Abstract
The impact of the local inflammatory response on the process of wound healing has been debated for decades. In particular, the question whether infiltrating macrophages and granulocytes promote or impede tissue repair has received much attention. In the present study, we show that wound healing is accelerated in mice deficient for the anti-inflammatory cytokine interleukin (IL)-10. IL-10−/− mice closed excisional wounds significantly earlier compared with IL-10-competent control littermates. This effect was attributable to accelerated epithelialization as well as enhanced contraction of the wound tissue in the mutant animals. Increased α-smooth muscle actin expression in IL-10-deficient mice suggests that augmented myofibroblast differentiation is responsible for the enhanced contraction of wounds in mutant mice. The number of macrophages infiltrating the wound tissue was significantly increased in IL-10−/− mice compared with control littermates suggesting that this cell type mediates the accelerated tissue repair. These results show for the first time that IL-10 can impede wound repair.
The process of wound repair requires a complex interplay of resident epithelial and mesenchymal cells with resident and recruited hematopoietic cells to accomplish the three stages of wound healing: inflammation, formation of new tissue, and tissue remodeling.1 The initial inflammatory phase is characterized by a local activation of innate immune mechanisms resulting in an initial influx of neutrophilic granulocytes into the damaged tissue followed by an accumulation of macrophages.1 The innate response of resident and recruited cells combats invading microbes but supposedly also critically influences the repair process by liberation of a wide spectrum of mediators. However, it remains unclear whether the inflammatory response hampers or accelerates wound repair and whether it affects the quality of the repaired tissues.2 Interestingly, analyses of wound healing in a number of murine knockout models deficient for individual inflammatory mediators or their receptor including tumor necrosis factor (TNF)-α, interleukin (IL)-6, monocyte chemotactic protein (MCP)-1, and interferon (IFN)-γ have yielded heterogeneous results.3 Wound healing is accelerated in TNF-receptor-554 or IFN-γ-deficient mice5 but is impaired in mice deficient for IL-66 or MCP-1.7
IL-10 is an immunoregulatory cytokine that limits innate as well as adaptive immune responses protecting the host from immune-mediated tissue damage.8 In a variety of different cell types, IL-10 mediates down-regulation of a broad spectrum of proinflammatory mediators such as IL-1, IL-6, IL-12, IFN-γ, TNF-α, regulated on activation normal T cell expressed and secreted, MCP-1, macrophage inflammatory protein (MIP)-1α, IL-8, interferon-γ-inducible protein 10 (IP-10), and prostaglandin E2 (PGE2), whereas anti-inflammatory molecules such as IL-1 receptor antagonist (IL-1 ra) or soluble TNF-receptor are up-regulated.8,9 In IL-10−/− mice, uncontrolled Th1 responses to intestinal bacterial antigens result in the spontaneous development of inflammatory bowel disease.10 On infection with a number of different pathogens these animals develop severe immunopathology.11–14 The importance of IL-10 for the control of innate responses was demonstrated by an increased sensitivity of IL-10-deficient animals to lipopolysaccharide. IL-10−/− mice develop lethal septic shock because of overproduction of TNF-α after the intraperitoneal administration of 20-fold lower doses of lipopolysaccharide compared with wild-type animals.15 In addition, the local inflammatory response to lipopolysaccharide and the local response to bacterial DNA or CpG oligodeoxynucleotides follow a more vigorous course in IL-10−/− mice.16 Likewise, the irritant response of the skin to tetradecanoylphorbol-acetate-containing irritants, which is a function of cutaneous innate immunity, is clearly enhanced in IL-10-deficient mice in comparison with wild-type animals.17
A role for IL-10 in wound repair has been suggested by the results of several earlier studies. An analysis of IL-10 expression during wound healing revealed an early peak of IL-10 production 3 hours after wounding and a second peak 3 days after injury.18 Keratinocytes at the wound margins and infiltrating mononuclear cells were identified as the major sources of IL-10 mRNA and protein. A function of IL-10 in repair was suggested by a study in which IL-10-neutralizing antibodies were locally applied to incisional wounds.18 In this model, the infiltration of myeloid cells toward the site of injury, as well as the expression of chemokines and proinflammatory mediators was increased, indicating a suppressive role for IL-10 in wound inflammation. Furthermore, a study of fetal wound healing in a skin transplant model suggested that IL-10 may be responsible for the scarless tissue repair observed in fetal skin.19 Herein we report that IL-10 deficiency results in accelerated wound closure. These results show for the first time that IL-10 can impede wound repair and support the view that the local inflammatory response can promote tissue repair.
Materials and Methods
Animals
IL-10−/− mice10 on the C57BL/6 background were maintained and bred under standard pathogen-free conditions and genotyped by Southern blotting. Ten- to 12-week-old male IL-10−/− mice and IL-10wt/− or IL-10w/w control littermates (offspring from heterozygote breedings) were used for the experiments. Only healthy mice without any sign of inflammatory bowel disease were included in the study.
Wounding and Preparation of Wound Tissue
Mice were anesthetized by intraperitoneal injection of Ketanest/Rompun (Ketanest S: Park Davis GmbH, Karlsruhe, Germany; Rompun 2%: Bayer, Leverkusen, Germany). The back was shaved and four circular excisional wounds of 6-mm diameter were generated that extended beyond the panniculus carnosus (full thickness wounds) using a standard biopsy punch (Stiefel, Offenbach, Germany). The four wounds on the back of one animal were at least 5 mm apart from each other. For histological analysis, mice were sacrificed, and an area of 8 μm in diameter, which included the complete epithelial margins, was excised. Wounds were bisected in caudocranial direction, and the tissue was either fixed overnight in 4% paraformaldehyde in phosphate-buffered saline (PBS) or embedded in OCT compound (Tissue Tek; Miles, Elkhart, IN), immediately frozen in liquid nitrogen, and stored at −80°C. Histological analysis was performed on serial sections from the central portion of the wound.
Histology
Immunohistochemistry
For immunohistochemical staining of macrophages, paraffin sections (5 μm) were incubated with rat anti-mouse F4/80 antibody (MCA497GA; Serotec, Duesseldorf, Germany) at 4°C overnight. The section was then incubated with biotin-labeled polyclonal rabbit anti-rat Ig (DakoCytomation, Hamburg, Germany) for 30 minutes followed by incubation with streptavidin-conjugated horseradish peroxidase (30 minutes) and aminoethyl carbazole as chromogen (10 minutes). Nuclei were counterstained with hematoxylin. The naphthol-AS-d-chloroacetate esterase reaction was performed according to standard procedures (IHC World, Online Information Center For Histochemistry) for detection of neutrophilic granulocytes. To process tissue sections for the detection of CD31 (PECAM-1), 5-μm cryosections were fixed in acetone, endogenous peroxidase was inactivated (0.03% H2O2, 0.15 mol/L NaN3), and unspecific binding sites were blocked with 3% bovine serum in PBS. Sections were incubated with polyclonal rat antisera against murine CD31 (1 hour, room temperature, 1:500) (Pharmingen, Heidelberg, Germany); bound primary antibodies were detected using a peroxidase-conjugated goat anti-rat antibody (Southern Biotechnology, Birmingham, AL). Aminoethyl carbazole was used as a substrate and sections were counterstained with hematoxylin. For immunofluorescent staining, bound primary CD31 antibody was detected using an Alexa Fluor 488-conjugated polyclonal goat anti-rat antibody (1 hour, 1:500; Molecular Probes, Leiden, The Netherlands). For staining of α-smooth muscle actin (α-SMA) the cryosections were fixed in acetone, blocked with 3% bovine serum albumin in PBS, and incubated with Cy3-conjugated monoclonal anti-α-SMA antibody (1 hour, room temperature, 1:200; Sigma, St. Louis, MO). For vascular endothelial growth factor (VEGF)-A detection, 5-μm cryosections were fixed in 4% paraformaldehyde, blocked with 3% bovine serum albumin in PBS, and incubated overnight at room temperature with polyclonal rabbit anti-VEGF-A antibody (1:100, sc-507; Santa Cruz, Heidelberg, Germany), followed by detection using the DakoCytomation Envision system (labeled polymer horseradish peroxidase anti-rabbit; DakoCytomation) following the provider’s instructions. For immunofluorescent staining of VEGF-A, sections were incubated with an Alexa Fluor 594-conjugated polyclonal goat anti-rabbit antisera (Molecular Probes). For immunofluorescent staining of macrophages, cryosections (5 μm) were incubated overnight at room temperature with rat anti-mouse F4/80 antibody (1:100, MCA497GA; Serotec), followed by detection using an Alexa Fluor 488-conjugated polyclonal goat anti-rat antibody (Molecular Probes). Double fluorescence was analyzed in a laser-scanning confocal microscope (True Confocal Scanner Leica TCS SL; Leica Microsystems, Heidelberg, Germany) at ×200 magnification (CD31 and α-SMA) or ×1000 magnification (VEGF-A and F4/80). Isotype-matched rat antibodies were used as negative controls. For Fizz1 and Ym1 detection, cryosections were incubated for 1 hour at room temperature with rabbit anti-Fizz1 (Peprotech, London, UK) or goat anti-Ym1 (R&R Systems, Wiesbaden, Germany) antibody (1:50); bound primary antibodies were detected using a peroxidase-conjugated anti-rabbit or anti-goat antibody (DakoCytomation), respectively. Organization and maturation of collagen bundles was assessed on paraffin sections of day 14 wounds stained by Masson trichrome or by polarized light microscopy after Sirius Red staining.
Morphometric Analysis
Immunofluorescence/immunohistochemical microscopy was conducted at indicated magnifications (Microscope Eclipse 800E; Nikon, Düsseldorf, Germany). Morphometric analysis was performed on digital images using the Imaging Software Lucia G 4.80 (Laboratory Imaging Ltd., Prague, Czechoslovakia). The wound area was quantified by processing of photographs taken at various time points and was calculated as the percentage of the wound area immediately after surgery. The extent of epithelialization and granulation tissue formation was determined on hematoxylin and eosin (H&E)-stained paraffin tissue sections. The length of the epithelial tongue was determined as the distance that the neo-epithelium had migrated from the margin of the wound as defined by the presence of hair follicles in nonwounded skin. In addition, the width of the gap between the epithelial tips was measured. The distance between the edges of the panniculus carnosus was determined as a measure of wound contraction. Numbers of macrophages and neutrophilic granulocytes in the wound tissues were determined by counting cells in representative squares of 250 × 250 μm2. Three squares were selected at each wound margin and the center of the wound, on each of three serial sections per wound. The data are presented as the mean number of macrophages counted in the nine squares; three serial wound sections per wound were analyzed. For quantitative analysis of CD31 or α-SMA expression, the percentage of the area of granulation tissue, which stained positive for CD31 or α-SMA, was calculated. All histomorphometric analyses were performed in a blinded manner by two independent investigators.
Wound Bursting Strength
Mice were anesthetized and the dorsal region was shaved and treated with a depilatory agent (Pilca Perfect; Stafford-Miller Continental, Oevel, Belgium). Two full-thickness incisions (1 cm) were made at one anterior and one posterior dorsal site, and the skin margins were closed with strips of a wound plaster (Fixomull stretch; Beiersdorf, Hamburg, Germany). Mice were sacrificed on day 5 after wounding, and bursting strength of the wounds was determined in situ using the BTC-2000 system (SRLI Technologies, Nashville, TN) according to the manufacturer’s protocol for the nonhuman disruptive linear incision analysis. The experiments were performed with permission from the local veterinary authorities of Zurich, Switzerland.
Analysis of Collagen Content
Circular excisional wounds of 6-mm diameter were generated as described above. The mice were sacrificed and wound tissue as well as nonwounded back skin was excised, frozen, and immediately lyophilized. Amino acid composition of 6 N HCl-hydrolyzed tissue specimens was determined by phenylisothiocyanate derivatization and reverse phase high performance liquid chromatography, as previously described.19 The percent collagen content was calculated based on the relative hydroxyproline and proline contents of collagenous and noncollagenous proteins.
Statistical Analysis
Statistical analyses were performed using SPSS version 12.0.2 (SPSS GmbH, München, Germany). Significance of difference was analyzed using the Mann-Whitney U-test for non-Gaussian distribution and the unpaired t-test for Gaussian distribution. All data are presented as mean ± SD. A P value less than 0.05 was considered significant. If several statistical tests will be performed with an unadjusted type I error rate, the P values may only be interpreted in an explorative way.
Results
Wound Closure Is Accelerated in IL-10−/− Mice
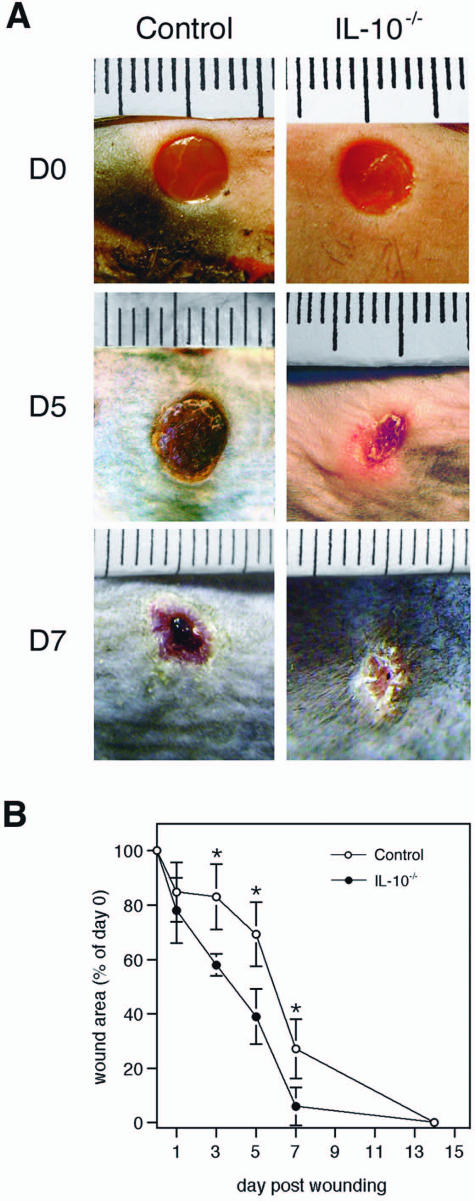
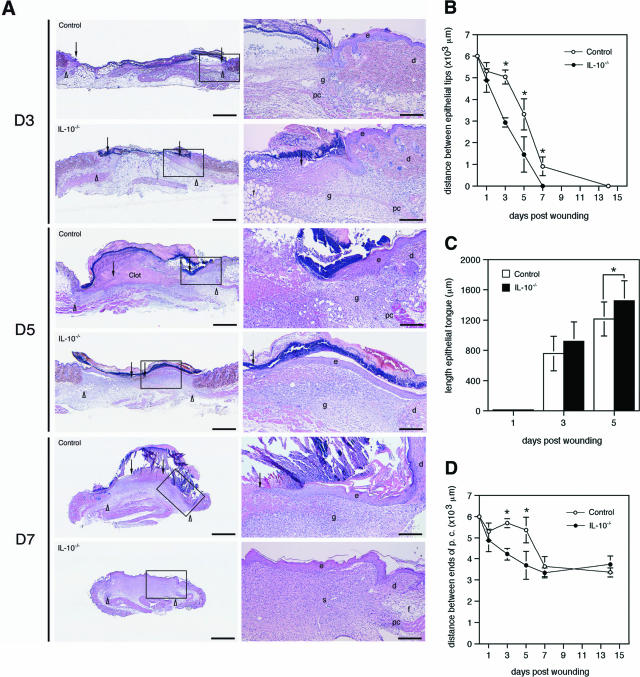
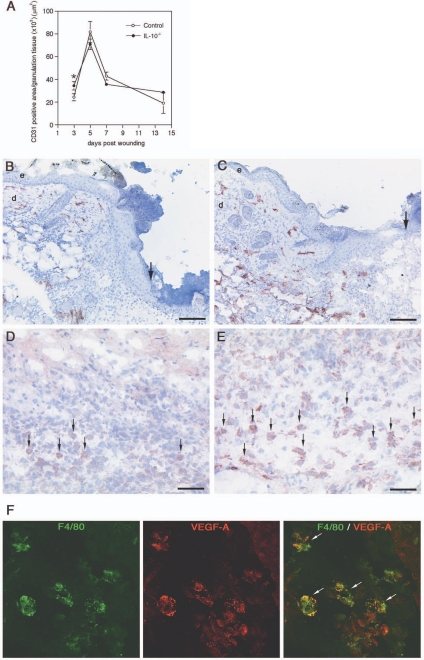
Full-thickness wounds (extending beyond the panniculus carnosus, ie, the subcutaneous muscle layer of murine skin) were generated by circular excisions of 6-mm diameter on the shaved back of IL-10−/− (20 wounds on five animals) or control mice (20 wounds on five animals). Macroscopic wound closure was accelerated in IL-10−/− mice compared with control animals (Figure 1A). On day 7 after wounding, the wounds of the mutant mice had already lost their eschar and appeared completely epithelialized, whereas the control wounds showed only partial epithelialization and were still carrying a scab (Figure 1A). Measurement of the wound area on digital images showed that the differences between mutant and control mice were statistically significant on days 3, 5, and 7 after wounding (P < 0.01) (Figure 1B). These macroscopic findings were confirmed by histological assessment of epithelialization (Figure 2A). Mutant and control animals were sacrificed on each of days 1, 3, 5, 7, and 14 after injury and the wound tissue (eight wounds on three mice per time point for each group) was excised. On H&E-stained paraffin sections, representing the longitudinal diameter of the wound, significantly shorter distances between the tips of the epithelial tongues were measured for the wounds of IL-10−/− mice compared with control animals on days 3, 5, and 7 after wounding (P < 0.04) (Figure 2B). Likewise, the length of the epithelial tongues (see Materials and Methods) was significantly increased in IL-10−/− mice in comparison with control mice on day 5 after injury (P < 0.018) (Figure 2C). These findings show that enhanced epithelialization contributed to the accelerated wound closure of IL-10-deficient mice. To analyze dermal repair, we determined granulation tissue formation and angiogenesis in wound tissue of mutant and control mice. Differences in the amount of granulation tissue were analyzed in H&E-stained sections and were shown to be not statistically different between wounds of mutant and control mice (not shown). Morphometric quantification of the expression of the endothelial cell marker CD31 within the area of granulation tissue was used as read-out for neovascular processes at the wound site. In knockout and control mice, vascular density within the granulation tissue increased during the healing response, peaking at day 5 after wounding (Figure 3A). At day 3 after wounding in IL-10-deficient mice (four wounds on two animals), the density of vascular structures was significantly increased when compared with control mice (three wounds in two animals), reflecting an accelerated angiogenic response during the early phase of repair (P < 0.04) (Figure 3, A–C). After day 3, the density of vascular structures at the wound site was not significantly different in knockout and control mice. To identify factors that might mediate the accelerated vascular response in mutant mice, we stained wound tissue for VEGF-A, one of the most potent angiogenic mediators. Numerous mononuclear leukocytes were detected that stained positive for VEGF-A within the early granulation tissue of knockout mice (Figure 3, D and E). Double staining for F4/80 and VEGF-A indicated that macrophages present the major fraction of VEGF-A expressing mononuclear cells in early granulation tissue of mutant mice (Figure 3F). These results suggest that macrophage-derived VEGF-A contributes to the accelerated angiogenic response in IL-10 knockout mice.
Figure 1.
Wound closure is accelerated in IL-10−/− versus control mice. A: Macroscopic appearance of wounds of IL-10−/− and control mice at indicated time points after injury. Whereas wounds of control mice still carry a firmly adherent scab, wounds of IL-10−/− mice had already lost the scab 5 days after wounding. At day 7, control wounds have not closed yet, whereas wounds of mutant mice are completely epithelialized. B: At the time points indicated, the wound area was determined using image analysis and expressed as percentage of the wound area immediately after injury for IL-10−/− and control mice. Data are expressed as mean ± SD, n = 20 wounds for each time point and genotype (*P < 0.01).
Figure 2.
IL-10 deficiency accelerates epithelialization. A: H&E staining of wounds of IL-10−/− and control mice at indicated time points after injury. In IL-10−/− mice, the length of the epithelial tongue was increased when compared with control mice. Correspondingly, the distance between the epithelial tips is reduced in knockout versus control mice. Whereas in control mice, the day 5 wound is filled with a fibrin-rich clot, the clot has been replaced by a cellular and highly vascularized granulation tissue in IL-10−/− mice; arrows point to the tips of epithelial tongues, arrowheads indicate wound edges defined by the presence of hair follicles in nonwounded skin. B: Distance between the epithelial tips (*P < 0.04); C: length of the epithelial tongue (*P < 0.018); D: distance between the edges of the panniculus carnosus (*P < 0.001) in wounds of IL-10−/− and control mice. Data are expressed as mean ± SD, n = 8 wounds for each time point and group. e, epidermis; d, dermis; f, subcutaneous fat layer; pc, panniculus carnosus; g, granulation tissue. Scale bars: 1 mm (left); 250 μm (right). Original magnifications: ×12.5 (A, left); ×50 (A, right).
Figure 3.
Angiogenesis in wounds of IL-10−/− and control mice. A: Computer-assisted morphometric quantification of CD31-positive cells within the granulation tissue of IL-10−/− and control mice during healing (*P < 0.04); data are expressed as mean ± SD, n = 3 wounds for each time point and group. B and C: CD31-stained blood vessels (red) 3 days after wounding; microvascular density is significantly increased in early granulation tissue of IL-10−/− mice when compared with control mice; arrows indicate the epithelial wound edge. D and E: VEGF-A staining (red) 3 days after wounding; arrows indicate VEGF-A-expressing mononuclear cells within the granulation tissue of control (D) and mutant mice (E). F: Double labeling for F4/80 (green) and VEGF-A (red) in granulation tissue of IL-10−/− mice indicates VEGF-A expression in macrophages. e, epidermis; d, dermis. Scale bars: 100 μm (B, C); 50 μm (D, E). Original magnifications, ×1000 (F).
Accelerated Wound Healing in IL-10−/− Mice Is Associated with Enhanced Macrophage Infiltration
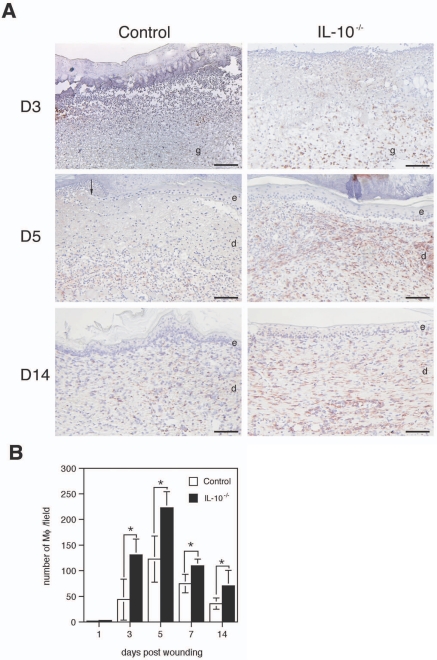
H&E staining (Figure 2A) as well as staining for the neutrophil-specific enzyme chloroacetate esterase (not shown) demonstrated a substantial influx of neutrophilic granulocytes into day 1 and day 3 wound tissue of mutant and control mice. Quantitative evaluation did not reveal significant differences in neutrophil numbers between mutant and control mice (not shown). In contrast, immunostaining of wound tissue for the macrophage marker F4/80 demonstrated that the influx of these cells occurred earlier, that they persisted longer at the wound site, and that the number of infiltrating macrophages was increased at the wound site of IL-10−/− mice in comparison with control wounds (Figure 4A). Thus, significantly more macrophages were counted in IL-10 mutant mice compared with controls on day 3 through day 14 after wounding (Figure 4B) (eight wounds from three animals for each experimental group, P < 0.024).
Figure 4.
Wounds of IL-10−/− mice contain increased numbers of macrophages. F4/80 immunostaining of wounds analyzed at the indicated time points after injury. A: The central part of day 3 wounds (top), the wound margins covered by neo-epidermis of day 5 wounds (middle), and the late granulation tissue of day 14 wounds (bottom) show increased numbers of infiltrating macrophages in IL-10−/− versus control mice. B: Macrophages within the center of the wound and wound margins were counted in high-power fields. Data are expressed as means ± SD, n = 8 wounds for each time point and group (*P < 0.024). e, epidermis; d, dermis; g, granulation tissue; Mφ, macrophages. Scale bars = 100 μm.
Alternatively Activated Macrophages Are Present in IL-10−/− Wound Tissue
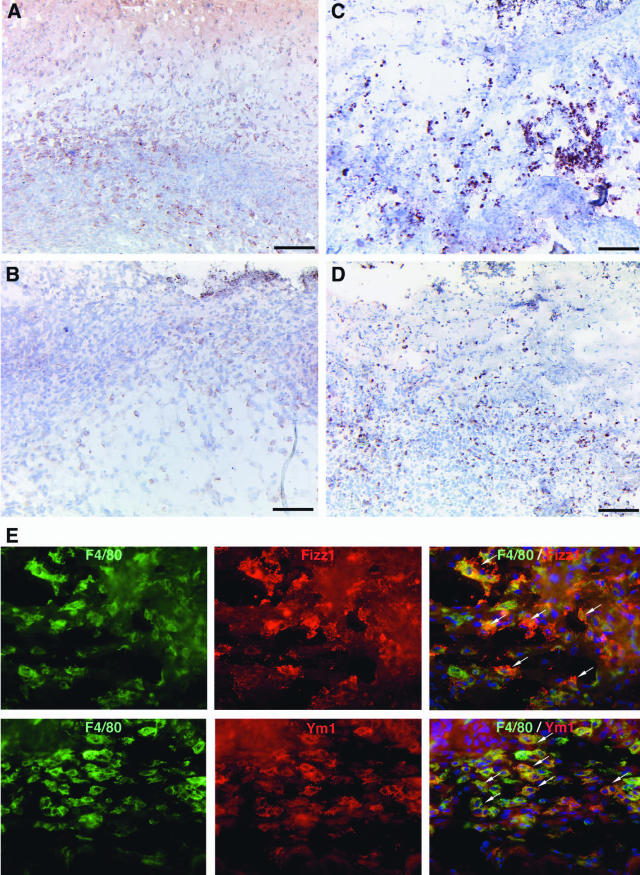
To phenotypically characterize the macrophages infiltrating the wound tissue, we analyzed wound sections for the expression of Fizz1 (found in inflammatory zone)/RELM-α and Ym1/ECF (eosinophil chemotactic factor). Expression of both factors was recently described as a reliable marker of alternatively activated macrophages.21,22 In wound tissue 5 days after injury, both control (three wounds on three animals) and IL-10-deficient wound tissue (three wounds on three animals) contained numerous Fizz1- and Ym1-positive cells (Figure 5, A–D). Double staining for F4/80 and Fizz1 or Ym1 revealed that most cells expressing these markers were macrophages (Figure 5G). The fraction of Fizz1- or Ym1-positive macrophages was similar in knockout and control animals.
Figure 5.
Fizz1- and Ym1-expressing macrophages in wound tissue of IL-10−/− and control mice. Day 5 wounds of control (A, B) and IL-10−/− mice (C, D) stained for Fizz1 (A, C) or Ym1 (B, D); numerous mononuclear cells within the granulation tissue of control and mutant mice stain positive for Fizz1 or Ym1. E: Double labeling for F4/80 (green) and Fizz1 or Ym1 (red) in granulation tissue of IL-10−/− mice demonstrates Fizz1 and Ym1 expression by macrophages. Scale bars = 100 μm. Original magnifications, ×400 (E).
Wound Contraction Is Enhanced in IL-10−/− Mice
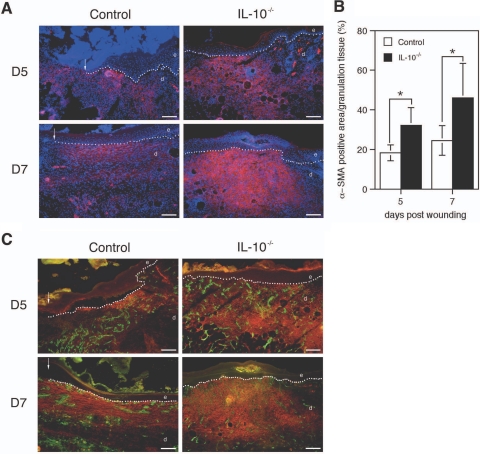
To investigate whether, in addition to more rapid epithelialization, enhanced contraction of the wound tissue also contributed to the accelerated wound closure of IL-10-deficient mice, we measured the distance between the edges of the panniculus carnosus at the wound margins (Figure 2D). This distance was significantly shorter in wounds from IL-10 mutant mice than in the control wounds on day 3 and 5 after wounding (P < 0.001). In 5-day old wounds of mutant and control mice, immunostaining for α-smooth muscle actin (α-SMA), a marker for myofibroblast differentiation, revealed that α-SMA was abundantly expressed at the wound margins and to a lesser extent in the wound bed (Figure 6A). In day 7 wounds, α-SMA was present throughout the entire layer of the late granulation tissue that had developed beneath the neoepidermis and which extended into deep dermal layers (Figure 6A). Although the pattern of α-SMA expression during repair was similar in both experimental groups, in day 5 and day 7 wounds from IL-10 mutant mice, the number of α-SMA-positive cells as well as the staining intensity of positive cells was significantly increased (Figure 6B), suggesting that augmented myofibroblast differentiation is responsible for the enhanced contraction of wounds from IL-10-deficient animals (five wounds from three animals for each experimental group per day, P < 0.03).
Figure 6.
α-SMA expression in wounds of IL-10−/− and control mice. A: α-SMA immunostaining (red) of day 5 and day 7 wound tissue, DAPI counterstaining of nuclei (blue). B: Morphometric quantification of the area within the granulation tissue that stained positive for α-SMA at indicated time points after injury; data are expressed as means ± SD, n = 5 wounds for each time point and group (*P < 0.03). C: Double labeling of CD31 (green) and α-SMA actin (red) of day 5 and day 7 wound tissue. Note that in wounds of both mouse strains the α-SMA staining is found in the areas between the capillary structures, indicating the nonendothelial cell origin of this staining and suggesting the presence of myofibroblasts. e, epidermis; d, dermis; g, granulation tissue. Scale bars = 100 μm.
To evaluate whether the increased staining for α-SMA in IL-10−/− wounds might be attributable to an increased number of vascular structures coated with α-SMA-positive perivascular cells, we performed immunofluorescent double labeling for CD31 and α-SMA. At days 5 and 7 after wounding, both IL-10−/− and control mice showed a highly vascularized granulation tissue (Figure 6C). In wounds of both mouse strains, the tissue between capillary structures stained positive for α-SMA, indicating a nonendothelial cell origin of this staining and suggesting the presence of myofibroblasts.
Reduced Mechanical Strength of the Granulation Tissue in IL-10−/− Wounds
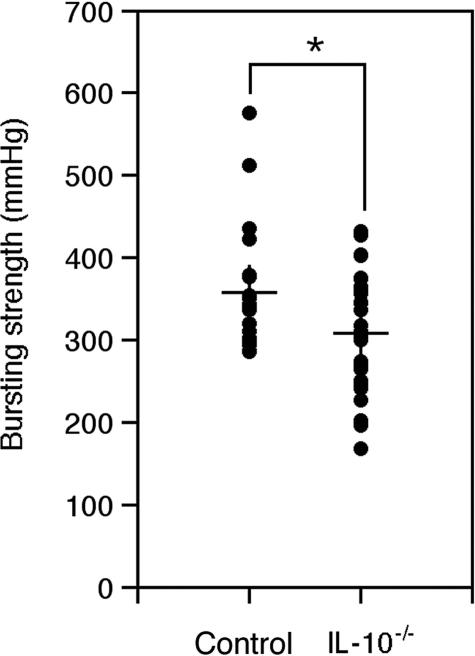
To analyze whether the accelerated wound healing observed in IL-10-deficient mice results in altered biomechanical qualities of the early wound tissue, we generated full-thickness incisional wounds (see Materials and Methods) on the shaved backs of IL-10 mutant (30 wounds from 15 mice) and control mice (20 wounds from 10 mice). On day 5 after injury, when the wounds were closed in both experimental groups, the animals were sacrificed, and the negative pressure at which the wounds ruptured was measured (see Materials and Methods). Wound tissue from IL-10-deficient mice had a significantly reduced bursting strength compared with wounds from wild-type mice (Figure 7), indicating that IL-10 affects the quality of the repaired tissue (P < 0.027).
Figure 7.
Reduced bursting strength of wound tissue in IL-10-deficient mice. The bursting strength of day 5 incisional wounds, measured using a suction device, was significantly reduced in IL-10−/− mice when compared with control mice; data are expressed as means ± SD, n = 20 control wounds, n = 30 IL-10−/− wounds (*P < 0.027).
Architecture of Late Granulation Tissue Is Altered in IL-10−/− and Control Mice
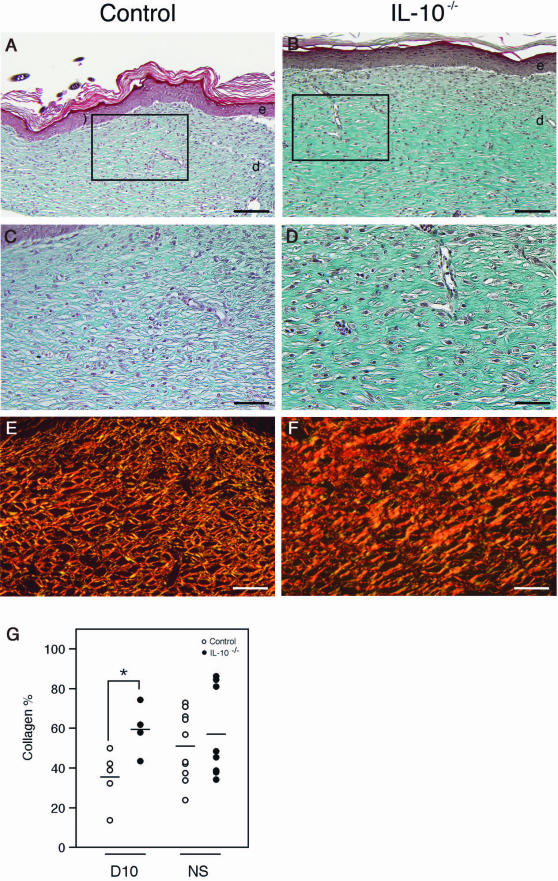
The macroscopic appearance of day 14 wound tissue in IL-10−/− and control mice was similar. In contrast, major differences between mutant and control mice became apparent when we assessed the dermal architecture by Masson trichrome staining in mutant (four wounds from two animals) and control mice (seven wounds from four animals) (Figure 8, A–D). Notably, at day 14 in wounds of IL-10−/− mice (three wounds of four) the basket-weave organization of collagen bundles characteristic for control mice was primarily absent. Instead, thick and densely packed parallel layers of collagen were present. Advanced maturation and organization of collagen bundles in knockout mice was revealed by Sirius Red staining of day 14 wound sections and examination with polarized light (Figure 8, E and F). In addition, in mutant mice the dermo-epidermal junction was flat, whereas in control mice rete ridges were partially present. To investigate whether altered organization of collagen bundles in mutant mice is caused by an increase of collagen deposition, we quantified the collagen content in day 10 wound tissue (five wounds from five control animals; four wounds from four IL-10−/− mice, see Materials and Methods). In wounds of IL-10−/− mice, relative collagen content was significantly increased when compared with control mice (P < 0.027) (Figure 8G), indicating that IL-10 deficiency promoted collagen deposition.
Figure 8.
Dermal architecture of late granulation tissue in IL-10−/− and control mice. A–D: Masson trichrome staining of day 14 wounds. In IL-10−/− mice, the basket-weave organization of collagen bundles is primarily absent and replaced by thick and densely packed parallel layers of collagen; in IL-10−/− mice the dermo-epidermal junction is flat, whereas in control mice rete ridges are present. E and F: Sirius Red staining and examination with polarized light revealed advanced organization and maturation of collagen bundles in day 14 wounds of IL-10−/− mice when compared with controls. G: In day 10 wounds, collagen content is significantly increased in IL-10−/− mice (n = 4 wounds) when compared with control mice (n = 5 wounds); each point represents the collagen content in tissue derived from one wound; horizontal bars represent the means of collagen content within each group of mice (*P < 0.024). NS, collagen content in nonwounded skin; e, epidermis; d, dermis. Scale bars = 100 μm.
Discussion
We show that wound healing is accelerated in IL-10−/− mice, which closed excisional wounds significantly earlier compared with IL-10-competent control littermates. This effect was attributable to a combination of accelerated epithelialization as well as increased wound contraction in the mutant animals. IL-10−/− wound tissue contained more macrophages than wounds from control mice and formed a highly cellular granulation tissue.
The impact of the local inflammatory response on the process of wound healing has been intensively discussed.2 In particular the question whether infiltrating macrophages and granulocytes promote or impede tissue repair has received much attention. Recent reports argued against an essential role of inflammatory cells in the wound repair process. In various experimental models of repair, inflammation has been shown to delay healing and to result in excessive fibrosis. A recent study analyzed wound healing in PU.1 mutant mice, which are deficient for multiple hemopoietic cell lineages and completely devoid of macrophages and neutrophilic granulocytes.23 Newborn PU.1-null mice, rescued by antibiotic treatment from perinatal lethal septicemia, repaired incisional and small (2 mm) excisional defects with similar kinetics as control animals. Moreover, wound healing followed an embryonic pattern with little scarring. These findings show that the presence of inflammatory hemopoietic cells in the wound tissue is not an essential prerequisite for repair—at least not for small wounds in neonatal repair under normal laboratory conditions. On the other hand, this experiment demonstrates that already in the neonate, and under the particular conditions of the animal model used, leukocytes significantly affect the outcome of wound repair. In addition, wound healing was analyzed in a number of mouse mutants with defects for molecules that affect the local inflammatory response. In mice deficient for the TNF receptor p55,4 for IFN-γ,5 or the transcriptional regulator Smad3,24 a reduced infiltration of the wound tissue by macrophages and/or neutrophils was observed. Wound closure, however, was found improved in these mutants. The inflammatory response was augmented, but wound healing was impaired in mutant mice constitutively expressing the chemokine IP-10 in the epidermis25 and in knockout mice deficient for the anti-inflammatory secretory leukocyte protease inhibitor.26 In contrast to these studies, experiments more than 30 years ago supported the concept that inflammation is essential for the establishment of cutaneous homeostasis after injury. In these studies, the functions of neutrophils and macrophages at the wound site were analyzed by depletion with antisera and/or treatment with anti-inflammatory steroids. Although depletion of neutrophils from guinea pig wounds did not significantly perturb healing of incisional wounds under sterile conditions,27 the depletion of macrophages prevented healing of incisional skin wounds.28 In more recent studies, mice deficient for endothelial cell adhesion molecules essential for the recruitment of myeloid cells from the vascular system were analyzed. P- and E-selectin double-deficient mice,29 mice deficient in β-1,4-galactosyltransferase, which glycosylates the P and E selectins30 as well as intercellular adhesion molecule-1-deficient mice31 showed a significantly reduced infiltration of neutrophils and macrophages as well as a dramatic delay in wound closure.
IL-10 is an immunomodulatory cytokine with potent anti-inflammatory effects, suppressing most facets of innate and T-cell-mediated inflammation. In a variety of different cell types, including macrophages, neutrophils, dendritic cells, and T cells, IL-10 blocks proinflammatory function and mediates down-regulation of most known proinflammatory mediators.8 Given these broad anti-inflammatory effects of IL-10, our finding of accelerated closure of wounds in IL-10-deficient mice provides strong evidence for beneficial effects of the local inflammatory response on the repair process.
In other models of skin inflammation, IL-10 deficiency8 resulted in exaggerated responses, leading to tissue destruction. Whereas the response of IL-10-deficient mice to a low dose of the irritant croton oil was characterized by edema and infiltration of macrophages and neutrophils, application of higher doses elicited a more vigorous response ultimately leading to tissue necrosis.17 Likewise, subcutaneous injections of the TLR ligands lipopolysaccharide and CpG resulted in extensive necrosis in IL-10-deficient mice.16 We speculate that the local inflammatory response to tissue injury, although beneficial under the conditions of our experiment, may exert destructive effects delaying wound healing, if activated beyond a threshold by additional stimuli for the innate immune system such as bacterial infection.
The wounds of IL-10-deficient mice contained higher numbers of macrophages at all time points tested, whereas the number of neutrophils was similar as in control mice. This finding is suggestive of an important role of macrophages in the promotion of tissue repair. The release of increased amounts of various growth factors by macrophages might result in enhanced stimulation of epithelial and mesenchymal cells supporting the healing response. Indeed, numerous macrophages stained positive for VEGF-A in the early granulation tissue of IL-10-deficient mice. Therefore, the accelerated angiogenic response observed in these mice during the early phase of repair might have been mediated by the increase in macrophage-derived VEGF-A. However, an altered function of neutrophilic granulocytes, dendritic cells, or mast cells may also contribute to the acceleration of tissue repair in the mutant animals. In addition, we cannot exclude direct effects of IL-10 on proliferation, migration, and function of resident cells including fibroblasts,32,33 endothelial cells,34 or keratinocytes.35
Based on distinct cytokine profiles and biological functions subsets of activated macrophages have been identified.36 The best-described population is the classically activated macrophage that differentiates on stimulation with IFN-γ and TNF-α and that is an effector cell in Th1 cellular immune responses.37 A second subset of alternatively activated macrophages is induced by IL-4 or glucocorticoids and is characterized by an anti-inflammatory capacity.36,38 The signature cytokines of this macrophage population are the immunosuppressive factors IL-10, IL-1 receptor antagonist, and TGF-β1.36 Alternatively activated macrophages were initially described in vitro and were implicated to play a role in tissue repair.39 Our study revealed strong expression of two newly identified markers of alternatively activated macrophages, Fizz1 and Ym1,21,22 by a fraction of macrophages in wounds of control and IL-10-deficient mice. To our knowledge, this is the first description of alternatively activated macrophages in skin wounds. Because IL-10 suppresses the production of IFN-γ and TNF-α which are key mediators of classical macrophage activation,8,10 one might have assumed that augmented classical and reduced alternative macrophage activation might occur in IL-10−/− mice after injury. Our results indicate that IL-10 is not required to induce or maintain the alternatively activated macrophage phenotype. Current studies in our group investigate the impact of IL-10 deficiency on macrophage activation pathways.
In addition to the enhanced epithelialization, also a more rapid contraction of the wound tissue contributed to the accelerated wound closure in IL-10−/− mice. Immunostaining for α-SMA revealed an enhanced differentiation of myofibroblasts in the wounds of IL-10−/− mice. TGF-β1 is the major growth factor inducing myofibroblast differentiation during repair, and infiltrating macrophages at the wound site are considered to be a major source for TGF-β1.40,41 In support of this view, a recent study by Peters and colleagues42 demonstrated that wound repair in CD18-deficient mice was significantly delayed because of impaired myofibroblast differentiation and wound contraction. This delay was probably attributable to reduced levels of TGF-β1 at the wound site.42 Our data suggest that in IL-10-deficient mice, the abundance of macrophages at the wound site results in an increased release of TGF-β1, which mediates enhanced myofibroblast differentiation and hence more rapid wound contraction.
Although IL-10 deficiency resulted in accelerated wound closure, biomechanical strength of day 5 wound tissue was significantly reduced in knockout mice when compared with controls, indicating that matrix deposition and maturation is not improved but rather impeded by IL-10 deficiency during the phase of early granulation tissue formation. Decreased biomechanical strength of wound tissue in IL-10-deficient mice during this early phase of repair might reflect the increased proportion of cells and provisional wound matrix versus collagen at the wound site. Our results suggest that IL-10 deficiency at the wound site might prolong the inflammatory phase and delay resolution of the early granulation tissue.
During later stages of repair, microscopic analysis of wound tissue revealed an increased thickness as well as advanced maturation and organization of collagen bundles in mutant versus control mice. This observation is suggestive of increased synthesis and/or altered molecular assembly of extracellular matrix components in the mutant mice. Several independent in vitro studies demonstrated that IL-10 directly regulates synthesis and degradation of various extracellular matrix molecules by different fibroblastic cell types.32,33,43,44 Whereas IL-10 down-regulated collagen I and fibronectin mRNA in dermal fibroblasts, IL-10 enhanced the expression of decorin, collagenase-1, as well as stromelysin-1.32,44 In addition, the cited studies showed that IL-10 suppressed the TGF-β1-elicited increase in type I collagen and fibronectin mRNA expression. Furthermore, in a recent in vivo study, IL-10 was shown to protect mice from bleomycin-induced TGF-β1-mediated fibrosis.45 Anti-fibrotic effects of IL-10 have also been described after skin injury. Liechty and colleagues19 grafted embryonic skin from IL-10−/− mice onto strain-matched adult mice and wounded this skin. Whereas skin grafts of controls showed little inflammation and normal restoration of the dermal architecture, wounded IL-10−/− grafts were characterized by a much more pronounced inflammatory cell infiltration and collagen deposition. These results suggest an important role of IL-10 in regulating the expression of proinflammatory cytokines in fetal wounds, leading to reduced matrix deposition and scar-free healing. In our model, the increased matrix deposition during later stages of repair, which might be attributable to abundant TGF-β1-producing macrophages as outlined above, may be further amplified by the lack of direct suppressive effects of IL-10 on matrix synthesis and deposition by fibroblasts. However, further studies are required to better understand potential anti-fibrotic mechanisms of IL-10 in skin.
Taken together, our data show that IL-10 has important effects on tissue repair that delay the closure of noncomplicated wounds in healthy animals. The effects of IL-10 on tissue repair under conditions of compromised wound healing such as infection or diabetes mellitus, however, may be different and require further experiments.
Acknowledgments
We thank Michael Piekarek and Tobias Häring for their technical assistance; Silke Kummer for excellent assistance preparing immunohistochemical stainings; and Hildegard Christ, Institute of Medical Statistics, Informatics, and Epidemiology, for advice in the statistical analysis.
Footnotes
Address reprint requests to Priv.-Doz. Dr. med. Sabine A. Eming, Department of Dermatology, University of Cologne, Joseph-Stelzmann Str. 9, 50931 Köln, Germany. E-mail: sabine.eming@uni-koeln.de.
Supported by the Deutsche Forschungsgemeinschaft (grant SFB 589 to S.A.E., A.R., and T.K.), the Swiss National Science Foundation (grant 3100A0-109340/1 to S.W.), the National Institutes of Health (grants AG-06528 and AR-44104 to J.M.D.), the Department of Veterans Affairs (to J.M.D.), and the European Community (LSHB-CT-2005-512102 to S.A.E. and S.W.).
References
- Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Eming SA, Krieg T, Davidson JM: Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol (in press) [DOI] [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Mori R, Kondo T, Ohshima T, Ishida Y, Mukaida N. Accelerated wound healing in tumor necrosis factor receptor p55-deficient mice with reduced leukocyte infiltration. FASEB J. 2002;16:963–974. doi: 10.1096/fj.01-0776com. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Takayasu T, Iwakura Y, Mukaida N. The essential involvement of cross-talk between IFN-gamma and TGF-beta in the skin wound-healing process. J Immunol. 2004;172:1848–1855. doi: 10.4049/jimmunol.172.3.1848. [DOI] [PubMed] [Google Scholar]
- Gallucci RM, Simeonova PP, Matheson JM, Kommineni C, Guriel JL, Sugawara T, Luster MI. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000;14:2525–2531. doi: 10.1096/fj.00-0073com. [DOI] [PubMed] [Google Scholar]
- Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, Kovacs EJ, DiPietro LA. Wound healing in MIP-1alpha(−/−) and MCP-1(−/−) mice. Am J Pathol. 2001;159:457–463. doi: 10.1016/s0002-9440(10)61717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefytqq R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Fiorentino DF, Zlotnik A, Mosmann TR, Howard, M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Kullberg MC, Ward JM, Gorelick PL, Caspar P, Hieny S, Cheever A, Jankovic D, Sher A. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kühn R, Müller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- Deckert M, Soltek S, Geginat G, Lutjen S, Montesinos-Rongen M, Hof H, Schlüter D. Endogenous interleukin-10 is required for prevention of a hyperinflammatory intracerebral immune response in Listeria monocytogenes meningoencephalitis. Infect Immun. 2001;69:4561–4571. doi: 10.1128/IAI.69.7.4561-4571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namangala B, Noel W, De Baetselier P, Brys L, Beschin A. Relative contribution of interferon-gamma and interleukin-10 to resistance to murine African trypanosomosis. J Infect Dis. 2001;183:1794–1800. doi: 10.1086/320731. [DOI] [PubMed] [Google Scholar]
- Berg DJ, Kühn R, Rajewsky K, Muller W, Menon S, Davidson N, Grunig G, Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewe L, Bollati-Fogolin M, Wickenhauser C, Krieg T, Müller W, Roers A: Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not to CpG oligonucleotides. Eur J Immunol (in press) [DOI] [PubMed] [Google Scholar]
- Berg DJ, Leach MW, Kühn R, Rajewsky K, Müller W, Davidson NJ, Rennick D. Interleukin 10 but not interleukin 4 is a natural suppressant of cutaneous inflammatory responses. J Exp Med. 1995;182:99–108. doi: 10.1084/jem.182.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Ohshima T, Kondo T. Regulatory role of endogenous interleukin-10 in cutaneous inflammatory response of murine wound healing. Biochem Biophys Res Commun. 1999;265:194–199. doi: 10.1006/bbrc.1999.1455. [DOI] [PubMed] [Google Scholar]
- Liechty KW, Kim HB, Adzick NS, Crombleholme TM. Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg. 2000;35:866–872. doi: 10.1053/jpsu.2000.6868. [DOI] [PubMed] [Google Scholar]
- Buckley A, Hill KE, Davidson JM. Collagen metabolism. Methods Enzymol. 1988;163:674–694. doi: 10.1016/0076-6879(88)63056-4. [DOI] [PubMed] [Google Scholar]
- Raes G, De Baetselier P, Noel W, Beschin A, Brombacher F, Hassanzadeh G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- Nair MG, Cochrane DW, Allen JE. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol Lett. 2003;85:173–180. doi: 10.1016/s0165-2478(02)00225-0. [DOI] [PubMed] [Google Scholar]
- Martin P, D’Souza D, Martin J, Grose R, Cooper L, Maki R, McKercher SR. Wound healing in the PU.1 null mouse—tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:1122–1128. doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, Roberts AB. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- Luster AD, Cardiff RD, MacLean JA, Crowe K, Granstein RD. Delayed wound healing and disorganized neovascularization in transgenic mice expressing the IP-10 chemokine. Proc Assoc Am Physicians. 1998;110:183–196. [PubMed] [Google Scholar]
- Ashcroft GS, Lei K, Jin W, Longenecker G, Kulkarni AB, Greenwell-Wild T, Hale-Donze H, McGrady G, Song XY, Wahl SM. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat Med. 2000;6:1147–1153. doi: 10.1038/80489. [DOI] [PubMed] [Google Scholar]
- Simpson DM, Ross R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum: the neutrophilic leukocyte in wound repair a study with antineutrophil serum. J Clin Invest. 1972;51:2009–2023. doi: 10.1172/JCI107007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- Subramaniam M, Saffaripour S, Van De Water L, Frenette PS, Mayadas TN, Hynes RO, Wagner DD. Role of endothelial selectins in wound repair. Am J Pathol. 1997;150:1701–1709. [PMC free article] [PubMed] [Google Scholar]
- Mori R, Kondo T, Nishie T, Ohshima T, Asano M. Impairment of skin wound healing in beta-1,4-galactosyltransferase-deficient mice with reduced leukocyte recruitment. Am J Pathol. 2004;164:1303–1314. doi: 10.1016/s0002-9440(10)63217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka T, Kaburagi Y, Hamaguchi Y, Hasegawa M, Takehara K, Steeber DA, Tedder TF, Sato S. Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. Am J Pathol. 2000;157:237–247. doi: 10.1016/S0002-9440(10)64534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Eckes B, Krieg T. Effect of interleukin-10 on the gene expression of type I collagen, fibronectin, and decorin in human skin fibroblasts: differential regulation by transforming growth factor-beta and monocyte chemoattractant protein-1. Biochem Biophys Res Commun. 2001;281:200–205. doi: 10.1006/bbrc.2001.4321. [DOI] [PubMed] [Google Scholar]
- Moroguchi A, Ishimura K, Okano K, Wakabayashi H, Maeba T, Maeta H. Interleukin-10 suppresses proliferation and remodeling of extracellular matrix of cultured human skin fibroblasts. Eur Surg Res. 2004;36:39–44. doi: 10.1159/000075073. [DOI] [PubMed] [Google Scholar]
- Cattaruzza M, Slodowski W, Stojakovic M, Krzesz R, Hecker M. Interleukin-10 induction of nitric-oxide synthase expression attenuates CD40-mediated interleukin-12 synthesis in human endothelial cells. J Biol Chem. 2003;278:37874–37880. doi: 10.1074/jbc.M301670200. [DOI] [PubMed] [Google Scholar]
- Michel G, Mirmohammadsadegh A, Olasz E, Jarzebska-Deussen B, Muschen A, Kemeny L, Abts HF, Ruzicka T. Demonstration and functional analysis of IL-10 receptors in human epidermal cells: decreased expression in psoriatic skin, down-modulation by IL-8, and up-regulation by an antipsoriatic glucocorticosteroid in normal cultured keratinocytes. J Immunol. 1997;159:6291–6297. [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-g genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Stein M, Keshav S, Harris N, Gordon S. IL-4 potently enhances murine macrophage mannose receptor activity; a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratchev A, Guillot P, Hakiy N, Politz O, Orfanos CE, Schledzewski K, Goerdt S. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein bIG-H3. Scand J Immunol. 2001;53:386–392. doi: 10.1046/j.1365-3083.2001.00885.x. [DOI] [PubMed] [Google Scholar]
- Assoian RK, Fleurdelys BE, Stevenson HC, Miller PJ, Madtes DK, Raines EW, Ross R, Sporn MB. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA. 1987;84:6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Peters T, Sindrilaru A, Hinz B, Hinrichs R, Menke A, Al-Azzeh EA, Holzwarth K, Oreshkova T, Wang H, Kess D, Walzog B, Sulyok S, Sunderkotter C, Friedrich W, Wlaschek M, Krieg T, Scharffetter-Kochanek K. Wound-healing defect of CD18(−/−) mice due to a decrease in TGF-beta1 and myofibroblast differentiation. EMBO J. 2005;24:3400–3410. doi: 10.1038/sj.emboj.7600809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T, Abe K, Matsuoka H, Yoshida M, Mori M, Goya S, Kida H, Nishino K, Osaki T, Tachibana I, Kaneda Y, Hayashi S. Introduction of the interleukin-10 gene into mice inhibited bleomycin-induced lung injury in vivo. Am J Physiol. 2000;278:L914–L922. doi: 10.1152/ajplung.2000.278.5.L914. [DOI] [PubMed] [Google Scholar]
- Reitamo S, Remitz A, Tamai K, Uitto J. Interleukin-10 modulates type I collagen and matrix metalloprotease gene expression in cultured human skin fibroblasts. J Clin Invest. 1994;94:2489–2492. doi: 10.1172/JCI117618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitani A, Fuss I, Nakamura K, Kumaki F, Usui T, Strober W. Transforming growth factor (TGF)-beta1-producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF-beta1-mediated fibrosis. J Exp Med. 2003;198:1179–1188. doi: 10.1084/jem.20030917. [DOI] [PMC free article] [PubMed] [Google Scholar]