Abstract
Background—Cytomegalovirus (CMV) is the prototype member of the ß-herpesvirinae, which can cause multiple organ dysfunction in the immunocompromised host. Human herpesvirus 6 (HHV-6) and HHV-7 are newer members of the ß-herpesvirinae that can cause febrile illness in young children and are also possible pathogens in the immunocompromised patient.
Aim—CMV is detected in histopathological sections by visualisation of owl's eye inclusion bodies. The aim of this study was to quantify the relation between CMV, HHV-6, and HHV-7 viral loads and the presence of owl's eye inclusions in histological sections.
Methods—Histopathological examination of postmortem material and recording of owl's eye inclusion bodies were performed. CMV, HHV-6, and HHV-7 were detected by qualitative and quantitative polymerase chain reaction (PCR) from the same postmortem samples. Statistical analysis of the histopathological and PCR results was performed.
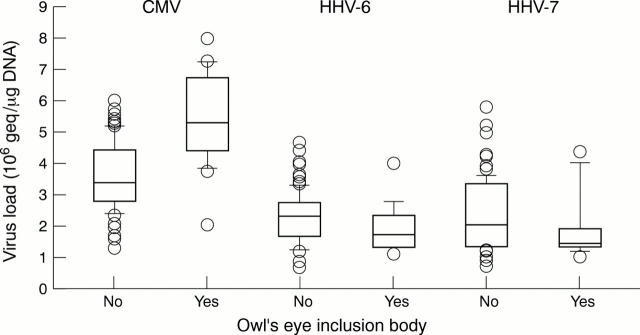
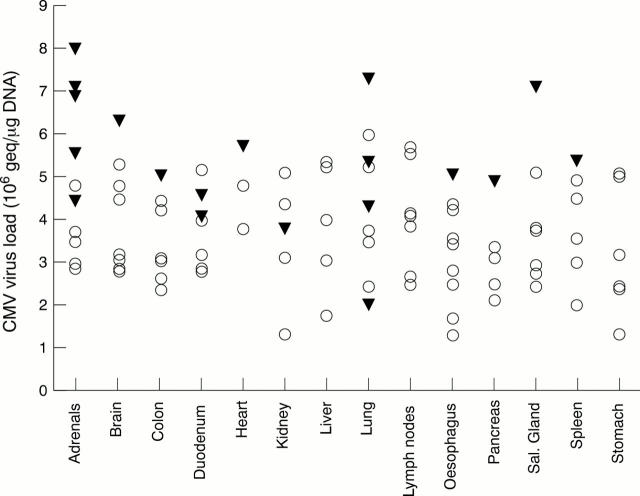
Results—There was a significant association between the detection of owl's eye inclusion bodies and positive CMV PCR (p < 0.001); the median CMV viral load was significantly higher in samples that were positive for owl's eye inclusions (p < 0.001). No association was found between the presence of owl's eye inclusions and HHV-6 or HHV-7 positivity.
Conclusion—Histological detection of owl's eye inclusion bodies is an insensitive but highly specific method for detecting CMV organ involvement. Owl's eye inclusion bodies are not associated with HHV-6 or HHV-7 infection.
Key Words: polymerase chain reaction • inclusion bodies • viral load
Full Text
The Full Text of this article is available as a PDF (141.4 KB).
Figure 1 Box plots illustrating the relations between viral loads for three ß-herpesviruses and the presence of owl's eye inclusions. The horizontal lines display the 10th, 25th, 50th (thick line), 75th, and 90th centiles; the boxes encompass 50% of the values; data points illustrate individual outlying values. CMV, cytomegalovirus; HHV, human herpesvirus.
Figure 2 Association between the quantity of cytomegalovirus (CMV) DNA and the presence of owl's eye inclusions in particular organs. Closed triangle, CMV load associated with owl's eye inclusions; open circle, CMV load associated with negative staining for owl's eye inclusions.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins M., Strappe P., Kaye S., Loveday C., McLaughlin J. E., Johnson M. A., Tedder R. S., Griffiths P. D., Emery V. C. Quantitative differences in the distribution of zidovudine resistance mutations in multiple post-mortem tissues from AIDS patients. J Med Virol. 1998 Jun;55(2):138–146. doi: 10.1002/(sici)1096-9071(199806)55:2<138::aid-jmv10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Black J. B., Pellett P. E. Human herpesvirus 7. Rev Med Virol. 1999 Oct-Dec;9(4):245–262. doi: 10.1002/(sici)1099-1654(199910/12)9:4<245::aid-rmv253>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Clark D. A., Ait-Khaled M., Wheeler A. C., Kidd I. M., McLaughlin J. E., Johnson M. A., Griffiths P. D., Emery V. C. Quantification of human herpesvirus 6 in immunocompetent persons and post-mortem tissues from AIDS patients by PCR. J Gen Virol. 1996 Sep;77(Pt 9):2271–2275. doi: 10.1099/0022-1317-77-9-2271. [DOI] [PubMed] [Google Scholar]
- Clark D. A., Kidd I. M., Collingham K. E., Tarlow M., Ayeni T., Riordan A., Griffiths P. D., Emery V. C., Pillay D. Diagnosis of primary human herpesvirus 6 and 7 infections in febrile infants by polymerase chain reaction. Arch Dis Child. 1997 Jul;77(1):42–45. doi: 10.1136/adc.77.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockrell D. H., Prada J., Jones M. F., Patel R., Badley A. D., Harmsen W. S., Ilstrup D. M., Wiesner R. H., Krom R. A., Smith T. F. Seroconversion to human herpesvirus 6 following liver transplantation is a marker of cytomegalovirus disease. J Infect Dis. 1997 Nov;176(5):1135–1140. doi: 10.1086/514104. [DOI] [PubMed] [Google Scholar]
- Emery V. C., Atkins M. C., Bowen E. F., Clark D. A., Johnson M. A., Kidd I. M., McLaughlin J. E., Phillips A. N., Strappe P. M., Griffiths P. D. Interactions between beta-herpesviruses and human immunodeficiency virus in vivo: evidence for increased human immunodeficiency viral load in the presence of human herpesvirus 6. J Med Virol. 1999 Mar;57(3):278–282. doi: 10.1002/(sici)1096-9071(199903)57:3<278::aid-jmv11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Fox J. C., Griffiths P. D., Emery V. C. Quantification of human cytomegalovirus DNA using the polymerase chain reaction. J Gen Virol. 1992 Sep;73(Pt 9):2405–2408. doi: 10.1099/0022-1317-73-9-2405. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Schirmer E. C., Wyatt L. S., Katsafanas G., Roffman E., Danovich R. M., June C. H. Isolation of a new herpesvirus from human CD4+ T cells. Proc Natl Acad Sci U S A. 1990 Jan;87(2):748–752. doi: 10.1073/pnas.87.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd I. M., Clark D. A., Ait-Khaled M., Griffiths P. D., Emery V. C. Measurement of human herpesvirus 7 load in peripheral blood and saliva of healthy subjects by quantitative polymerase chain reaction. J Infect Dis. 1996 Aug;174(2):396–401. doi: 10.1093/infdis/174.2.396. [DOI] [PubMed] [Google Scholar]
- Knox K. K., Carrigan D. R. HHV-6 and CMV pneumonitis in immunocompromised patients. Lancet. 1994 Jun 25;343(8913):1647–1647. [PubMed] [Google Scholar]
- Macasaet F. F., Holley K. E., Smith T. F., Keys T. F. Cytomegalovirus studies of autopsy tissue. II. Incidence of inclusion bodies and related pathologic data. Am J Clin Pathol. 1975 Jun;63(6):859–865. doi: 10.1093/ajcp/63.6.859. [DOI] [PubMed] [Google Scholar]
- Osman H. K., Peiris J. S., Taylor C. E., Warwicker P., Jarrett R. F., Madeley C. R. "Cytomegalovirus disease" in renal allograft recipients: is human herpesvirus 7 a co-factor for disease progression? J Med Virol. 1996 Apr;48(4):295–301. doi: 10.1002/(SICI)1096-9071(199604)48:4<295::AID-JMV1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Ablashi D. V., Markham P. D., Josephs S. F., Sturzenegger S., Kaplan M., Halligan G., Biberfeld P., Wong-Staal F., Kramarsky B. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986 Oct 31;234(4776):596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- Smith T. F., Holley K. E., Keys T. F., Macasaet F. F. Cytomegalovirus studies of autopsy tissue. I. Virus isolation. Am J Clin Pathol. 1975 Jun;63(6):854–858. doi: 10.1093/ajcp/63.6.854. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Kondo T., Torigoe S., Okada S., Mukai T., Yamanishi K. Human herpesvirus 7: another causal agent for roseola (exanthem subitum). J Pediatr. 1994 Jul;125(1):1–5. doi: 10.1016/s0022-3476(94)70113-x. [DOI] [PubMed] [Google Scholar]
- Umene K. Mechanism and application of genetic recombination in herpesviruses. Rev Med Virol. 1999 Jul;9(3):171–182. doi: 10.1002/(sici)1099-1654(199907/09)9:3<171::aid-rmv243>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Yamanishi K., Okuno T., Shiraki K., Takahashi M., Kondo T., Asano Y., Kurata T. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988 May 14;1(8594):1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]