Abstract
Enterobacter cloacae strains producing chromosomally mediated beta-lactamase constitutively show high degrees of resistance to most of the third-generation beta-lactams. It has been proposed that this resistance is due to the nonhydrolytic binding or trapping of beta-lactams by the enzyme. We found that the outer membrane of E. cloacae strain 55M indeed had permeability to cefazolin about 14-fold lower than that of Escherichia coli, and that the number of beta-lactamase molecules produced by this constitutive mutant was exceptionally large (2 X 10(5) per cell). These conditions are expected to produce a low degree of resistance, but could not explain the high resistance level of the mutant. We showed that the beta-lactamase of this strain hydrolyzed third-generation beta-lactams at measurable rates. Although the V max for these compounds was less than 0.01% of that for cefazolin, the enzyme could hydrolyze them at rates comparable to the rate for cefazolin when the substrate concentration was near 0.1 microM, a concentration thought to be physiologically relevant for the inhibition of cell growth, because of the exceptionally high affinity of the enzyme to many third-generation compounds. Calculations based on kinetic parameters of the enzyme, outer membrane permeability, and affinity toward penicillin-binding proteins succeeded in predicting the MICs for several third-generation beta-lactams. The data suggest that hydrolysis may be more important than nonhydrolytic binding for the expression of the resistant phenotype, and that studies on the susceptibility of beta-lactams to beta-lactamases should be carried out at physiologically relevant, very low concentrations of the drug, rather than the customary very high concentrations, such as 100 microM.
Full text
PDF
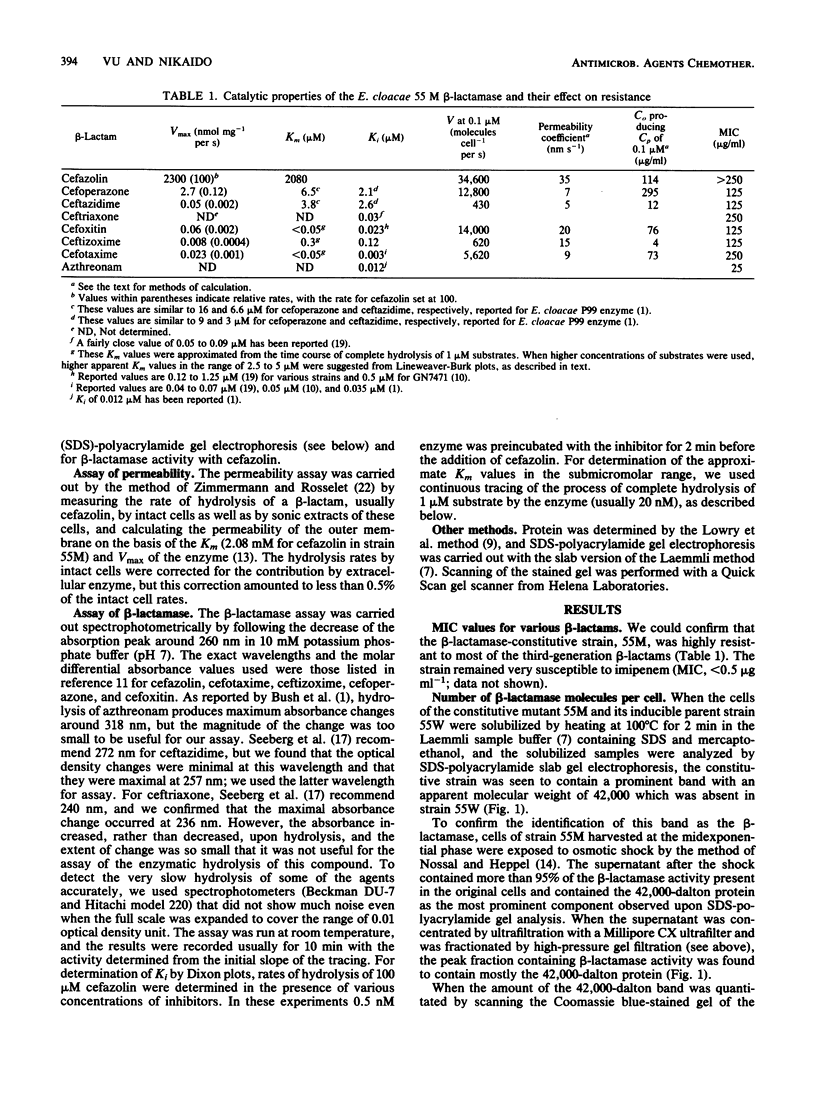
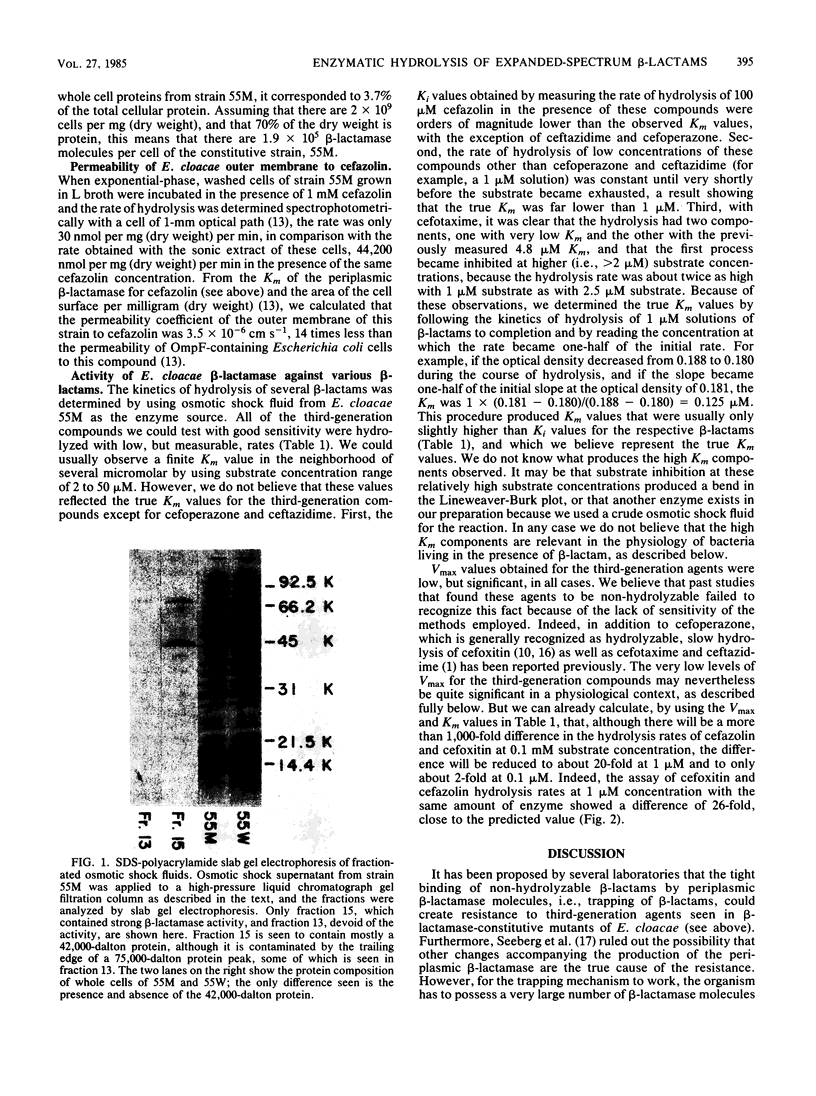
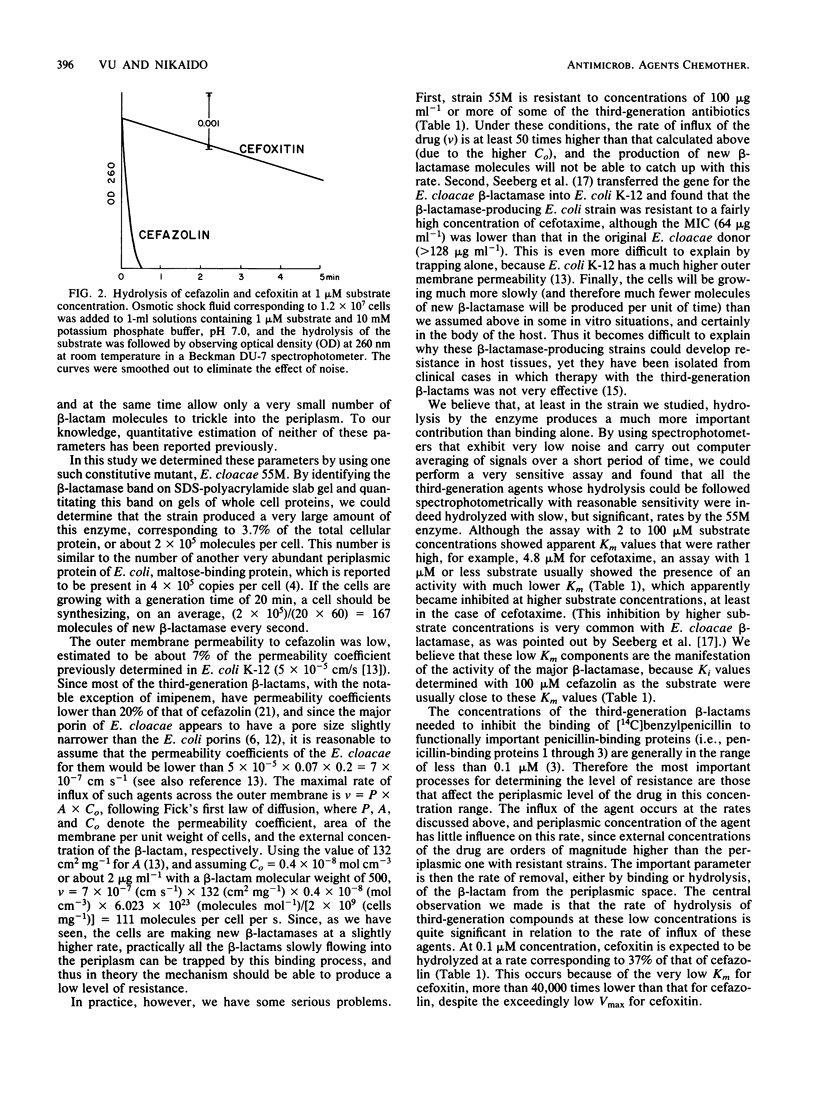


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bush K., Freudenberger J. S., Sykes R. B. Interaction of azthreonam and related monobactams with beta-lactamases from gram-negative bacteria. Antimicrob Agents Chemother. 1982 Sep;22(3):414–420. doi: 10.1128/aac.22.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Curtis N. A., Orr D., Ross G. W., Boulton M. G. Affinities of penicillins and cephalosporins for the penicillin-binding proteins of Escherichia coli K-12 and their antibacterial activity. Antimicrob Agents Chemother. 1979 Nov;16(5):533–539. doi: 10.1128/aac.16.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzel I., Kolb V., Boos W. Pole cap formation in Escherichia coli following induction of the maltose-binding protein. Arch Microbiol. 1978 Aug 1;118(2):207–218. doi: 10.1007/BF00415731. [DOI] [PubMed] [Google Scholar]
- Gootz T. D., Sanders C. C., Goering R. V. Resistance to cefamandole: derepression of beta-lactamases by cefoxitin and mutation in Enterobacter cloacae. J Infect Dis. 1982 Jul;146(1):34–42. doi: 10.1093/infdis/146.1.34. [DOI] [PubMed] [Google Scholar]
- Kaneko M., Yamaguchi A., Sawai T. Purification and characterization of two kinds of porins from the Enterobacter cloacae outer membrane. J Bacteriol. 1984 Jun;158(3):1179–1181. doi: 10.1128/jb.158.3.1179-1181.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Livermore D. M. Kinetics and significance of the activity of the Sabath and Abrahams' beta-lactamase of Pseudomonas aeruginosa against cefotaxime and cefsulodin. J Antimicrob Chemother. 1983 Feb;11(2):169–179. doi: 10.1093/jac/11.2.169. [DOI] [PubMed] [Google Scholar]
- Minami S., Inoue M., Mitsuhashi S. Purification and properties of a cephalosporinase from Enterobacter cloacae. Antimicrob Agents Chemother. 1980 Dec;18(6):853–857. doi: 10.1128/aac.18.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J Bacteriol. 1983 Jan;153(1):241–252. doi: 10.1128/jb.153.1.241-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Sanders C. C. Novel resistance selected by the new expanded-spectrum cephalosporins: a concern. J Infect Dis. 1983 Mar;147(3):585–589. doi: 10.1093/infdis/147.3.585. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Emergence of resistance to cefamandole: possible role of cefoxitin-inducible beta-lactamases. Antimicrob Agents Chemother. 1979 Jun;15(6):792–797. doi: 10.1128/aac.15.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg A. H., Tolxdorff-Neutzling R. M., Wiedemann B. Chromosomal beta-lactamases of Enterobacter cloacae are responsible for resistance to third-generation cephalosporins. Antimicrob Agents Chemother. 1983 Jun;23(6):918–925. doi: 10.1128/aac.23.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Sawai T., Ando T., Yamagishi S. Cefoxitin resistance by a chromosomal cephalosporinase in Escherichia coli. J Antibiot (Tokyo) 1980 Sep;33(9):1037–1042. doi: 10.7164/antibiotics.33.1037. [DOI] [PubMed] [Google Scholar]
- Then R. L., Angehrn P. Trapping of nonhydrolyzable cephalosporins by cephalosporinases in Enterobacter cloacae and Pseudomonas aeruginosa as a possible resistance mechanism. Antimicrob Agents Chemother. 1982 May;21(5):711–717. doi: 10.1128/aac.21.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Diffusion of beta-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob Agents Chemother. 1985 Jan;27(1):84–92. doi: 10.1128/aac.27.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]