Abstract
Human T lymphotropic virus type 1 (HTLV-1) causes disabling and fatal diseases, yet there is no vaccine, no satisfactory treatment, and no means of assessing the risk of disease or prognosis in infected people. Recent research on the molecular virology and immunology of HTLV-1 shows the importance of the host's immune response in reducing the risk of these diseases, and is beginning to explain why some HTLV-1 infected people develop serious illnesses whereas most remain healthy life long carriers of the virus. These findings might be applicable to other persistent virus infections such as human immunodeficiency virus, hepatitis B, and hepatitis C.
Key Words: human T lymphotropic virus type 1 • adult T cell leukaemia/lymphoma • tropical spastic paraparesis • myelopathy
Full Text
The Full Text of this article is available as a PDF (141.8 KB).
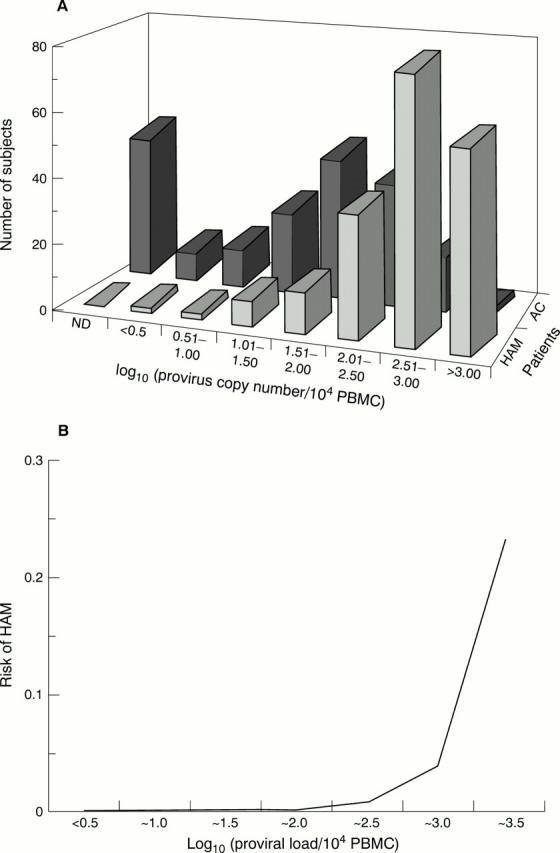
Figure 1 (A) Distribution of human T lymphotropic virus type 1 (HTLV-1) proviral load in 202 patients with tropical spastic paraparesis (HAM/TSP) and 200 asymptomatic carriers of HTLV-1 (AC) in Kagoshima, southern Japan.56 The lower limit of detection was one provirus copy/104 peripheral blood mononuclear cells (PBMCs).56 The median proviral load in patients with HAM/TSP was 16 times greater than in asymptomatic carriers. ND, not determined. (B) The risk of HAM/TSP depends on the proviral load of HTLV-1. The vertical axis shows the risk of HAM/TSP at a given log10 (HTLV-1 proviral load). The risk remains very low until the proviral load reaches 1% PBMCs; above this apparent threshold, the risk of disease rises rapidly with increasing load. The prevalence of HAM/TSP among the seropositive adult population was assumed to be 0.01.57
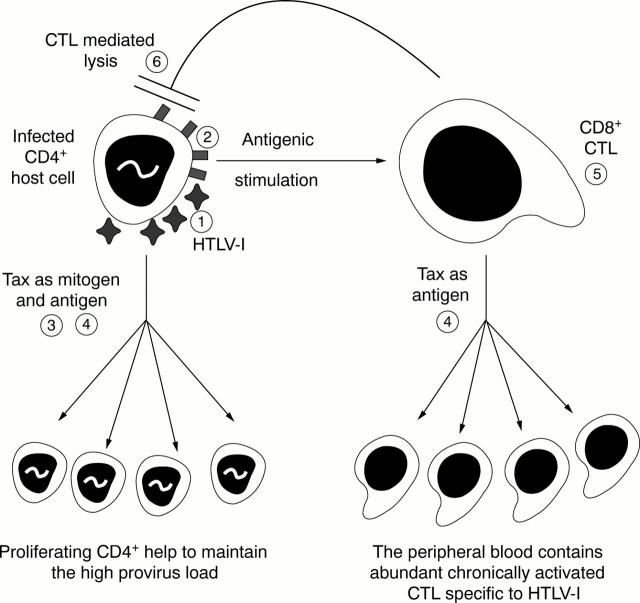
Figure 2 Human T lymphotropic virus type 1 (HTLV-1) infection establishes a dynamic equilibrium between virus replication and immune destruction. The infected CD4 positive host cell produces new HTLV-1 virus particles (1), and HTLV-1 derived antigenic peptides complexed with class I and class II human major histocompatibility complex (HLA) proteins on its surface (2). The HTLV-1 virions infect susceptible neighbouring CD4 positive cells. The Tax protein of HTLV-1 acts both as mitogen and antigen, to drive the division of infected CD4 positive and CD8 positive T cells that are specific for Tax (4). The stimulated CD8 positive cytotoxic T cells (CTLs) recognise and kill host cells that express HTLV-1 Tax–HLA protein complexes on the cell surface (5). Tax is not the only HTLV-1 antigen recognised by lymphocytes, but it has a position of special importance because it is the first HTLV-1 protein to be produced, and is a powerful mitogen and antigen.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangham C. R., Hall S. E., Jeffery K. J., Vine A. M., Witkover A., Nowak M. A., Wodarz D., Usuku K., Osame M. Genetic control and dynamics of the cellular immune response to the human T-cell leukaemia virus, HTLV-I. Philos Trans R Soc Lond B Biol Sci. 1999 Apr 29;354(1384):691–700. doi: 10.1098/rstb.1999.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddison W. E., Kubota R., Kawanishi T., Taub D. D., Cruikshank W. W., Center D. M., Connor E. W., Utz U., Jacobson S. Human T cell leukemia virus type I (HTLV-I)-specific CD8+ CTL clones from patients with HTLV-I-associated neurologic disease secrete proinflammatory cytokines, chemokines, and matrix metalloproteinase. J Immunol. 1997 Aug 15;159(4):2018–2025. [PubMed] [Google Scholar]
- Blattner W. A., Kalyanaraman V. S., Robert-Guroff M., Lister T. A., Galton D. A., Sarin P. S., Crawford M. H., Catovsky D., Greaves M., Gallo R. C. The human type-C retrovirus, HTLV, in Blacks from the Caribbean region, and relationship to adult T-cell leukemia/lymphoma. Int J Cancer. 1982 Sep 15;30(3):257–264. doi: 10.1002/ijc.2910300302. [DOI] [PubMed] [Google Scholar]
- CRUICKSHANK E. K. A neuropathic syndrome of uncertain origin; review of 100 cases. West Indian Med J. 1956 Sep;5(3):147–158. [PubMed] [Google Scholar]
- Cleghorn F. R., Manns A., Falk R., Hartge P., Hanchard B., Jack N., Williams E., Jaffe E., White F., Bartholomew C. Effect of human T-lymphotropic virus type I infection on non-Hodgkin's lymphoma incidence. J Natl Cancer Inst. 1995 Jul 5;87(13):1009–1014. doi: 10.1093/jnci/87.13.1009. [DOI] [PubMed] [Google Scholar]
- Daenke S., Bangham C. R. Do T cells cause HTLV-1-associated disease?: a taxing problem. Clin Exp Immunol. 1994 May;96(2):179–181. doi: 10.1111/j.1365-2249.1994.tb06538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daenke S., Kermode A. G., Hall S. E., Taylor G., Weber J., Nightingale S., Bangham C. R. High activated and memory cytotoxic T-cell responses to HTLV-1 in healthy carriers and patients with tropical spastic paraparesis. Virology. 1996 Mar 1;217(1):139–146. doi: 10.1006/viro.1996.0101. [DOI] [PubMed] [Google Scholar]
- Daenke S., McCracken S. A., Booth S. Human T-cell leukaemia/lymphoma virus type 1 syncytium formation is regulated in a cell-specific manner by ICAM-1, ICAM-3 and VCAM-1 and can be inhibited by antibodies to integrin beta2 or beta7. J Gen Virol. 1999 Jun;80(Pt 6):1429–1436. doi: 10.1099/0022-1317-80-6-1429. [DOI] [PubMed] [Google Scholar]
- Daenke S., Nightingale S., Cruickshank J. K., Bangham C. R. Sequence variants of human T-cell lymphotropic virus type I from patients with tropical spastic paraparesis and adult T-cell leukemia do not distinguish neurological from leukemic isolates. J Virol. 1990 Mar;64(3):1278–1282. doi: 10.1128/jvi.64.3.1278-1282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovaara I., Koenig S., Brewah A. Y., Woods R. M., Lehky T., Jacobson S. High human T cell lymphotropic virus type 1 (HTLV-1)-specific precursor cytotoxic T lymphocyte frequencies in patients with HTLV-1-associated neurological disease. J Exp Med. 1993 Jun 1;177(6):1567–1573. doi: 10.1084/jem.177.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini G. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood. 1995 Nov 15;86(10):3619–3639. [PubMed] [Google Scholar]
- Gessain A., Barin F., Vernant J. C., Gout O., Maurs L., Calender A., de Thé G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985 Aug 24;2(8452):407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Gessain A., Saal F., Gout O., Daniel M. T., Flandrin G., de The G., Peries J., Sigaux F. High human T-cell lymphotropic virus type I proviral DNA load with polyclonal integration in peripheral blood mononuclear cells of French West Indian, Guianese, and African patients with tropical spastic paraparesis. Blood. 1990 Jan 15;75(2):428–433. [PubMed] [Google Scholar]
- Gill P. S., Harrington W., Jr, Kaplan M. H., Ribeiro R. C., Bennett J. M., Liebman H. A., Bernstein-Singer M., Espina B. M., Cabral L., Allen S. Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N Engl J Med. 1995 Jun 29;332(26):1744–1748. doi: 10.1056/NEJM199506293322603. [DOI] [PubMed] [Google Scholar]
- Gout O., Baulac M., Gessain A., Semah F., Saal F., Périès J., Cabrol C., Foucault-Fretz C., Laplane D., Sigaux F. Rapid development of myelopathy after HTLV-I infection acquired by transfusion during cardiac transplantation. N Engl J Med. 1990 Feb 8;322(6):383–388. doi: 10.1056/NEJM199002083220607. [DOI] [PubMed] [Google Scholar]
- Gout O., Gessain A., Iba-Zizen M., Kouzan S., Bolgert F., de Thé G., Lyon-Caen O. The effect of zidovudine on chronic myelopathy associated with HTLV-1. J Neurol. 1991 Apr;238(2):108–109. doi: 10.1007/BF00315691. [DOI] [PubMed] [Google Scholar]
- Harrington W. J., Jr, Sheremata W. A., Snodgrass S. R., Emerson S., Phillips S., Berger J. R. Tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM): treatment with an anabolic steroid danazol. AIDS Res Hum Retroviruses. 1991 Dec;7(12):1031–1034. doi: 10.1089/aid.1991.7.1031. [DOI] [PubMed] [Google Scholar]
- Heredia A., Soriano V., Weiss S. H., Bravo R., Vallejo A., Denny T. N., Epstein J. S., Hewlett I. K. Development of a multiplex PCR assay for the simultaneous detection and discrimination of HIV-1, HIV-2, HTLV-I and HTLV-II. Clin Diagn Virol. 1996 Nov;7(2):85–92. doi: 10.1016/s0928-0197(96)00255-3. [DOI] [PubMed] [Google Scholar]
- Hermine O., Bouscary D., Gessain A., Turlure P., Leblond V., Franck N., Buzyn-Veil A., Rio B., Macintyre E., Dreyfus F. Brief report: treatment of adult T-cell leukemia-lymphoma with zidovudine and interferon alfa. N Engl J Med. 1995 Jun 29;332(26):1749–1751. doi: 10.1056/NEJM199506293322604. [DOI] [PubMed] [Google Scholar]
- Hildreth J. E., Subramanium A., Hampton R. A. Human T-cell lymphotropic virus type 1 (HTLV-1)-induced syncytium formation mediated by vascular cell adhesion molecule-1: evidence for involvement of cell adhesion molecules in HTLV-1 biology. J Virol. 1997 Feb;71(2):1173–1180. doi: 10.1128/jvi.71.2.1173-1180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino S., Katamine S., Miyata H., Tsuji Y., Yamabe T., Miyamoto T. Primary prevention of HTLV-1 in Japan. Leukemia. 1997 Apr;11 (Suppl 3):57–59. [PubMed] [Google Scholar]
- Hinuma Y., Komoda H., Chosa T., Kondo T., Kohakura M., Takenaka T., Kikuchi M., Ichimaru M., Yunoki K., Sato I. Antibodies to adult T-cell leukemia-virus-associated antigen (ATLA) in sera from patients with ATL and controls in Japan: a nation-wide sero-epidemiologic study. Int J Cancer. 1982 Jun 15;29(6):631–635. doi: 10.1002/ijc.2910290606. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höllsberg P., Wucherpfennig K. W., Ausubel L. J., Calvo V., Bierer B. E., Hafler D. A. Characterization of HTLV-I in vivo infected T cell clones. IL-2-independent growth of nontransformed T cells. J Immunol. 1992 May 15;148(10):3256–3263. [PubMed] [Google Scholar]
- Ijichi S., Izumo S., Eiraku N., Machigashira K., Kubota R., Nagai M., Ikegami N., Kashio N., Umehara F., Maruyama I. An autoaggressive process against bystander tissues in HTLV-I-infected individuals: a possible pathomechanism of HAM/TSP. Med Hypotheses. 1993 Dec;41(6):542–547. doi: 10.1016/0306-9877(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y. Human T cell leukemia virus type I infection and chronic myelopathy. Brain Pathol. 1993 Jan;3(1):1–10. doi: 10.1111/j.1750-3639.1993.tb00719.x. [DOI] [PubMed] [Google Scholar]
- Jeffery K. J., Usuku K., Hall S. E., Matsumoto W., Taylor G. P., Procter J., Bunce M., Ogg G. S., Welsh K. I., Weber J. N. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):3848–3853. doi: 10.1073/pnas.96.7.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamihira S., Nakasima S., Oyakawa Y., Moriuti Y., Ichimaru M., Okuda H., Kanamura M., Oota T. Transmission of human T cell lymphotropic virus type I by blood transfusion before and after mass screening of sera from seropositive donors. Vox Sang. 1987;52(1-2):43–44. doi: 10.1111/j.1423-0410.1987.tb02987.x. [DOI] [PubMed] [Google Scholar]
- Kataoka A., Imai H., Inayoshi S., Tsuda T. Intermittent high-dose vitamin C therapy in patients with HTLV-I associated myelopathy. J Neurol Neurosurg Psychiatry. 1993 Nov;56(11):1213–1216. doi: 10.1136/jnnp.56.11.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai H., Nishida Y., Takagi M., Nakamura K., Masuda K., Saito S., Shirakami A. HTLV-I-associated myelopathy with adult T-cell leukemia. Neurology. 1989 Aug;39(8):1129–1131. doi: 10.1212/wnl.39.8.1129. [DOI] [PubMed] [Google Scholar]
- Kawano F., Yamaguchi K., Nishimura H., Tsuda H., Takatsuki K. Variation in the clinical courses of adult T-cell leukemia. Cancer. 1985 Feb 15;55(4):851–856. doi: 10.1002/1097-0142(19850215)55:4<851::aid-cncr2820550424>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kira J., Koyanagi Y., Yamada T., Itoyama Y., Goto I., Yamamoto N., Sasaki H., Sakaki Y. Increased HTLV-I proviral DNA in HTLV-I-associated myelopathy: a quantitative polymerase chain reaction study. Ann Neurol. 1991 Feb;29(2):194–201. doi: 10.1002/ana.410290214. [DOI] [PubMed] [Google Scholar]
- Kondo T., Kono H., Miyamoto N., Yoshida R., Toki H., Matsumoto I., Hara M., Inoue H., Inatsuki A., Funatsu T. Age- and sex-specific cumulative rate and risk of ATLL for HTLV-I carriers. Int J Cancer. 1989 Jun 15;43(6):1061–1064. doi: 10.1002/ijc.2910430618. [DOI] [PubMed] [Google Scholar]
- LaGrenade L., Hanchard B., Fletcher V., Cranston B., Blattner W. Infective dermatitis of Jamaican children: a marker for HTLV-I infection. Lancet. 1990 Dec 1;336(8727):1345–1347. doi: 10.1016/0140-6736(90)92896-p. [DOI] [PubMed] [Google Scholar]
- Maloney E. M., Cleghorn F. R., Morgan O. S., Rodgers-Johnson P., Cranston B., Jack N., Blattner W. A., Bartholomew C., Manns A. Incidence of HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) in Jamaica and Trinidad. J Acquir Immune Defic Syndr Hum Retrovirol. 1998 Feb 1;17(2):167–170. doi: 10.1097/00042560-199802010-00011. [DOI] [PubMed] [Google Scholar]
- Manns A., Murphy E. L., Wilks R., Haynes G., Figueroa J. P., Hanchard B., Barnett M., Drummond J., Waters D., Cerney M. Detection of early human T-cell lymphotropic virus type I antibody patterns during seroconversion among transfusion recipients. Blood. 1991 Feb 15;77(4):896–905. [PubMed] [Google Scholar]
- Manns A., Wilks R. J., Murphy E. L., Haynes G., Figueroa J. P., Barnett M., Hanchard B., Blattner W. A. A prospective study of transmission by transfusion of HTLV-I and risk factors associated with seroconversion. Int J Cancer. 1992 Jul 30;51(6):886–891. doi: 10.1002/ijc.2910510609. [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Watanabe T., Yamaguchi K., Takatsuki K., Yoshimura K., Shirao M., Nakashima S., Mori S., Araki S., Miyata N. HTLV-I uveitis: a distinct clinical entity caused by HTLV-I. Jpn J Cancer Res. 1992 Mar;83(3):236–239. doi: 10.1111/j.1349-7006.1992.tb00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G. R., Traugott U., Scheinberg L. C., Raine C. S. Tropical spastic paraparesis: a model of virus-induced, cytotoxic T-cell-mediated demyelination? Ann Neurol. 1989 Oct;26(4):523–530. doi: 10.1002/ana.410260405. [DOI] [PubMed] [Google Scholar]
- Morgan O. S., Rodgers-Johnson P., Mora C., Char G. HTLV-1 and polymyositis in Jamaica. Lancet. 1989 Nov 18;2(8673):1184–1187. doi: 10.1016/s0140-6736(89)91793-5. [DOI] [PubMed] [Google Scholar]
- Nagai M., Usuku K., Matsumoto W., Kodama D., Takenouchi N., Moritoyo T., Hashiguchi S., Ichinose M., Bangham C. R., Izumo S. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol. 1998 Dec;4(6):586–593. doi: 10.3109/13550289809114225. [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Izumo S., Ijichi S., Kubota H., Arimura K., Kawabata M., Osame M. HTLV-I-associated myelopathy: analysis of 213 patients based on clinical features and laboratory findings. J Neurovirol. 1995 Mar;1(1):50–61. doi: 10.3109/13550289509111010. [DOI] [PubMed] [Google Scholar]
- Niewiesk S., Bangham C. R. Evolution in a chronic RNA virus infection: selection on HTLV-I tax protein differs between healthy carriers and patients with tropical spastic paraparesis. J Mol Evol. 1996 Apr;42(4):452–458. doi: 10.1007/BF02498639. [DOI] [PubMed] [Google Scholar]
- Niewiesk S., Daenke S., Parker C. E., Taylor G., Weber J., Nightingale S., Bangham C. R. Naturally occurring variants of human T-cell leukemia virus type I Tax protein impair its recognition by cytotoxic T lymphocytes and the transactivation function of Tax. J Virol. 1995 Apr;69(4):2649–2653. doi: 10.1128/jvi.69.4.2649-2653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiesk S., Daenke S., Parker C. E., Taylor G., Weber J., Nightingale S., Bangham C. R. The transactivator gene of human T-cell leukemia virus type I is more variable within and between healthy carriers than patients with tropical spastic paraparesis. J Virol. 1994 Oct;68(10):6778–6781. doi: 10.1128/jvi.68.10.6778-6781.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka K., Maruyama I., Sato K., Kitajima I., Nakajima Y., Osame M. Chronic inflammatory arthropathy associated with HTLV-I. Lancet. 1989 Feb 25;1(8635):441–441. doi: 10.1016/s0140-6736(89)90038-x. [DOI] [PubMed] [Google Scholar]
- Nowak M. A., Bangham C. R. Population dynamics of immune responses to persistent viruses. Science. 1996 Apr 5;272(5258):74–79. doi: 10.1126/science.272.5258.74. [DOI] [PubMed] [Google Scholar]
- Okochi K., Sato H., Hinuma Y. A retrospective study on transmission of adult T cell leukemia virus by blood transfusion: seroconversion in recipients. Vox Sang. 1984;46(5):245–253. doi: 10.1111/j.1423-0410.1984.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Osame M., Janssen R., Kubota H., Nishitani H., Igata A., Nagataki S., Mori M., Goto I., Shimabukuro H., Khabbaz R. Nationwide survey of HTLV-I-associated myelopathy in Japan: association with blood transfusion. Ann Neurol. 1990 Jul;28(1):50–56. doi: 10.1002/ana.410280110. [DOI] [PubMed] [Google Scholar]
- Osame M., Usuku K., Izumo S., Ijichi N., Amitani H., Igata A., Matsumoto M., Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986 May 3;1(8488):1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- Paul W. E. Can the immune response control HIV infection? Cell. 1995 Jul 28;82(2):177–182. doi: 10.1016/0092-8674(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87). Br J Haematol. 1991 Nov;79(3):428–437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- Sommerfelt M. A., Williams B. P., Clapham P. R., Solomon E., Goodfellow P. N., Weiss R. A. Human T cell leukemia viruses use a receptor determined by human chromosome 17. Science. 1988 Dec 16;242(4885):1557–1559. doi: 10.1126/science.3201246. [DOI] [PubMed] [Google Scholar]
- Stuver S. O., Tachibana N., Okayama A., Shioiri S., Tsunetoshi Y., Tsuda K., Mueller N. E. Heterosexual transmission of human T cell leukemia/lymphoma virus type I among married couples in southwestern Japan: an initial report from the Miyazaki Cohort Study. J Infect Dis. 1993 Jan;167(1):57–65. doi: 10.1093/infdis/167.1.57. [DOI] [PubMed] [Google Scholar]
- Tajima K. The 4th nation-wide study of adult T-cell leukemia/lymphoma (ATL) in Japan: estimates of risk of ATL and its geographical and clinical features. The T- and B-cell Malignancy Study Group. Int J Cancer. 1990 Feb 15;45(2):237–243. doi: 10.1002/ijc.2910450206. [DOI] [PubMed] [Google Scholar]
- Taylor G. P., Hall S. E., Navarrete S., Michie C. A., Davis R., Witkover A. D., Rossor M., Nowak M. A., Rudge P., Matutes E. Effect of lamivudine on human T-cell leukemia virus type 1 (HTLV-1) DNA copy number, T-cell phenotype, and anti-tax cytotoxic T-cell frequency in patients with HTLV-1-associated myelopathy. J Virol. 1999 Dec;73(12):10289–10295. doi: 10.1128/jvi.73.12.10289-10295.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. P., Tosswill J. H., Matutes E., Daenke S., Hall S., Bain B. J., Davis R., Thomas D., Rossor M., Bangham C. R. Prospective study of HTLV-I infection in an initially asymptomatic cohort. J Acquir Immune Defic Syndr. 1999 Sep 1;22(1):92–100. doi: 10.1097/00042560-199909010-00012. [DOI] [PubMed] [Google Scholar]
- Uchiyama T. Human T cell leukemia virus type I (HTLV-I) and human diseases. Annu Rev Immunol. 1997;15:15–37. doi: 10.1146/annurev.immunol.15.1.15. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Yodoi J., Sagawa K., Takatsuki K., Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977 Sep;50(3):481–492. [PubMed] [Google Scholar]
- Umehara F., Izumo S., Nakagawa M., Ronquillo A. T., Takahashi K., Matsumuro K., Sato E., Osame M. Immunocytochemical analysis of the cellular infiltrate in the spinal cord lesions in HTLV-I-associated myelopathy. J Neuropathol Exp Neurol. 1993 Jul;52(4):424–430. doi: 10.1097/00005072-199307000-00010. [DOI] [PubMed] [Google Scholar]
- Usuku K., Nishizawa M., Matsuki K., Tokunaga K., Takahashi K., Eiraku N., Suehara M., Juji T., Osame M., Tabira T. Association of a particular amino acid sequence of the HLA-DR beta 1 chain with HTLV-I-associated myelopathy. Eur J Immunol. 1990 Jul;20(7):1603–1606. doi: 10.1002/eji.1830200729. [DOI] [PubMed] [Google Scholar]
- Usuku K., Sonoda S., Osame M., Yashiki S., Takahashi K., Matsumoto M., Sawada T., Tsuji K., Tara M., Igata A. HLA haplotype-linked high immune responsiveness against HTLV-I in HTLV-I-associated myelopathy: comparison with adult T-cell leukemia/lymphoma. Ann Neurol. 1988;23 (Suppl):S143–S150. doi: 10.1002/ana.410230733. [DOI] [PubMed] [Google Scholar]
- Wharfe G., Hanchard B. Zidovudine and interferon therapy for adult T-cell leukaemia/lymphoma. Results of a preliminary study at UHWI-Mona. West Indian Med J. 1996 Dec;45(4):107–109. [PubMed] [Google Scholar]
- Wodarz D., Nowak M. A., Bangham C. R. The dynamics of HTLV-I and the CTL response. Immunol Today. 1999 May;20(5):220–227. doi: 10.1016/s0167-5699(99)01446-2. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Nishimura H., Kohrogi H., Jono M., Miyamoto Y., Takatsuki K. A proposal for smoldering adult T-cell leukemia: a clinicopathologic study of five cases. Blood. 1983 Oct;62(4):758–766. [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Osame M., Kawai H., Toita M., Kuwasaki N., Nishida Y., Hiraki Y., Takahashi K., Nomura K., Sonoda S. Increased replication of HTLV-I in HTLV-I-associated myelopathy. Ann Neurol. 1989 Sep;26(3):331–335. doi: 10.1002/ana.410260304. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Seiki M., Yamaguchi K., Takatsuki K. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2534–2537. doi: 10.1073/pnas.81.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Thé G., Bomford R. An HTLV-I vaccine: why, how, for whom? AIDS Res Hum Retroviruses. 1993 May;9(5):381–386. doi: 10.1089/aid.1993.9.381. [DOI] [PubMed] [Google Scholar]