Abstract
Background: Few dose ranging studies have investigated optimal dosing with inhaled corticosteroids in children with asthma.
Aims: To compare the efficacy and tolerability of fluticasone propionate 100 or 200 µg twice daily in children with moderate to severe asthma for one year.
Methods: One year, randomised, double blind, parallel group, multicentre study. Children aged 4–11 years (n = 528) with moderate to severe asthma who had previously received high dose inhaled corticosteroids were given fluticasone propionate 100 or 200 µg twice daily for the 52 week treatment period. Efficacy (exacerbations, lung function, and symptoms) and tolerability (adverse events and cortisol levels) were measured.
Results: There was a non-significant decreased risk of experiencing an exacerbation at any time with fluticasone propionate 200 µg twice daily compared with fluticasone propionate 100 µg twice daily. This difference reached significance among patients with more severe asthma (defined by previous inhaled corticosteroid dose >800 µg/day). Daily record card morning peak expiratory flow (PEF) in the total population improved significantly more with the higher dose of fluticasone propionate (between group difference, weeks 1–52: 11.4 l/min). Clinic visit mean PEF improved from baseline with both doses, but the response was significantly greater with the higher dose (between group difference, week 52: 17.8 l/min). Both doses were equally well tolerated and overnight urinary cortisol concentrations were unchanged or slightly increased during treatment with either dose.
Conclusion: This long term dose comparison study shows that treatment with fluticasone propionate 200 µg twice daily may offer benefits over a lower dose, particularly in children with more severe asthma.
Full Text
The Full Text of this article is available as a PDF (250.1 KB).
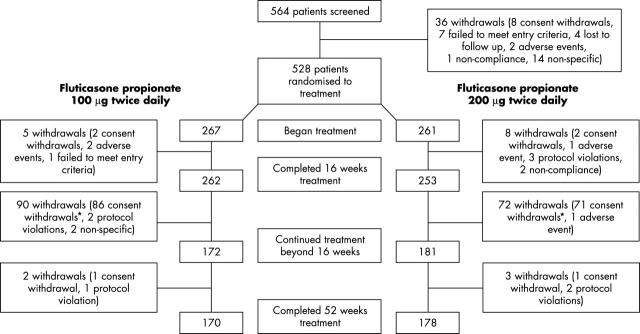
Figure 1.
Flow of patients through the study. *Consent withdrawal: centres did not participate in the extension of the study to 52 weeks or patients did not wish to continue in the study beyond the original planned 16 weeks.
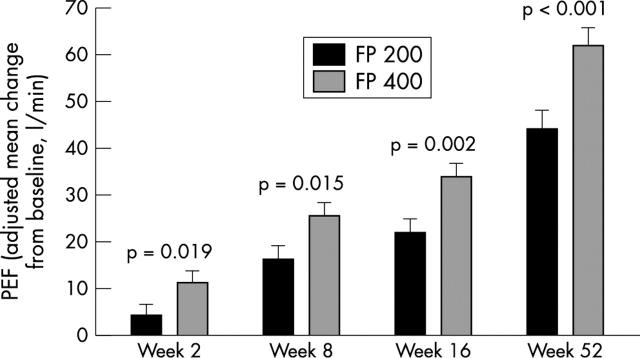
Figure 2.
Change from baseline in mean adjusted clinic peak expiratory flow (PEF) in children randomised to fluticasone propionate 100 µg twice daily (FP 200; n = 267) or 200 µg twice daily (FP 400; n = 261). Error bars represent standard errors.
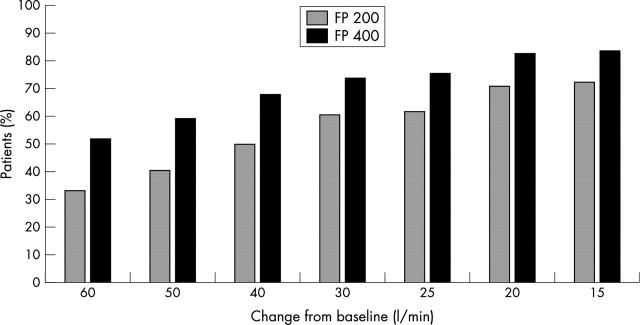
Figure 3.
Cumulative proportion of patients showing the specified threshold change from baseline in adjusted clinic peak expiratory flow (PEF) after 52 weeks therapy with fluticasone propionate 100 µg twice daily (FP 200; n = 170) or 200 µg twice daily (FP 400; n = 178). Between group difference: p < 0.05 for each PEF threshold analysed.
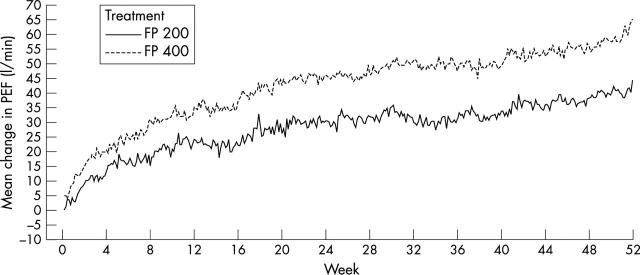
Figure 4.
Change from baseline in daily record card mean morning peak expiratory flow (PEF) in children randomised to fluticasone propionate 100 µg twice daily (FP 200; n = 267) or 200 µg twice daily (FP 400; n = 261) (unadjusted data).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. B., Bronsky E. A., LaForce C. F., Nathan R. A., Tinkelman D. G., Vandewalker M. L., Konig P. Growth in asthmatic children treated with fluticasone propionate. Fluticasone Propionate Asthma Study Group. J Pediatr. 1998 Mar;132(3 Pt 1):472–477. doi: 10.1016/s0022-3476(98)70023-x. [DOI] [PubMed] [Google Scholar]
- Baker J. W., Mellon M., Wald J., Welch M., Cruz-Rivera M., Walton-Bowen K. A multiple-dosing, placebo-controlled study of budesonide inhalation suspension given once or twice daily for treatment of persistent asthma in young children and infants. Pediatrics. 1999 Feb;103(2):414–421. doi: 10.1542/peds.103.2.414. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. Efficacy of inhaled corticosteroids in asthma. J Allergy Clin Immunol. 1998 Oct;102(4 Pt 1):531–538. doi: 10.1016/s0091-6749(98)70268-4. [DOI] [PubMed] [Google Scholar]
- Bisgaard H., Gillies J., Groenewald M., Maden C. The effect of inhaled fluticasone propionate in the treatment of young asthmatic children: a dose comparison study. Am J Respir Crit Care Med. 1999 Jul;160(1):126–131. doi: 10.1164/ajrccm.160.1.9811024. [DOI] [PubMed] [Google Scholar]
- Boe J., Rosenhall L., Alton M., Carlsson L. G., Carlsson U., Hermansson B. A., Hetta L., Kiviloog J., Karlson B. W., Lundbäck B. Comparison of dose-response effects of inhaled beclomethasone dipropionate and budesonide in the management of asthma. Allergy. 1989 Jul;44(5):349–355. doi: 10.1111/j.1398-9995.1989.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Cochrane G. M., Horne R., Chanez P. Compliance in asthma. Respir Med. 1999 Nov;93(11):763–769. doi: 10.1016/s0954-6111(99)90260-3. [DOI] [PubMed] [Google Scholar]
- Crowley S., Hindmarsh P. C., Honour J. W., Brook C. G. Reproducibility of the cortisol response to stimulation with a low dose of ACTH(1-24): the effect of basal cortisol levels and comparison of low-dose with high-dose secretory dynamics. J Endocrinol. 1993 Jan;136(1):167–172. doi: 10.1677/joe.0.1360167. [DOI] [PubMed] [Google Scholar]
- Dahl R., Lundback B., Malo J. L., Mazza J. A., Nieminen M. M., Saarelainen P., Barnacle H. A dose-ranging study of fluticasone propionate in adult patients with moderate asthma. International Study Group. Chest. 1993 Nov;104(5):1352–1358. doi: 10.1378/chest.104.5.1352. [DOI] [PubMed] [Google Scholar]
- Donahue J. G., Weiss S. T., Livingston J. M., Goetsch M. A., Greineder D. K., Platt R. Inhaled steroids and the risk of hospitalization for asthma. JAMA. 1997 Mar 19;277(11):887–891. [PubMed] [Google Scholar]
- Drake A. J., Howells R. J., Shield J. P. H., Prendiville A., Ward P. S., Crowne E. C. Symptomatic adrenal insufficiency presenting with hypoglycaemia in children with asthma receiving high dose inhaled fluticasone propionate. BMJ. 2002 May 4;324(7345):1081–1082. doi: 10.1136/bmj.324.7345.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarsh Peter. Commentary: Exogenous glucocorticoids influence adrenal function, but assessment can be difficult. BMJ. 2002 May 4;324(7345):1083–1083. [PubMed] [Google Scholar]
- Holt S., Suder A., Weatherall M., Cheng S., Shirtcliffe P., Beasley R. Dose-response relation of inhaled fluticasone propionate in adolescents and adults with asthma: meta-analysis. BMJ. 2001 Aug 4;323(7307):253–256. doi: 10.1136/bmj.323.7307.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz Y., Lebas F. X., Medley H. V., Robson R. Fluticasone propionate 50 micrograms BID versus 100 micrograms BID in the treatment of children with persistent asthma. Fluticasone Propionate Study Group. Clin Ther. 1998 May-Jun;20(3):424–437. doi: 10.1016/s0149-2918(98)80053-2. [DOI] [PubMed] [Google Scholar]
- MacKenzie C. A., Tsanakas J., Tabachnik E., Radford M., Berdel D., Götz M. H., Parker C. An open study to assess the long-term safety of fluticasone propionate in asthmatic children. International Study Group. Br J Clin Pract. 1994 Jan-Feb;48(1):15–18. [PubMed] [Google Scholar]
- MacKenzie C. A., Weinberg E. G., Tabachnik E., Taylor M., Havnen J., Crescenzi K. A placebo controlled trial of fluticasone propionate in asthmatic children. Eur J Pediatr. 1993 Oct;152(10):856–860. doi: 10.1007/BF02073387. [DOI] [PubMed] [Google Scholar]
- Nielsen L. P., Dahl R. Therapeutic ratio of inhaled corticosteroids in adult asthma. A dose-range comparison between fluticasone propionate and budesonide, measuring their effect on bronchial hyperresponsiveness and adrenal cortex function. Am J Respir Crit Care Med. 2000 Dec;162(6):2053–2057. doi: 10.1164/ajrccm.162.6.9912072. [DOI] [PubMed] [Google Scholar]
- Patel L., Wales J. K., Kibirige M. S., Massarano A. A., Couriel J. M., Clayton P. E. Symptomatic adrenal insufficiency during inhaled corticosteroid treatment. Arch Dis Child. 2001 Oct;85(4):330–334. doi: 10.1136/adc.85.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman D. S., Noonan M. J., Tashkin D. P., Goldstein M. F., Hamedani A. G., Kellerman D. J., Schaberg A. Comparative efficacy and safety of twice daily fluticasone propionate powder versus placebo in the treatment of moderate asthma. Ann Allergy Asthma Immunol. 1997 Apr;78(4):356–362. doi: 10.1016/S1081-1206(10)63196-1. [DOI] [PubMed] [Google Scholar]
- Peden D. B., Berger W. E., Noonan M. J., Thomas M. R., Hendricks V. L., Hamedani A. G., Mahajan P., House K. W. Inhaled fluticasone propionate delivered by means of two different multidose powder inhalers is effective and safe in a large pediatric population with persistent asthma. J Allergy Clin Immunol. 1998 Jul;102(1):32–38. doi: 10.1016/s0091-6749(98)70052-1. [DOI] [PubMed] [Google Scholar]
- Rao R., Gregson R. K., Jones A. C., Miles E. A., Campbell M. J., Warner J. O. Systemic effects of inhaled corticosteroids on growth and bone turnover in childhood asthma: a comparison of fluticasone with beclomethasone. Eur Respir J. 1999 Jan;13(1):87–94. doi: 10.1183/09031936.99.13108799. [DOI] [PubMed] [Google Scholar]
- Suissa S., Ernst P., Benayoun S., Baltzan M., Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000 Aug 3;343(5):332–336. doi: 10.1056/NEJM200008033430504. [DOI] [PubMed] [Google Scholar]
- Tanner J. M., Whitehouse R. H., Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. I. Arch Dis Child. 1966 Oct;41(219):454–471. doi: 10.1136/adc.41.219.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G. R. G., Acerini C. L., Buck J. J., Murphy N. P., Ross-Russell R., Warner J. T., McCance D. R. Acute adrenal crisis in asthmatics treated with high-dose fluticasone propionate. Eur Respir J. 2002 Jun;19(6):1207–1209. doi: 10.1183/09031936.02.00274402. [DOI] [PubMed] [Google Scholar]
- Wilson A. M., McFarlane L. C., Lipworth B. J. Effects of repeated once daily dosing of three intranasal corticosteroids on basal and dynamic measures of hypothalamic-pituitary-adrenal-axis activity. J Allergy Clin Immunol. 1998 Apr;101(4 Pt 1):470–474. doi: 10.1016/S0091-6749(98)70354-9. [DOI] [PubMed] [Google Scholar]