Abstract
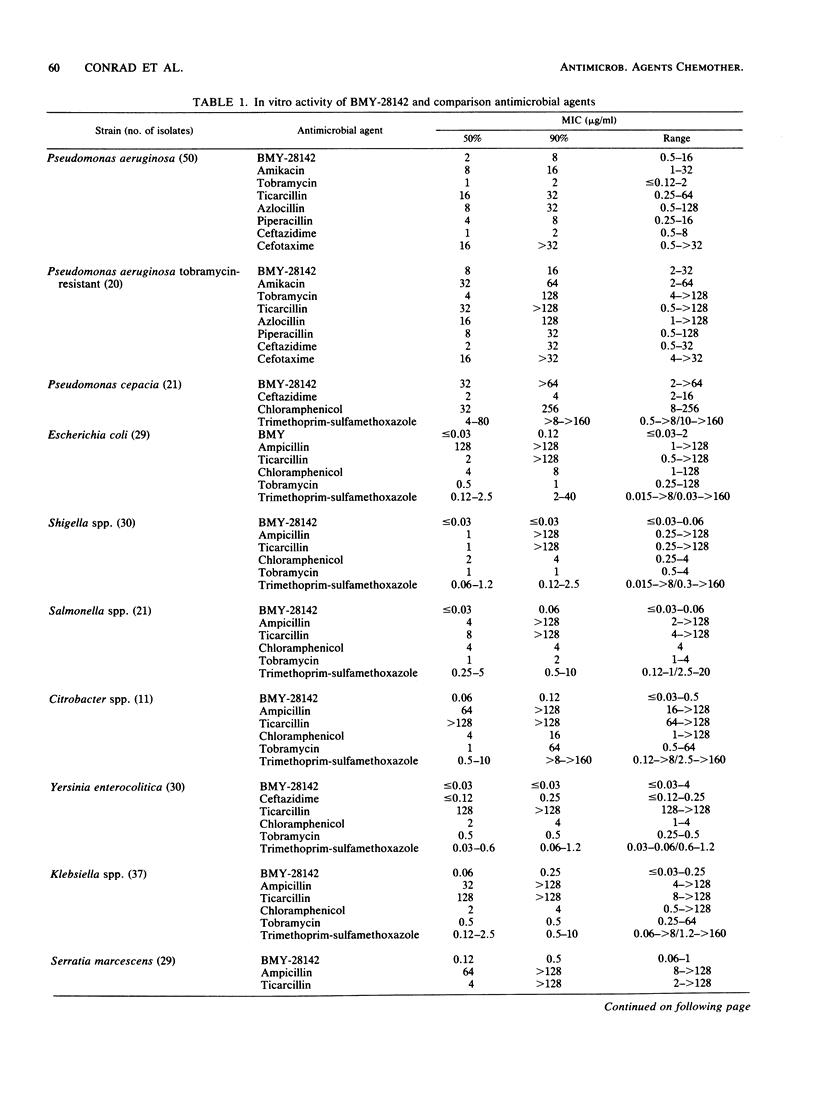
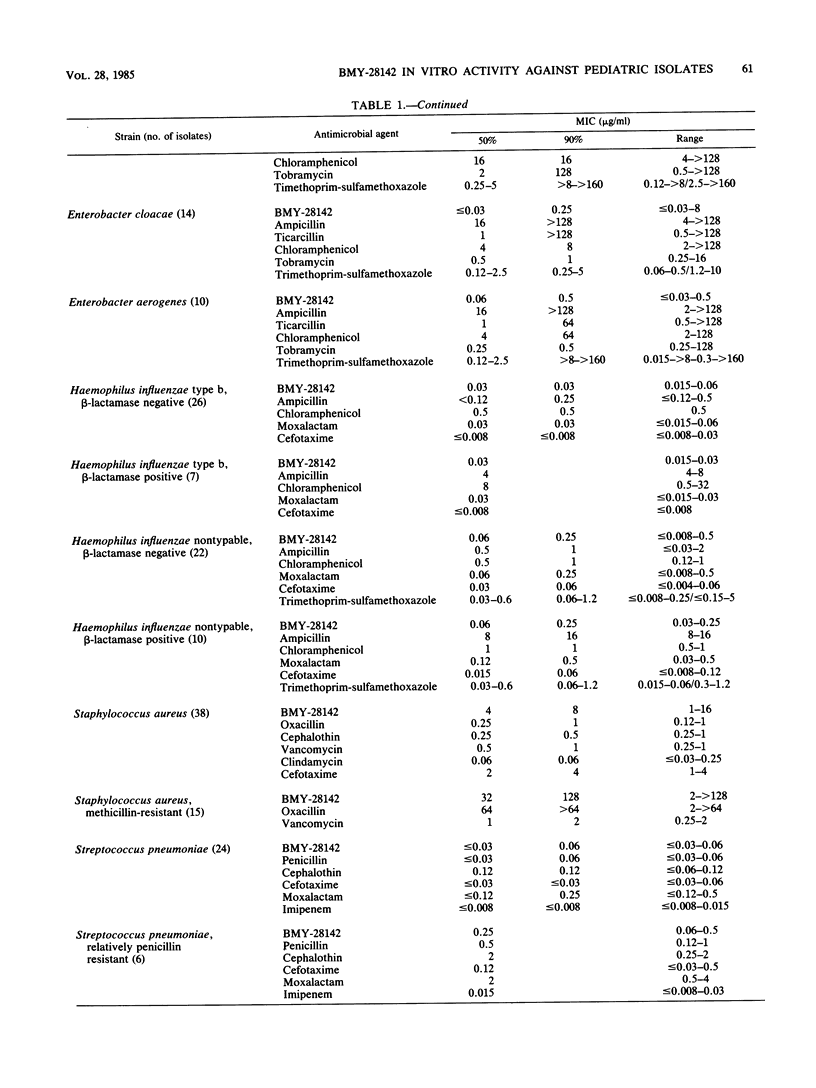
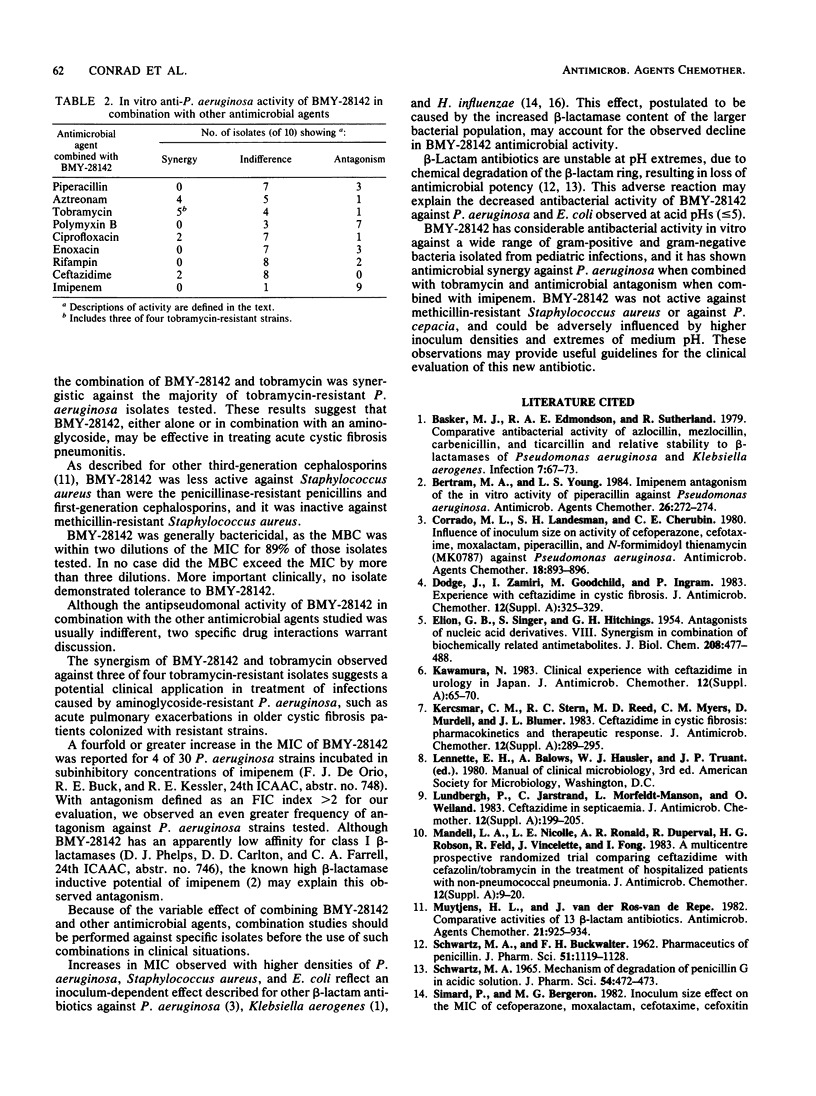
The antibacterial activity of BMY-28142, a new aminothiazole cephalosporin, was measured by standardized broth microdilution and agar dilution methods against 450 gram-positive and gram-negative bacteria isolated from pediatric infections, including acute pulmonary exacerbations of cystic fibrosis. BMY-28142 activity was compared with that of aminoglycosides, beta-lactams, chloramphenicol, trimethoprim-sulfamethoxazole, vancomycin, and clindamycin. The activity of BMY-28142 in combination with other antimicrobial agents against Pseudomonas aeruginosa was also determined. Furthermore, the effects of inoculum and pH on BMY-28142 activity were evaluated. BMY-21842 was active against most of the gram-positive and gram-negative isolates, with the exception of methicillin-resistant Staphylococcus aureus and Pseudomonas cepacia. The combination of BMY-28142 with tobramycin was often synergistic, and combinations of BMY-28142 with either polymyxin B or imipenem were usually antagonistic. BMY-28142 antibacterial activity could be adversely affected at extremes of medium pH and by high inoculum densities.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basker M. J., Edmondson R. A., Sutherland R. Comparative antibacterial activity of azlocillin, mezlocillin, carbenicillin and ticarcillin and relative stability to beta-lactamases of pseudomonas aeruginosa and klebsiella aerogenes. Infection. 1979;7(2):67–73. doi: 10.1007/BF01641616. [DOI] [PubMed] [Google Scholar]
- Bertram M. A., Young L. S. Imipenem antagonism of the in vitro activity of piperacillin against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Aug;26(2):272–274. doi: 10.1128/aac.26.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrado M. L., Landesman S. H., Cherubin C. E. Influence of inoculum size on activity of cefoperazone, cefotaxime, moxalactam, piperacillin, and N-formimidoyl thienamycin (MK0787) against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1980 Dec;18(6):893–896. doi: 10.1128/aac.18.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge J., Zamiri I., Goodchild M., Ingram P. Experience with ceftazidime in cystic fibrosis. J Antimicrob Chemother. 1983 Jul;12 (Suppl A):325–329. doi: 10.1093/jac/12.suppl_a.325. [DOI] [PubMed] [Google Scholar]
- ELION G. B., SINGER S., HITCHINGS G. H. Antagonists of nucleic acid derivatives. VIII. Synergism in combinations of biochemically related antimetabolites. J Biol Chem. 1954 Jun;208(2):477–488. [PubMed] [Google Scholar]
- Kawamura N. Clinical experience with ceftazidime in urology in Japan. J Antimicrob Chemother. 1983 Jul;12 (Suppl A):65–70. doi: 10.1093/jac/12.suppl_a.65. [DOI] [PubMed] [Google Scholar]
- Kercsmar C. M., Stern R. C., Reed M. D., Myers C. M., Murdell D., Blumer J. L. Ceftazidime in cystic fibrosis: pharmacokinetics and therapeutic response. J Antimicrob Chemother. 1983 Jul;12 (Suppl A):289–295. doi: 10.1093/jac/12.suppl_a.289. [DOI] [PubMed] [Google Scholar]
- Lundbergh P., Jarstrand C., Morfeldt-Månson L., Weiland O. Ceftazidime in septicaemia. J Antimicrob Chemother. 1983 Jul;12 (Suppl A):199–205. doi: 10.1093/jac/12.suppl_a.199. [DOI] [PubMed] [Google Scholar]
- Mandell L. A., Nicolle L. E., Ronald A. R., Duperval R., Robson H. G., Feld R., Vincelette J., Fong I. A multicentre prospective randomized trial comparing ceftazidime with cefazolin/tobramycin in the treatment of hospitalized patients with non-pneumococcal pneumonia. J Antimicrob Chemother. 1983 Jul;12 (Suppl A):9–20. doi: 10.1093/jac/12.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- Muytjens H. L., van der Ros-van de Repe J. Comparative activities of 13 beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Jun;21(6):925–934. doi: 10.1128/aac.21.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ M. A., BUCKWALTER F. H. Pharmaceutics of penicillin. J Pharm Sci. 1962 Dec;51:1119–1128. doi: 10.1002/jps.2600511202. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ M. A. MECHANISM OF DEGRADATION OF PENICILLIN G IN ACIDIC SOLUTION. J Pharm Sci. 1965 Mar;54:472–473. doi: 10.1002/jps.2600540336. [DOI] [PubMed] [Google Scholar]
- Simard P., Bergeron M. G. Inoculum size effect on the MIC of cefoperazone, moxalactam, cefotaxime, cefoxitin and cephalothin for 118 strains of Haemophilus influenzae including 'tolerant' micro-organisms. J Antimicrob Chemother. 1982 Nov;10(5):397–402. doi: 10.1093/jac/10.5.397. [DOI] [PubMed] [Google Scholar]
- Tarpay M. M., Welch D. F., Marks M. I. Antimicrobial susceptibility testing of Streptococcus pneumoniae by micro-broth dilution. Antimicrob Agents Chemother. 1980 Oct;18(4):579–581. doi: 10.1128/aac.18.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., 2nd Discrepancies between in vitro activity of and in vivo response to antimicrobial agents. Diagn Microbiol Infect Dis. 1983 Mar;1(1):25–31. doi: 10.1016/0732-8893(83)90029-9. [DOI] [PubMed] [Google Scholar]


