qiagen).
Synthetic peptides encompassing CC2, designated as CC2P1-CC2P12 (Table 1), were synthesized using a peptide synthesizer (Shimadzu, Kyoto, Japan). All of the peptides used in this study were >90% pure as determined and were purified if necessary using HPLC.
Table 1.
Amino Acid Sequences of Synthetic Peptides Encompassing CC2 Used in the Study
| Peptide | Residue | Sequence |
|---|---|---|
| P1 | 317–348 | AEEDVWEILRQAPPSEYERIAFQYGVTDLRGM |
| P2 | 344–375 | DLRGMLKRLKGMRRDEKKSTAFQKKLEPAYQV |
| P3 | 371–402 | PAYQVSKGHKIRLTVELADHDAEVKWLKNGQE |
| P4 | 398–429 | KNGQEIQMSGSKYIFESIGAKRTLTISQCSLA |
| P5 | 425–456 | QCSLADDAAYQCVVGGEKCSTELFVKEPPVLI |
| P6 | 452–483 | PPVLITRPLEDQLVMVGQRVEFECEVSEEGAQ |
| P7 | 479–510 | EEGAQVKWLKDGVELTREETFKYRFKKDGQRH |
| P8 | 506–537 | DGQRHHLIINEAMLEDAGHYALCTSGGQALRE |
| P9 | 533–564 | QALRELIVQEKKLEVYQSIADLMVGAKDQAVF |
| P10 | 560–591 | DQAVFKCEVSDENVRGVWLKNGKELVPDSRIK |
| P11 | 587–619 | DSRIKVSHIGRVHKLTIDDVTPADEADYSFVPE |
| P12 | 615–647 | SFVPEGFACNLSAKLHFMEVKIDFVPRQEPPKI |
C-protein fragment 2 encompasses amino acid 317–647 residues of human cardiac C-protein.
Conjugation of CC2P12 with KLH
To increase immunogenicity of CC2P12, the peptide was conjugated with keyhole limpet hemocyanin (KLH; Wako, Tokyo, Japan) as described previously.6 KLH (in 0.083 mol/L sodium phosphate, 0.9 mol/L NaCl, and 0.1 mol/L ethylenediamine tetraacetic acid, pH 7.2) and m-maleimidobenzoyl-N-hydrosuccinimide ester in dimethyl sulfoxide (MBS; Pierce, Chicago, IL) at concentrations of 10 and 20 mg/ml, respectively, were incubated at a ratio of 10:1 for 1 hour at room temperature. Then, excess MBS was removed on a HiTrap desalting column (Amersham Biosciences, Tokyo, Japan). Finally, the KLH-CC2P12 complex was formed by incubating MBS-KLH with CC2P12 for 2 hours at room temperature.
EAC Induction and Tissue Sampling
Lewis rats were immunized once on day 0 with the indicated antigen with complete Freund’s adjuvant (CFA) (2.5 mg/ml Mycobacterium tuberculosis) in the hind foot pads. At the time of immunization, rats received an intraperitoneal injection of 2 μg of pertussis toxin (PT; Seikagaku Corp., Tokyo, Japan). The numbers of rats used for experiments are shown in the footnotes of tables and the figure legends. Histological and immunohistochemical examinations were performed at the indicated time points using frozen and paraffin-embedded sections of the heart. Although evaluation of EAC and DCM was mainly based on histological examinations (see below), clinical score was also recorded: grade 1, dyspnea; grade 2, dyspnea plus ruffling of fur; and grade 3, moribund condition or death.
Histological Grading of Inflammatory Lesions and Immunohistochemistry
EAC inflammatory lesions were evaluated using hematoxylin and eosin (H&E)-stained sections according to the following criteria: grade 1, rare focal inflammatory lesions; grade 2, multiple isolated foci of inflammation frequently associated with pericarditis; grade 3, diffuse inflammation involving the outer layer of the muscle; grade 4, grade 3 plus focal transmural inflammation; and grade 5; diffuse inflammation with necrosis. CC2 immunization induced pericarditis that was frequently associated with pericardial and pleural effusion. However, we did not include the findings in the scores because the above grading system covered the whole range of mild to severe EAC. The extent of fibrosis revealed by Azan staining was graded into five categories: grade 1, rare scattered foci of fibrosis; grade 2, multiple isolated foci of fibrosis; grade 3, fibrosis involving the outer layer of the muscle; grade 4, grade 3 plus partial transmural fibrosis; and grade 5, diffuse fibrosis.
Establishment of T-Cell Lines and the Proliferative Assay
CC2- or CC2P12-specific T-cell lines were established from draining (popliteal) lymph node cells taken from CC2- or CC2P12-immunized rats by cycle stimulations with CC2 or CC2P12 in the presence of mitomycin C-treated thymocytes as antigen-presenting cells. Between antigen stimulations, T cells were propagated in culture medium containing 5% Con A supernatant.
Proliferative responses of lymph node cells were assayed in microtiter wells by the uptake of [3H]thymidine. After being washed with phosphate-buffered saline, lymph node cells (2 × 105 cells/well) were cultured with the indicated concentrations of CC2 or CC2 peptides for 3 days, with the last 18 hours in the presence of 0.5 μCi of [3H]thymidine (Amersham Pharmacia Biotech, Tokyo, Japan). In some experiments, the proliferative responses of CC2- or CC2P12-specific T-cell lines (3 × 104 cells/well) were assayed in the presence of the antigens and antigen-presenting cells (5 × 105 cells/well). The cells were harvested on glass-fiber filters, and the label uptake was determined using standard liquid scintillation techniques.
CDR3 Spectratyping
CDR3 spectratyping was performed as described previously.7,8 In brief, PCR products were added to an equal volume of formamide/dye loading buffer and heated at 94°C for 2 minutes. The amplified PCR products were electrophoresed on polyacrylamide sequencing gels, and the fluorescence-labeled DNA profile on the gels was directly recorded using an FMBIO fluorescence image analyzer (Hitachi, Yokohama, Japan). The presence or absence of contaminations of the reagents used in PCR was examined every 10 PCR analyses by performing PCR without the templates. When contaminations were present, all reagents used and the results obtained during the period were discarded.
ELISA
The levels of anti-CC2 and anti-CC2 peptide antibodies were measured using the standard ELISA test. Recombinant CC2 and CC2P1-P12 (10 μg/ml) were coated onto microtiter plates, and serially diluted sera from normal and immunized animals were applied. After washing, appropriately diluted horseradish-conjugated anti-rat IgG, IgG1, or IgG2a was applied. The reaction products were then visualized after incubation with the substrate. The absorbance was read at 450 nm.
Generation of Polyclonal Antibodies Against CC2 and CC2 Peptides
Polyclonal antibodies against CC2 and CC2 peptides were raised by immunizing rats with the antigens/CFA four times on a weekly basis. Sera were obtained 1 week after the last immunization, and ammonium sulfate-precipitated preparations were used for the transfer experiments. The presence of antibodies against the indicated antigens was confirmed by ELISA.
Statistical Analysis
Unless otherwise indicated, Student’s t-test or Mann-Whitney’s U-test was used for the statistical analysis.
Results
Autoimmune Carditis-Inducing Ability of Recombinant C-Protein and Synthetic Peptides
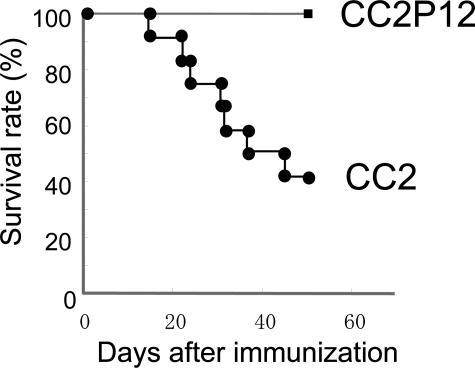
As reported in our previous study,5 recombinant CC2 (amino acid residues 317–647 of human cardiac C-protein) possessed the strongest carditogenic activity among four recombinant proteins encompassing the entire molecule. In the present study, we prepared 12 overlapping synthetic peptides (Table 1) covering the CC2 molecule and examined their carditis-inducing ability. As shown in Table 2, we first screened all of the peptides using the peptide mixtures (groups A through D). Only mixture 4 containing peptides 10, 11, and 12 at 100 μg of each peptide (CC2P10 to -P12) induced EAC in all of the immunized rats (group D), whereas mixtures 1, 2, and 3 induced mild EAC in one of three rats (groups A through C). Then, we tested the carditogenicity of each peptide in mixture 4 and found that only peptide 12 (CC2P12) possessed a carditis-inducing ability (group G). However, it should be noted that compared with CC2, both inflammation and fibrosis induced with CC2P12 were significantly milder as estimated on day 17 and 6 weeks after immunization (group G versus group I). Because immunization with 300 μg of CC2P12 did not differ to 100 μg of CC2P12 immunization in terms of the histological severity, the pooled data are shown in Table 2. In addition, CC2P12-immunized rats did not develop DCM at 6 weeks postimmunization (PI) (see below). Another important aspect was the survival rate. As shown in Figure 1, 75% of the rats immunized with CC2 died of cardiac failure by day 50 PI. In sharp contrast, all of the rats immunized with CC2P12 had survived by day 50. Furthermore, CC2P12 was conjugated with KLH to increase the immunogenicity, and rats were immunized with the conjugate. However, this procedure did not augment the carditis-inducing ability of CC2P12 (group H). In an additional experiment, we immunized rats with a mixture of P1, P5, P8, P11, and P12, but the histological score was not significantly different from that of P12-immunized rats (data not shown). Collectively, these findings suggest that substances induced after CC2P12 immunization lack some aggravation factors for EAC/DCM induced by immunization with CC2.
Table 2.
Histological Severities of EAC Induced by Immunization with the Peptide Mixtures, Synthetic Peptides, and Recombinant CC2*
| Group | Antigen | Sampling | Incidence | Inflammation | Fibrosis |
|---|---|---|---|---|---|
| A | Mix 1 (P1 to P3) | 3 weeks | 1/3 | 0.7 ± 0.7 | 0 |
| B | Mix 2 (P4 to P6) | 3 weeks | 1/3 | 0.3 ± 0.3 | 0 |
| C | Mix 3 (P7 to P9) | 3 weeks | 1/3 | 0.2 ± 0.2 | 0 |
| D | Mix 4 (P10 to P12) | 3 weeks | 3/3 | 2.5 ± 1.2 | 0 |
| E | P10 | Day 17 | 0/3 | 0† | 0 |
| F | P11 | Day 17 | 1/3 | 0.2 ± 0.2† | 0 |
| G | P12 | Day 17 | 4/4 | 2.0 ± 0.4† | 0 |
| 6 weeks | 6/6 | 1.8 ± 0.4† | 1.8 ± 0.6‡ | ||
| H | P12-KLH | 4 weeks | 2/3 | 0.7 ± 0.3 | 0.7 ± 0.3 |
| 6 weeks | 3/3 | 1.3 ± 0.3† | 0.8 ± 0.4‡ | ||
| I | CC2 | Day 17 | 5/5 | 4.1 ± 0.4† | 1.5 ± 0.4 |
| 6 weeks | 6/6 | 3.8 ± 0.2† | 4.1 ± 0.2‡ |
Lewis rats were immunized once with mixtures 1, 2, 3, and 4 that had consisted of peptides 1 to 3, 4 to 6, 7 to 9, and 10 to 12, respectively (100 μg of each peptide), in CFA in the foot pads along with intraperitoneal injection of pertussis toxin (2 μg). Because mix 4 showed carditogenicity, each peptide in the mixture (P10, P11, and P12) was tested in a similar manner. For comparison, the results obtained with recombinant C-protein fragment 2 are also shown. The denominators in the incidence column represent the number of rats used for each experiment.
Analysis of variance and multiple comparison (Scheffe’s F-test) were performed, and significant differences were noted in the following combinations: P10 versus CC2, P = 0.002; P11 versus CC2, P = 0.0001; P12 versus CC2, P = 0.008 on day 17; P12 versus CC2, P = 0.003; P12-KLH versus CC2, P = 0.003 at 6 weeks.
Significant differences were noted in the following combinations: P12 versus CC2, P = 0.011; P12-KLH versus CC2, P = 0.004 at 6 weeks.
Figure 1.
The survival rate of rats immunized with recombinant CC2/CFA or CC2P12/CFA with the intraperitoneal injection of pertussis toxin. Seventy-five percent of rats immunized with CC2 died between days 15 and 49 PI, whereas all of the rats immunized with CC2P12 survived during the observation period.
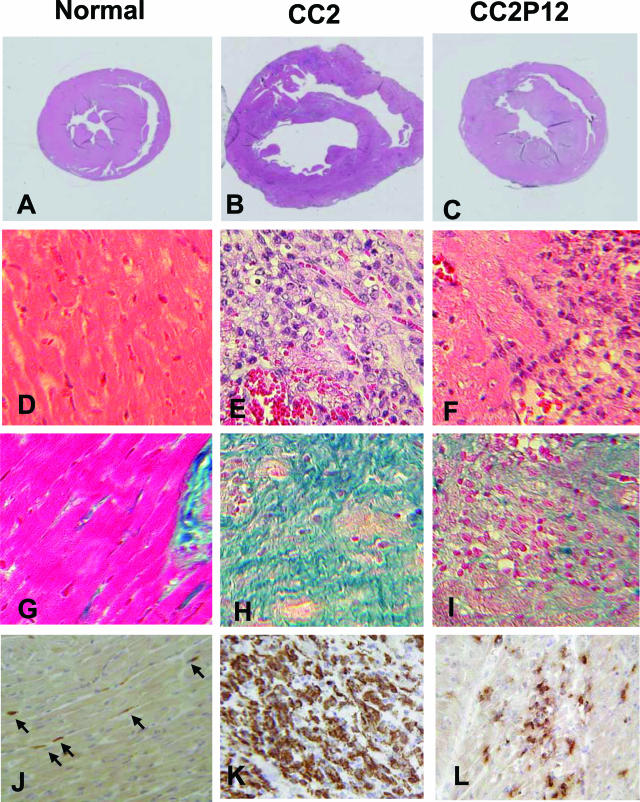
Figure 2 depicts normal histology of the heart (A, D, G, and J) and pathology of EAC induced by CC2 (B, E, H, and K) and CC2P12 (C, F, I, and L). At 2 weeks PI, when EAC was at the acute inflammatory stage, the hearts taken from CC2-immunized rats showed marked hypertrophy (Figure 1B), whereas the hearts from CC2P12-immunized rats showed slight enlargement (Figure 1C). We measured the long and short axes of the middle portion of normal, CC2-immunized, and CC2P12-immunized rats and calculated the heart area at this level. As shown in Table 3, there were significant differences between normal and CC2-immunized rats (P = 0.01) and between CC2- and CC2P12-immunized rats (P = 0.005), indicating that the hearts from CC2-immunized rats showed marked hypertrophy. In contrast, there was no significant difference between the normal and CC2P12-immunized groups. H&E (Figure 2, E and F) and azan (Figure 2, H and I) stainings showed that compared with CC2-induced EAC (Figure 2, E and H), both inflammation and fibrosis were mild (Figure 2, F and I). In sections immunostained for macrophages, there was extensive and diffuse macrophage infiltration in CC2-induced EAC (Figure 2K), whereas macrophage infiltration in CC2P12-induced EAC was mild and focal (Figure 2L). B-cell infiltration was absent in both types of EAC (data not shown).
Figure 2.
Normal histology (A, D, G, and J) and pathology of EAC induced by immunization with CC2 (B, E, H, and K) and CC2P12 (C, F, I, and L). At 2 weeks PI, when EAC was at the acute inflammatory stage, the hearts taken from CC2-immunized rats showed marked hypertrophy (B) compared with the normal heart (A), whereas the hearts from CC2P12-immunized rats showed only slight enlargement (C). H&E (E and F) and azan (H and I) stainings showed that compared with CC2-induced EAC (E and H), both inflammation and fibrosis of the heart were mild in CC2P12-immunized rats (F and I). In sections immunostained for macrophages, there was extensive and diffuse macrophage infiltration in CC2-induced EAC (J), whereas macrophage infiltration in CC2P12-induced EAC was mild and focal (K). A–C: H&E staining; the photographs were taken at the same magnification. D–F: H&E staining, ×240. G–I: Azan staining, ×240. J–L: ED1 staining, ×240.
Table 3.
Measurements of Hearts under Normal and Diseased Conditions*
| Condition | No. of rats examined | Diameter (mm)
|
Estimated area of hearts (mm2)† | |
|---|---|---|---|---|
| Long axis | Short axis | |||
| Normal | 3 | 0.83 ± 0.10 | 0.72 ± 0.12 | 0.47 ± 0.14‡ |
| CC2 | 6 | 1.20 ± 0.19 | 0.97 ± 0.08 | 0.91 ± 0.19‡ |
| CC2P12 | 6 | 0.91 ± 0.08 | 0.80 ± 0.11 | 0.58 ± 0.13‡ |
Rats were immunized once with CC2 or CC2P12, and the hearts were taken at 6 weeks after immunization. The long and short axes of the diameter were measured at the middle portion of the hearts. The hearts from CC2P12-immunized rats were measured before and after fixation, and those from normal and CC2-immunized rats were measured only after fixation. Because there was no significant change before and after fixation, all the values shown in the table are those measured after fixation.
The heart area was calculated as long axis/2 × short axis × 3.14.
Significant differences were noted between normal and CC2-immunized rats (P = 0.01) and between CC2- and CC2P12-immunized rats (P = 0.005). However, there was no significant difference between normal and CC2P12-immunized rats.
Characterization of Pathogenic T Cells
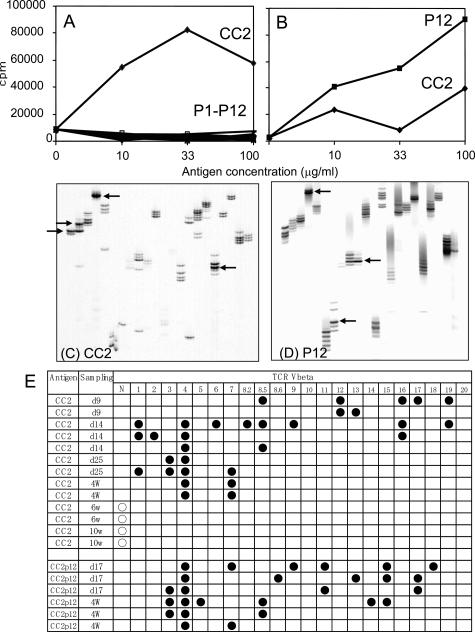
We next tried to determine whether a carditogenic peptide, CC2P12, contains an immunodominant or cryptic T-cell epitope. The representative results of three experiments are shown in Figure 3, A and B. When CC2 was immunized, the draining lymph node cells responded vigorously to CC2 but not to all of the overlapping peptide (P1 to P12 in Figure 3A). Similar experiments were performed using CC2-specific T-line cells after four to five cycles of antigen stimulation, and essentially the same results were obtained (data not shown). After CC2P12 immunization, lymph node cells responded well to CC2P12 and also to CC2 to a lesser extent (Figure 3B). These findings suggest that CC2P12 is processed and presented to T cells from CC2P12-immunized rats but is a cryptic epitope in the CC2 molecule for T cells from CC2-immunized rats.
Figure 3.
A and B: The proliferative responses of lymph node cells taken from CC2-immunized (A) and CC2P12-immunized (B) rats on day 12 PI. Lymph node cells (2 × 105 cells/well) were cultured with the indicated antigen for 72 hours, with the last 18 hours in the presence of [3H]thymidine. The cells were harvested on glass-fiber filters, and the label uptake was determined using standard liquid scintillation techniques. Each symbol represents the mean value of triplicate assays, and SEMs were within 10% of the mean values. C and D: CDR3 spectratyping profiles of heart-infiltrating T cells taken from CC2-immunized (C) and CC2P12-immunized (D) rats. Marked spectratype expansions are indicated by arrows. E: Summary of the results of CDR3 spectratyping of T cells in the hearts from CC2- and CC2P12-immunized rats. Each line represents the result obtained from one rat. Closed circles represent Vβ clonal expansion. N, the normal spectratype pattern; w, weeks.
In a previous study, we showed with CDR3 spectratyping analysis that in cardiac myosin-induced EAC, Vβ8.2 and Vβ10 TCR were clonally expanded in the inflamed heart and that Vβ8.2- and Vβ10-targeted immunotherapy was effective in ameliorating the severity of EAC.7 We performed a similar analysis to characterize the nature of clonally expanded T cells in the heart with C-protein-induced EAC. The representative profiles are depicted in Figure 3, C and D, and all of the results are summarized in Figure 3E. Infiltrating T cells in the heart of CC2-immunized rats on day 25 PI showed Vβ3 and Vβ4 expansion (Figure 3C, arrows), and those of CC2P12-immunized rats on day 17 PI showed Vβ4, Vβ8.6, and Vβ17 expansion (Figure 3D, arrows). The longitudinal study of CC2-immunized rats revealed interesting findings. On day 9 PI, T cells infiltrating the heart were rather heterogonous, and no particular Vβ expansion was noted (Figure 3E). In contrast, between days 14 and 28 PI when the inflammatory lesion reached at the maximal level,5 Vβ4 expansion was detected in all of the cases examined. Other Vβs such as Vβ1, -7, -8.5, and -16 were expanded in one-half of the cases. Interestingly, T cells found in the heart at the later stage (6 and 10 weeks), when there was extensive fibrosis, showed the normal spectratype pattern (Figure 3E). These findings suggested that infiltrating T cells showing oligoclonal expansion play an important role in the development of EAC lesions (see the results of the transfer experiments below). However, T cells found at the later stage may be less involved in the disease progression. CDR3 spectratyping analysis of heart-infiltrating T cells of CC2P12-immunized rats was performed on day 17 and at 4 weeks PI and revealed that there was Vβ4 expansion in all of the cases examined. Taken together, Vβ4-positive T cells appear to play an important role in lesion formation in both CC2- and CC2P12-induced EAC, and there was no significant difference in the T-cell characteristics between the two groups.
Characterization of Pathogenic B Cells and Anti-C-Protein Antibodies
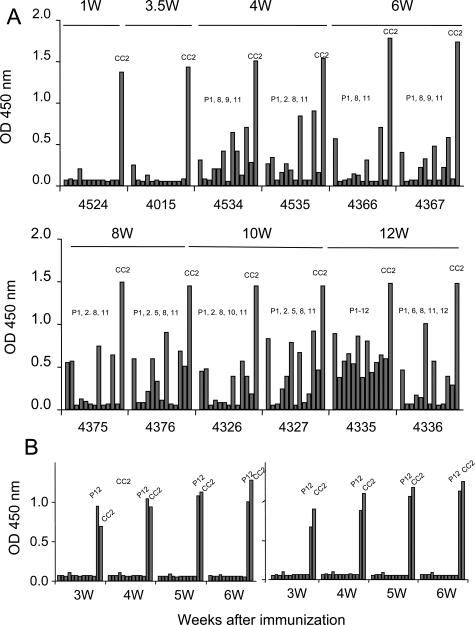
As shown in Table 2 and Figure 2, CC2P12-immunized rats showed mild to moderate EAC without subsequent DCM. Moreover, unlike CC2-induced EAC, CC2P12-induced EAC was not fatal. These findings suggest that there are factors in CC2, but not in CC2P12, that aggravate EAC and induce DCM. Because we did not find clear differences between CC2- and P12-reactive T cells in the clonality analysis, we next examined the nature of antibodies raised by CC2 (Figure 4A) and CC2P12 (Figure 4B) immunization. From 1 to 12 weeks PI, sera were collected from CC2-immunized rats, and the levels of antibodies against CC2 and CC2P1 to -P12 were determined by ELISA (Figure 4A). At the early stage (1 to 3.5 weeks), only anti-CC2 antibodies were elevated in CC2-immunized rats. Then, anti-P8 and anti-P11 antibodies rose between 4 and 6 weeks, and some others showed at a high level thereafter. In one case examined at 12 weeks PI (4335 in Figure 4A), antibodies against all of the peptides were detected. This finding clearly showed that there was B-cell epitope spreading in CC2-immunized rats. Because this phenomenon was detected in the peripheral blood and there was no B-cell infiltration in the heart (data not shown), B-cell epitope spreading would take place in the lymphoid organ. In sharp contrast, in CC2P12-immunized rats, only antibodies against CC2P12 and CC2 were recognized in all of the rats examined by 6 weeks PI (Figure 4B).
Figure 4.
The kinetics of anti-CC2 and anti-CC2 peptide antibodies in CC2-immunized (A) and CC2P12-immunized (B) rats. A: The levels of anti-CC2 and anti-CC2 peptide antibodies were determined using ELISA. Recombinant CC2 and CC2P1-P12 (10 μg/ml) were coated onto microtiter plates, and diluted sera from CC2-immunized rats were applied. After washing, horseradish peroxidase-conjugated anti-rat IgG was allowed to react. The reaction products were then visualized after incubation with the substrate. The absorbance was read at 450 nm. B: The kinetics of anti-CC2 and anti-CC2 peptide antibodies in CC2P12-immunized rats. During the observation period, only anti-CC2P12 and anti-CC2 antibodies were elevated.
CC2P12 Immunization and Cotransfer of Anti-CC2 or CC2 Peptide Antibodies Elicited Severe EAC
We further tried to identify factors that are responsible for the development of full-blown EAC and subsequent DCM. We already obtained the following findings. First, although CC2P12 was the sole carditis-inducing peptide in the CC2 molecule, CC2P12 induced relatively mild EAC without subsequent DCM. Second, there was no significant difference in the T-cell specificity between CC2-immunized and CC2P12-immunized rats. Finally, CC2-immunized rats showed marked intramolecular epitope spreading in the antibody production, whereas CC2P12-immunized rats developed antibodies that reacted with the immunizing antigen and the CC2 molecule. These findings raised the possibility that the generation of CC2-reacting T cells and generation of antibodies against various parts of the CC2 molecule are essential for full-blown EAC and subsequent DCM.
To test this possibility, we performed transfer experiments using various types of T cells and antibodies. The results are summarized in Table 4. Adoptive transfer of spleen and lymph node cells induced mild EAC in the recipients, whereas adoptive transfer of CC2-specific T-line cells did not elicit inflammation (Table 4, groups A and B). This finding suggests that not only T cells but also B cells are required for the development of inflammation in the heart. Cotransfer of anti-CC2 or anti-CC2P1–12 antisera after CC2P12 immunization aggravated both inflammation (Table 4, group D versus F, P = 0.03; group E versus F, P = 0.005) and fibrosis (Table 4, group D versus F, P = 0.049; group D versus F, P = 0.01) of EAC. There was no significant difference in the carditis-exacerbating ability between anti-CC2P1–12 and anti-CC2 antisera (Table 4, group D versus E). It should be noted that the transfer of anti-CC2 antisera alone did not induce EAC at all (group G). These findings strongly suggest that T cells are required for the initiation of inflammation in the heart and that anti-CC2 antibodies aggravate both inflammation and fibrosis.
Table 4.
Summary of Cell and Antibody Transfer Experiments in EAC
| Group | Immunization | Cell transfer
|
Ab transfer
|
|
|---|---|---|---|---|
| Cells | Dose | Ab | ||
| A | — | SpC + LNC* | 107 | — |
| B | — | CC2 TCL* | 102 to 6 × 106 | — |
| C | — | CC2P12 TCL* | 3.5 to 6 × 106 | — |
| D | CC2P12‡ | — | — | Anti-P1-P12 sera |
| E | CC2P12‡ | — | — | Anti-CC2 sera |
| F | CC2P12‡ | — | — | Normal sera |
| G | — | — | — | Anti-CC2 sera |
CC2-reactive T cells or CC2P12 with or without antibodies were administered, and the degree of inflammation and fibrosis was evaluated 3 weeks after cell transfer. The denominators in the incidence column represent the number of rats used for each experiment. SpC, spleen cells; LNC, lymph node cells; TCL, T-cell line; —, not performed.
n.e., not examined.
CC2P12 was immunized, and the indicated sera (1 ml after 5-fold dilution) were injected intravenously twice a week for 5 weeks. Rats were examined histologically 6 weeks after the immunization.
Significant differences were noted in the following comparisons: D versus F, P = 0.03; E versus F, P = 0.005.
Significant differences were noted in the following comparisons: D versus F, P = 0.049; E versus F, P = 0.01.
(Table continues)
Table 4.
Continued
| Ab transfer
|
Inflammation
|
Fibrosis
|
||
|---|---|---|---|---|
| Dose | Incidence | Grade | Incidence | Grade |
| — | 2/2 | 2 | n.e.† | n.e. |
| — | 0/5 | 0 | n.e. | n.e. |
| — | 2/3 | 0.8 ± 0.6 | 0/3 | 0 |
| 5 × dilution (1 ml) × 2/week × 5 weeks | 3/3 | 3.3 ± 0.3§ | 3/3 | 3.3 ± 0.2¶ |
| 5 × dilution (1 ml) × 2/week × 5 weeks | 4/4 | 3.4 ± 0.3§ | 4/4 | 3.3 ± 0.3¶ |
| 5 × dilution (1 ml) × 2/week × 5 weeks | 4/4 | 1.5 ± 0.5§ | 4/4 | 1.6 ± 0.4¶ |
| 5 × dilution (1 ml) × 2/week × 5 weeks | 0/3 | 0 | 0/3 | 0 |
Discussion
DCM is a serious problem for patients with heart failure because the disease progresses irreversibly and often ends in death. To develop effective therapies, it is essential to elucidate the pathomechanisms of the development of DCM. However, there are few good experimental models for DCM. In a previous study, we succeeded in inducing severe EAC with a high fatality rate and subsequent DCM in survivors by immunizing Lewis rats with cardiac C-protein.5 This animal model is useful not only for the elucidation of the pathomechanisms of DCM but also for the development of effective immunotherapies.5
In the present study, we first tried to determine the carditis-inducing epitopes in the CC2 molecule and found that only peptide 12 (CC2P12), covering the residues 615–647, contains carditogenic epitope(s). Interestingly, immunization with CC2P12 induced moderate EAC but did not lead to subsequent DCM. Here, we demonstrated in a C-protein-induced animal model that B-cell epitope spreading occurred in CC2-immunized rats with DCM but not in CC2P12-immunized rats without DCM and that elevation of antibodies against various parts of the CC2 molecule is essential for the induction of more severe inflammation and fibrosis. However, it should be noted that activation of pathogenic T cells as demonstrated by CDR3 spectratyping is essential for the initiation of lesion formation because adoptive transfer of anti-CC2 antisera alone did not induce pathology at all.
Epitope spreading was first described in detail by Lehmann et al9 as a key process for the development of chronic autoimmune encephalomyelitis. Initially, T-cell epitope spreading was intensively investigated, and this immunological event was thought to be highly involved in the relapse and chronicity of autoimmune diseases.10–12 Later, it was reported that B-cell epitope spreading is also involved in the pathogenesis of autoimmune diseases.13,14 Notably, Bischof et al15 have shown that immunization of mice with myelin oligodendrocyte glycoprotein, but not with myelin basic protein and proteolipid protein, induced extensive B-cell epitope spreading and chronic autoimmune encephalomyelitis. Furthermore, they observed that diversification of the B-cell reactivity did not follow a sequential cascade that is seen in T-cell epitope spreading but represented a simultaneous spread toward a broad range of antigenic epitopes. In the present study, we also observed a similar mode of B-cell epitope spreading. Many reports have suggested that autoantibodies against cardiac components play an important role in the formation of DCM.16–20 This assumption was also supported by the finding that immunoadsorption therapy to remove IgG ameliorated myocardial inflammation4 and improved the cardiac performance and clinical status.21 In addition, we have also observed that intravenous immunoglobulin administration suppressed the development of CC2-induced DCM and down-regulated anti-CC2 antibody production (our unpublished observation).
It is important to analyze the nature of DCM-inducing antibodies. We induced severe EAC with extensive fibrosis by immunization with CC2P12 plus transfer of anti-P1 to -P12 antisera that had been raised by peptide mixture immunization, but could not fully reconstitute the features of EAC and DCM produced by CC2 immunization. One of the reasons for this was that in our treatment protocol, it was difficult to maintain anti-peptide antibodies at a high level (unpublished observation). Although the reconstitution experiments demonstrated that anti-CC2P1–12 antisera possessed almost the same carditis-exacerbating ability as anti-CC2 antisera, there is a possibility that antibodies recognizing the conformational epitopes with high titers elicited by CC2 immunization but not by CC2P12 immunization are involved in the processes of DCM formation. In this regard, we are currently generating monoclonal antibodies against conformational epi-topes of the CC2 molecule to test their ability of producing DCM. The conformational epitope mapping analysis would be helpful to identify pathogenic antibodies.
Increasing information about the pathogenesis of DCM will provide more chance for immunotherapies for the prevention and/or cessation of DCM. If pathogenic antibodies are identified more accurately, then specific and selective immunoadsorption could be achieved effectively with minimal side effects. In cases of DCM developed in a manner similar to that shown in the present study, intravenous immunoglobulin therapy, which is already in clinical trials,22 is expected to be effective. Another important aspect is the timing of treatment initiation. As demonstrated in the previous5 and present studies, histological examination revealed that fibrosis of the heart starts at 4 weeks PI and establishes at 6 to 8 weeks PI. Generation of the full range of pathogenic antibodies starts at the same period of time. Therefore, this time point is critical for the start of treatment. Improvements in the image analysis and functional studies are expected to greatly increase the effect of DCM therapy.
In summary, we identified the amino acid residue containing carditis-inducing epitope(s) in the CC2 molecule. By comparing CC2-induced EAC and subsequent DCM with peptide-induced EAC, it was demonstrated that B-cell epitope spreading is critical for the development of DCM. Importantly, by down-regulating pathogenic antibodies, it is possible to control the disease processes. Information obtained in the present study will provide useful information for the development of effective immunotherapies against human DCM.
Acknowledgments
We thank Y. Kawazoe for technical assistance.
Footnotes
Address reprint requests to Yoh Matsumoto, Department of Molecular Neuropathology, Tokyo Metropolitan Institute for Neuroscience, Musashidai 2-6, Fuchu, Tokyo 183-8526, Japan. E-mail: matyoh@tmin.ac.jp.
Supported in part by grants-in-aid from the Japan Society for the Promotion of Science.
References
- Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation. 2001;104:1076–1082. doi: 10.1161/hc3401.095198. [DOI] [PubMed] [Google Scholar]
- Parrillo JE, Cunnion RE, Epstein SE, Parker MM, Suffredini AF, Brenner M, Schaer GL, Palmeri ST, Cannon RO, Alling D, Wittes JT, Ferrnas VJ, Rodriguez ER, Fauci AS. A prospective, randomized, controlled trial of predonisone for dilated cardiomyopathy. N Engl J Med. 1989;321:1061–1068. doi: 10.1056/NEJM198910193211601. [DOI] [PubMed] [Google Scholar]
- Wojnicz R, Nowalany-Kozielska E, Wojciechowska C, Glanowska G, Wilczewski P, Niklewski T, Zembala M, Polonske L, Rozek MM, Wodniecki J. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy. Circulation. 2001;104:39–45. doi: 10.1161/01.cir.104.1.39. [DOI] [PubMed] [Google Scholar]
- Staudt A, Schaper F, Stangl V, Plagemann A, Bohm M, Merkel K, Wallukat G, Wernecke KD, Stangl K, Baumann G, Felix SB. Immunohistological changes in dilated cardiomyopathy induced by immunoadsorption therapy and subsequent immunoglobulin substitution. Circulation. 2001;103:2681–2686. doi: 10.1161/01.cir.103.22.2681. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Tsukada Y, Miyakoshi A, Sakuma H, Kohyama K. C protein-induced myocarditis and subsequent dilated cardiomyopathy: rescue from death and prevention of dilated cardiomyopathy by chemokine receptor DNA therapy. J Immunol. 2004;173:3535–3541. doi: 10.4049/jimmunol.173.5.3535. [DOI] [PubMed] [Google Scholar]
- Edwards RJ, Singleton AM, Boobis AR, Davies DS. Cross-reaction of antibodies to coupling groups used in the production of anti-peptide antibodies. J Immunol Methods. 1989;117:215–220. doi: 10.1016/0022-1759(89)90143-9. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Jee Y, Sugisaki M. Successful TCR-based immunotherapy for autoimmune myocarditis with DNA vaccines after rapid identification of pathogenic TCR. J Immunol. 2000;164:2248–2254. doi: 10.4049/jimmunol.164.4.2248. [DOI] [PubMed] [Google Scholar]
- Kim G, Tanuma N, Kojima T, Kohyama K, Suzuki Y, Kawazoe Y, Matsumoto Y. CDR3 size spectratyping and sequencing of spectratype-derived T cell receptor of spinal cord T cells in autoimmune encephalomyelitis. J Immunol. 1998;160:509–513. [PubMed] [Google Scholar]
- Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SD, Vanderlugt CL, Begolka WS, Pao W, Yauch RL, Neville KL, Katz-Levy Y, Carrizosa A, Kim BS. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- Vanderlugt CL, Neville KL, Nikcevich KM, Eagar TN, Bluestone JA, Miller SD. Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. J Immunol. 2000;164:670–678. doi: 10.4049/jimmunol.164.2.670. [DOI] [PubMed] [Google Scholar]
- Naserke HE, Ziegler AG, Lampasona V, Bonifacio E. Early development and spreading of autoantibodies to epitopes of IA-2 and their association with progression to type 1 diabetes. J Immunol. 1998;161:6963–6969. [PubMed] [Google Scholar]
- Li N, Aoki V, Hans-Filho G, Rivitti EA, Diaz LA. The role of intramolecular epitope spreading in the pathogenesis of endemic pemphigus foliaceus (fogo selvagem). J Exp Med. 2003;197:1501–1510. doi: 10.1084/jem.20022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof F, Bins A, Durr M, Zevering Y, Melms A, Kruisbeek AM. A structurally available encephalitogenic epitope of myelin oligodendrocyte glycoprotein specifically induces a diversified pathogenic autoimmune response. J Immunol. 2004;173:600–606. doi: 10.4049/jimmunol.173.1.600. [DOI] [PubMed] [Google Scholar]
- Warraich RS, Dunn MJ, Yacoub MH. Subclass specificity of autoantibodies against myosin in patients with idiopathic dilated cardiomyopathy: pro-inflammatory antibodies in DCM patients. Biochem Biophys Res Commun. 1999;259:255–261. doi: 10.1006/bbrc.1999.0761. [DOI] [PubMed] [Google Scholar]
- Baba A, Yoshikawa T, Chino M, Murayama A, Mitani K, Nakagawa S, Fujii I, Shimada M, Akaishi M, Iwanaga S, Asakura Y, Fukuda K, Mitamura H, Ogawa S. Characterization of anti-myocardial autoantibodies in Japanese patients with dilated cardiomyopathy. Jpn Circ J. 2001;65:867–873. doi: 10.1253/jcj.65.867. [DOI] [PubMed] [Google Scholar]
- Caforio AL, Mahon NJ, Tona F, McKenna WJ. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail. 2002;4:411–417. doi: 10.1016/s1388-9842(02)00010-7. [DOI] [PubMed] [Google Scholar]
- Staudt A, Bohm M, Knebel F, Grosse Y, Bischoff C, Hummel A, Dahm JB, Borges A, Jochmann N, Wernecke KD, Wallukat G, Baumann G, Felix SB. Potential role of autoantibodies belonging to the immunoglobulin G-3 subclass in cardiac dysfunction among patients with dilated cardiomyopathy. Circulation. 2002;106:2448–2453. doi: 10.1161/01.cir.0000036746.49449.64. [DOI] [PubMed] [Google Scholar]
- Staudt A, Staudt Y, Dorr M, Bohm M, Knebel F, Hummel A, Wunderle L, Tiburcy M, Wernecke KD, Baumann G, Felix SB. Potential role of humoral immunity in cardiac dysfunction of patients suffering from dilated cardiomyopathy. J Am Coll Cardiol. 2004;44:829–836. doi: 10.1016/j.jacc.2004.04.055. [DOI] [PubMed] [Google Scholar]
- Müller J, Wallukat G, Dandel M, Bieda H, Brandes K, Spiegelsberger S, Nissen E, Kunze R, Hetzer R. Immunoglobulin adsorption in patients with idiopathic dilated cardiomyopathy. Circulation. 2000;101:385–391. doi: 10.1161/01.cir.101.4.385. [DOI] [PubMed] [Google Scholar]
- Larsson L, Mobini R, Aukrust P, Gullestad L, Wallukat G, Waagstein F, Fu M. Beneficial effect on cardiac function by intravenous immunoglobulin treatment in patients with dilated cardiomyopathy is not due to neutralization of anti-receptor autoantibody. Autoimmunity. 2004;37:489–493. doi: 10.1080/08916930400011684. [DOI] [PubMed] [Google Scholar]