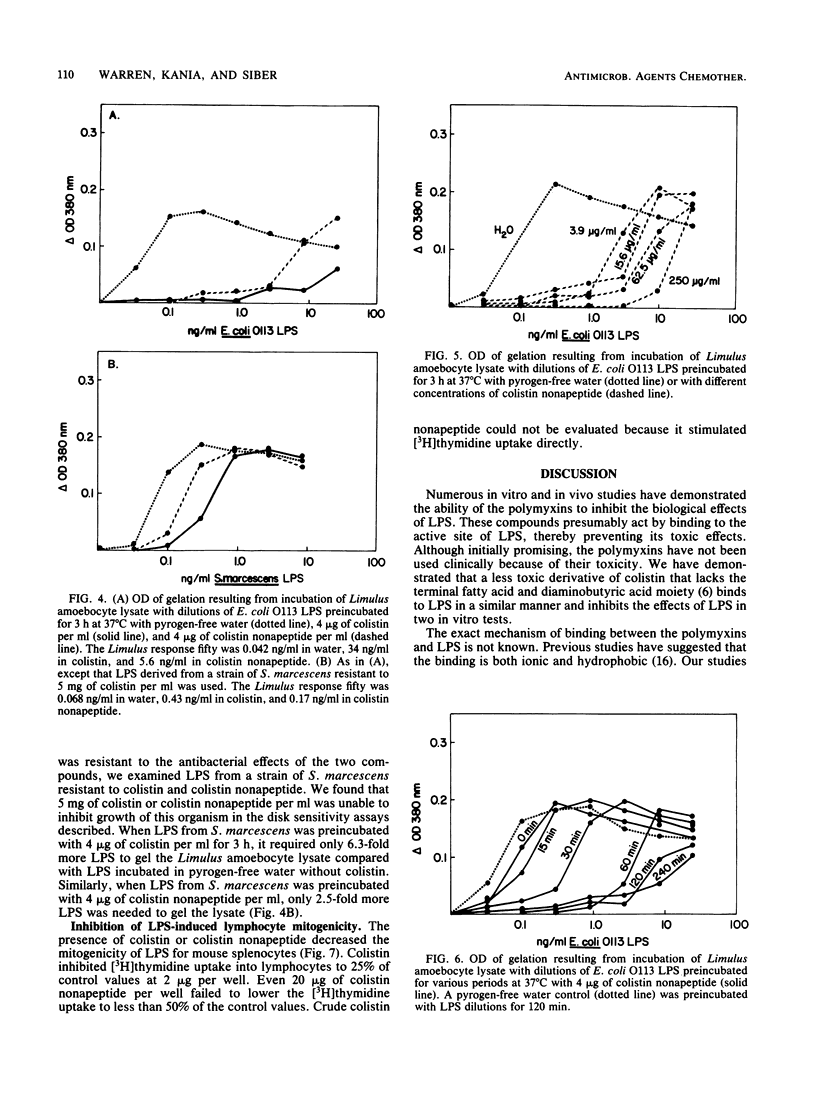
Abstract
Polymyxin nonapeptides, proteolytic derivatives of polymyxin antibiotics, are less toxic than their parent compounds but retain some of their antibacterial activities. To confirm and expand observations that polymyxin nonapeptides have anti-endotoxin activity, we studied the ability of colistin nonapeptide to bind to bacterial lipopolysaccharide (LPS) and to inhibit the effects of LPS on Limulus amoebocyte lysate and lymphocyte mitogenicity. Colistin nonapeptide was purified by high-pressure liquid chromatography and was demonstrated to bind to LPS by equilibrium dialysis. The ability of colistin nonapeptide to render E. coli ATCC 25922 cells sensitive to erythromycin was abrogated by 50% after incubation with E. coli O18 LPS in a ratio by weight of LPS to colistin nonapeptide of 3.9:1. The presence of 4 micrograms of colistin nonapeptide or colistin per ml increased by 130- and 800-fold, respectively, the concentration of E. coli O113 LPS required to produce 50% gelation of Limulus amoebocyte lysate as measured by a spectrophotometric assay. Neutralization of LPS by colistin nonapeptide was time and concentration dependent. In contrast to the neutralization seen with LPS derived from a colistin-sensitive organism, colistin nonapeptide neutralized very little LPS extracted from a strain of Serratia marcescens that was resistant to colistin. Colistin nonapeptide also inhibited LPS-induced [3H]thymidine uptake by splenic lymphocytes, but its activity was less than 1/10 that of colistin. We conclude that colistin nonapeptide binds to LPS and possesses antiendotoxin activity. However, the anti-endotoxin activity of the nonapeptide is considerably less than that of its parent compound, colistin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARNETT M., BUSHBY S. R., WILKINSON S. SODIUM SULPHOMETHYL DERIVATIVES OF POLYMYXINS. Br J Pharmacol Chemother. 1964 Dec;23:552–574. doi: 10.1111/j.1476-5381.1964.tb01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne R. M., Cheung R. Protective effect of polymyxin B sulfate in experimental enterobacterial infection in mice. Can J Microbiol. 1979 Sep;25(9):995–998. doi: 10.1139/m79-153. [DOI] [PubMed] [Google Scholar]
- Bannatyne R. M., Harnett N. M., Lee K. Y., Biggar W. D. Inhibition of the biologic effects of endotoxin on neutrophils by polymyxin B sulfate. J Infect Dis. 1977 Oct;136(4):469–474. doi: 10.1093/infdis/136.4.469. [DOI] [PubMed] [Google Scholar]
- Butler T., Möller G. Mitogenic response of mouse spleen cells and gelation of limulus lysate by lipopolysaccharide of Yersinia pestis and evidence for neutralization of lipopolysaccharide by polymyxin B. Infect Immun. 1977 Nov;18(2):400–404. doi: 10.1128/iai.18.2.400-404.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny G., Teuber M. Differential release of periplasmic versus cytoplasmic enzymes from Escherichia coli B by polymixin B. Arch Mikrobiol. 1971;78(2):166–179. doi: 10.1007/BF00424873. [DOI] [PubMed] [Google Scholar]
- Cooperstock M. S. Inactivation of endotoxin by polymyxin B. Antimicrob Agents Chemother. 1974 Oct;6(4):422–425. doi: 10.1128/aac.6.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooperstock M., Riegle L. Polymyxin B inactivation of lipopolysaccharide in vaccines of Gram-negative bacteria. Infect Immun. 1981 Jul;33(1):315–318. doi: 10.1128/iai.33.1.315-318.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan J. J., Jr, Bell B. M. Endotoxin-induced intravascular coagulation: prevention with polymyxin B sulfate. J Lab Clin Med. 1971 May;77(5):802–810. [PubMed] [Google Scholar]
- Corrigan J. J., Jr, Kiernat J. F. Effect of polymyxin B sulfate on endotoxin activity in a gram-negative septicemia model. Pediatr Res. 1979 Jan;13(1):48–51. doi: 10.1203/00006450-197901000-00011. [DOI] [PubMed] [Google Scholar]
- Craig W. A., Turner J. H., Kunin C. M. Prevention of the generalized Shwartzman reaction and endotoxin lethality by polymyxin B localized in tissues. Infect Immun. 1974 Aug;10(2):287–292. doi: 10.1128/iai.10.2.287-292.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund P. T., King T. P., Craig L. C., Walti A. Studies on ficin. I. Its isolation and characterization. Biochemistry. 1968 Jan;7(1):163–175. doi: 10.1021/bi00841a021. [DOI] [PubMed] [Google Scholar]
- Jacobs D. M., Morrison D. C. Inhibition of the mitogenic response to lipopolysaccharide (LPS) in mouse spleen cells by polymyxin B. J Immunol. 1977 Jan;118(1):21–27. [PubMed] [Google Scholar]
- Jacobs M. D., Morrison D. C. Dissociation between mitogenicity and immunogenicity of TNP-lipopolysaccharide, a T-independent antigen. J Exp Med. 1975 Jun 1;141(6):1453–1458. doi: 10.1084/jem.141.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounatmaa K., Mäkelä P. H., Sarvas M. Effect of polymyxin on the ultrastructure of the outer membrane of wild-type and polymyxin-resistant strain of Salmonella. J Bacteriol. 1976 Sep;127(3):1400–1407. doi: 10.1128/jb.127.3.1400-1407.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Rifkind D. Prevention by polymyxin B of endotoxin lethality in mice. J Bacteriol. 1967 Apr;93(4):1463–1464. doi: 10.1128/jb.93.4.1463-1464.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind D. Studies on the interaction between endotoxin and polymyxin B. J Infect Dis. 1967 Dec;117(5):433–438. doi: 10.1093/infdis/117.5.433. [DOI] [PubMed] [Google Scholar]
- Rudbach J. A., Akiya F. I., Elin R. J., Hochstein H. D., Luoma M. K., Milner E. C., Milner K. C., Thomas K. R. Preparation and properties of a national reference endotoxin. J Clin Microbiol. 1976 Jan;3(1):21–25. doi: 10.1128/jcm.3.1.21-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Outer membrane permeability barrier disruption by polymyxin in polymyxin-susceptible and -resistant Salmonella typhimurium. Antimicrob Agents Chemother. 1981 Apr;19(4):578–583. doi: 10.1128/aac.19.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations as outer membrane-disorganizing agents. Antimicrob Agents Chemother. 1983 Jul;24(1):114–122. doi: 10.1128/aac.24.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations sensitize enteric bacteria to antibiotics. Antimicrob Agents Chemother. 1983 Jul;24(1):107–113. doi: 10.1128/aac.24.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Sensitization of Gram-negative bacteria to antibiotics and complement by a nontoxic oligopeptide. Nature. 1983 Jun 9;303(5917):526–528. doi: 10.1038/303526a0. [DOI] [PubMed] [Google Scholar]
- Vaara M., Viljanen P., Vaara T., Mäkelä P. H. An outer membrane-disorganizing peptide PMBN sensitizes E. coli strains to serum bactericidal action. J Immunol. 1984 May;132(5):2582–2589. [PubMed] [Google Scholar]
- Viljanen P., Vaara M. Susceptibility of gram-negative bacteria to polymyxin B nonapeptide. Antimicrob Agents Chemother. 1984 Jun;25(6):701–705. doi: 10.1128/aac.25.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]


