Abstract
Aims: To examine the relation between enteroviral infection, especially group A coxsackieviral infection, and acute febrile illness over two summers using tissue culture and polymerase chain reaction (PCR).
Methods: Throat swabs were collected from 246 children from June to August 1997 and 1998.
Results: Enteroviruses were isolated from 33/246 samples and 35 other viruses were isolated. Enteroviral genomes were detected in 54/178 samples from which no virus was isolated. Of 41 enteroviral genotypes identified by sequence analysis of PCR products, 38 were group A coxsackieviruses, which are usually difficult to isolate using tissue culture.
Conclusion: Results indicate that viral detection and identification based on PCR is useful in the diagnosis of group A coxsackieviral infection.
Full Text
The Full Text of this article is available as a PDF (139.2 KB).
Figure 1 .
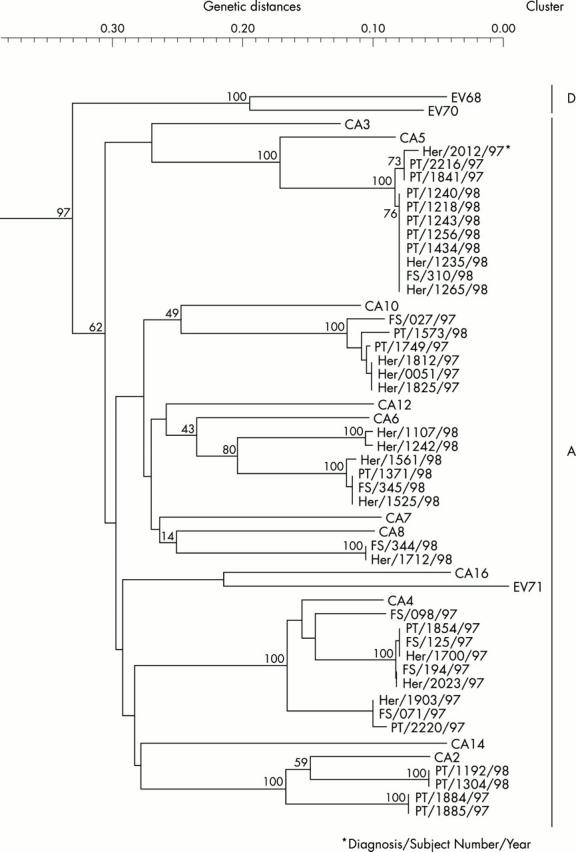
Phylogenetic tree of 64 prototype enteroviruses and 38 enteroviruses detected from patients with febrile illness during two summers. The phylogenetic tree was constructed based on the nucleotide sequences of the VP4 protein coding region. Sixty four prototype enterovirus strains were classified into four genetic clusters (A, B, C, and D). All 14 enteroviruses detected from patients with herpangina (Her), 8/11 enteroviruses detected from patients with febrile seizures (FS), and all 16 enteroviruses detected from patients with pharyngitis/tonsillitis (PT) were within the A cluster of enteroviruses (coxsackievirus group A-like genotype). Coxsackievirus group A-like genotypes detected from herpangina, febrile seizures, and pharyngitis/tonsillitis in two summers were distinctly clustered depending on the year when the samples were taken (1997 or 1998), rather than on the type of illness.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gjøen K. V., Bruu A. L. Specific detection of Coxsackie viruses A by the polymerase chain reaction. Clin Diagn Virol. 1997 Nov;8(3):183–188. doi: 10.1016/s0928-0197(97)00017-2. [DOI] [PubMed] [Google Scholar]
- HUEBNER R. J., COLE R. M., BEEMAN E. A., BELL J. A., PEERS J. H. Herpangina; etiological studies of a specific infectious disease. J Am Med Assoc. 1951 Mar 3;145(9):628–633. doi: 10.1001/jama.1951.02920270022005. [DOI] [PubMed] [Google Scholar]
- Hosoya M., Honzumi K., Suzuki H. Detection of enterovirus by polymerase chain reaction and culture in cerebrospinal fluid of children with transient neurologic complications associated with acute febrile illness. J Infect Dis. 1997 Mar;175(3):700–703. doi: 10.1093/infdis/175.3.700. [DOI] [PubMed] [Google Scholar]
- Hosoya M., Sato M., Honzumi K., Katayose M., Kawasaki Y., Sakuma H., Kato K., Shimada Y., Ishiko H., Suzuki H. Association of nonpolio enteroviral infection in the central nervous system of children with febrile seizures. Pediatrics. 2001 Jan;107(1):E12–E12. doi: 10.1542/peds.107.1.e12. [DOI] [PubMed] [Google Scholar]
- Iizuka N., Kuge S., Nomoto A. Complete nucleotide sequence of the genome of coxsackievirus B1. Virology. 1987 Jan;156(1):64–73. doi: 10.1016/0042-6822(87)90436-3. [DOI] [PubMed] [Google Scholar]
- KRAVIS L. P., HUMMELER K., SIGEL M. M., LECKS H. I. Herpangina; clinical and laboratory aspects of an outbreak caused by group A Coxsackie viruses. Pediatrics. 1953 Feb;11(2):113–119. [PubMed] [Google Scholar]
- Nomoto A., Omata T., Toyoda H., Kuge S., Horie H., Kataoka Y., Genba Y., Nakano Y., Imura N. Complete nucleotide sequence of the attenuated poliovirus Sabin 1 strain genome. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5793–5797. doi: 10.1073/pnas.79.19.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste M. S., Maher K., Kilpatrick D. R., Pallansch M. A. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J Virol. 1999 Mar;73(3):1941–1948. doi: 10.1128/jvi.73.3.1941-1948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive D. M., Al-Mufti S., Al-Mulla W., Khan M. A., Pasca A., Stanway G., Al-Nakib W. Detection and differentiation of picornaviruses in clinical samples following genomic amplification. J Gen Virol. 1990 Sep;71(Pt 9):2141–2147. doi: 10.1099/0022-1317-71-9-2141. [DOI] [PubMed] [Google Scholar]
- Rotbart H. A. Enzymatic RNA amplification of the enteroviruses. J Clin Microbiol. 1990 Mar;28(3):438–442. doi: 10.1128/jcm.28.3.438-442.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Melnick J. L., Wenner H. A., Ho H. H., Burkhardt M. A. Evaluation of enterovirus immune horse serum pools for identification of virus field strains. Bull World Health Organ. 1971;45(3):317–330. [PMC free article] [PubMed] [Google Scholar]
- Zoll G. J., Melchers W. J., Kopecka H., Jambroes G., van der Poel H. J., Galama J. M. General primer-mediated polymerase chain reaction for detection of enteroviruses: application for diagnostic routine and persistent infections. J Clin Microbiol. 1992 Jan;30(1):160–165. doi: 10.1128/jcm.30.1.160-165.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


