Abstract
Aims—To investigate interlaboratory variance in the immunohistochemical (IHC) detection of oestrogen receptors so as to determine the rate of false negatives, which could adversely influence the decision to give adjuvant tamoxifen treatment.
Methods—To ensure that similar results are obtained by different institutions, 200 laboratories from 26 countries have joined the UK national external quality assessment scheme for immunocytochemistry (NEQAS-ICC). Histological sections from breast cancers having low, medium, and high levels of oestrogen receptor expression were sent to each of the laboratories for immunohistochemical staining. The results obtained were evaluated for the sensitivity of detection, first by estimating threshold values of 1% and 10% of stained tumour cells, and second by the Quick score method, by a panel of four assessors judging individual sections independently on a single blind basis. The results were also evaluated using participants' own threshold values.
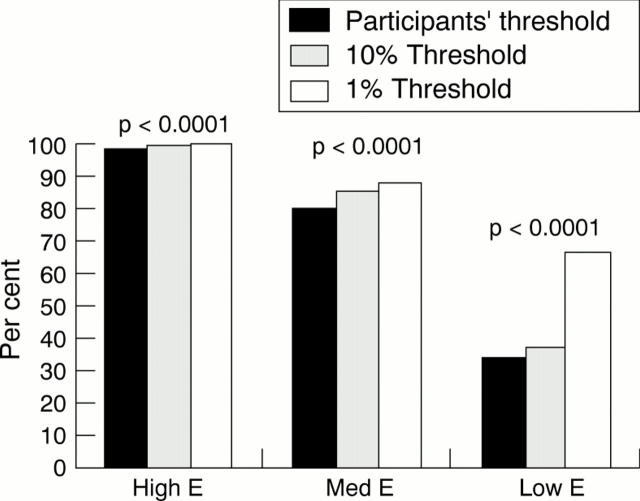
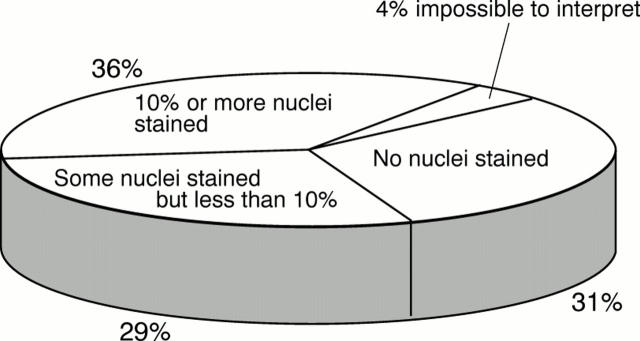
Results—Over 80% of laboratories were able to demonstrate oestrogen receptor positivity on the medium and high expressing tumours, but only 37% of laboratories scored adequately on the low expressing tumour. Approximately one third of laboratories failed to register any positive staining in this tumour, while one third showed only minimal positivity.
Conclusions—There is considerable interlaboratory variability, especially in relation to the detection of breast cancers with low oestrogen receptor positivity, with a false negative rate of between 30% and 60%. This variability appears to be caused by minor differences in methodology that may be rectified by fine adjustment of overall technique.
Key Words: immunohistochemistry • oestrogen receptors • interlaboratory variation
Full Text
The Full Text of this article is available as a PDF (158.0 KB).
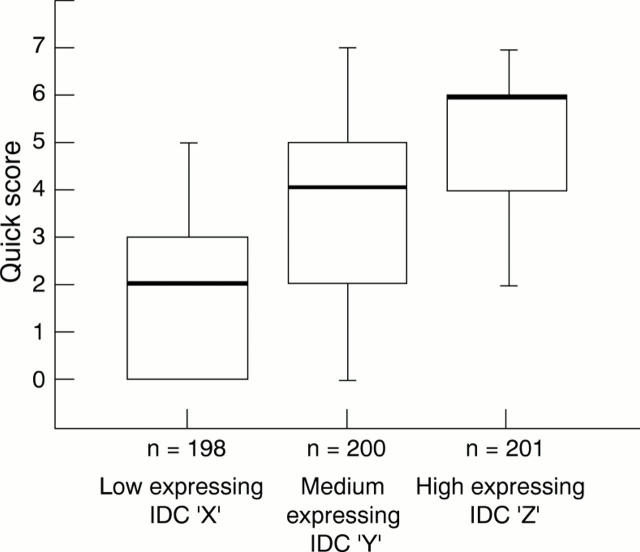
Figure 1 Distribution of the results of the "Quick" score evaluation conducted on the three infiltrating ductal carcinomas (IDC), X, Y, Z, used at assessment. The bold line represents the median score, the bottom and top of the boxes, the 1st and 3rd quartiles, respectively, and the range bars, the lowest and highest scores, respectively. The slightly different numbers for the three tumours reflect loss of tissue from the microscope slides; n, number of participating laboratories.
Figure 2 The proportion of laboratories from which immunohistochemistry reliably demonstrated the intraductal carcinomas X, Y, Z as being oestrogen receptor positive. χ2 values were as follows:
Figure 3 The proportions of 200 laboratories from which immunohistochemistry demonstrated either no nuclei, some nuclei but less than 10%, and 10% of more, in the "low" oestrogen receptor expressing infiltrating ductal carcinoma.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred D. C., Harvey J. M., Berardo M., Clark G. M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998 Feb;11(2):155–168. [PubMed] [Google Scholar]
- Barnes D. M., Harris W. H., Smith P., Millis R. R., Rubens R. D. Immunohistochemical determination of oestrogen receptor: comparison of different methods of assessment of staining and correlation with clinical outcome of breast cancer patients. Br J Cancer. 1996 Nov;74(9):1445–1451. doi: 10.1038/bjc.1996.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D. M., Millis R. R., Beex L. V., Thorpe S. M., Leake R. E. Increased use of immunohistochemistry for oestrogen receptor measurement in mammary carcinoma: the need for quality assurance. Eur J Cancer. 1998 Oct;34(11):1677–1682. doi: 10.1016/s0959-8049(98)00149-x. [DOI] [PubMed] [Google Scholar]
- Elledge R. M., Osborne C. K. Oestrogen receptors and breast cancer. BMJ. 1997 Jun 28;314(7098):1843–1844. doi: 10.1136/bmj.314.7098.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernö M., Andersson C., Fallenius G., Idvall I. Oestrogen receptor analysis of paraffin sections and cytosol samples of primary breast cancer in relation to outcome after adjuvant tamoxifen treatment. The South Sweden Breast Cancer Group. Acta Oncol. 1996;35(1):17–22. doi: 10.3109/02841869609098474. [DOI] [PubMed] [Google Scholar]
- Hendricks J. B., Wilkinson E. J. Comparison of two antibodies for evaluation of estrogen receptors in paraffin-embedded tumors. Mod Pathol. 1993 Nov;6(6):765–770. [PubMed] [Google Scholar]
- Kinsel L. B., Szabo E., Greene G. L., Konrath J., Leight G. S., McCarty K. S., Jr Immunocytochemical analysis of estrogen receptors as a predictor of prognosis in breast cancer patients: comparison with quantitative biochemical methods. Cancer Res. 1989 Feb 15;49(4):1052–1056. [PubMed] [Google Scholar]
- McCarty K. S., Jr, Miller L. S., Cox E. B., Konrath J., McCarty K. S., Sr Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985 Aug;109(8):716–721. [PubMed] [Google Scholar]
- Pertschuk L. P., Feldman J. G., Kim Y. D., Braithwaite L., Schneider F., Braverman A. S., Axiotis C. Estrogen receptor immunocytochemistry in paraffin embedded tissues with ER1D5 predicts breast cancer endocrine response more accurately than H222Sp gamma in frozen sections or cytosol-based ligand-binding assays. Cancer. 1996 Jun 15;77(12):2514–2519. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2514::AID-CNCR14>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Reiner A., Neumeister B., Spona J., Reiner G., Schemper M., Jakesz R. Immunocytochemical localization of estrogen and progesterone receptor and prognosis in human primary breast cancer. Cancer Res. 1990 Nov 1;50(21):7057–7061. [PubMed] [Google Scholar]
- Soubeyran I., Quénel N., Coindre J. M., Bonichon F., Durand M., Wafflart J., Mauriac L. pS2 protein: a marker improving prediction of response to neoadjuvant tamoxifen in post-menopausal breast cancer patients. Br J Cancer. 1996 Oct;74(7):1120–1125. doi: 10.1038/bjc.1996.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al Saati T., Clamens S., Cohen-Knafo E., Faye J. C., Prats H., Coindre J. M., Wafflart J., Caverivière P., Bayard F., Delsol G. Production of monoclonal antibodies to human estrogen-receptor protein (ER) using recombinant ER (RER). Int J Cancer. 1993 Oct 21;55(4):651–654. doi: 10.1002/ijc.2910550423. [DOI] [PubMed] [Google Scholar]