Abstract
Background—Familial amyloidosis of the Finnish type (FAF, Finnish hereditary amyloidosis) is caused by a 654G-A mutation in the gelsolin gene on chromosome 9 resulting in the expression of mutant Asn-187 gelsolin which is abnormally proteolytically processed generating amyloidogenic fragments that polymerise into amyloid fibrils. We have recently shown that in a Danish and a Czech family with a clinical syndrome similar to FAF, including corneal lattice dystrophy, cranial neuropathy and skin changes, the disease is caused by another mutation at the same position, namely 654G-T predicting a Tyr-for-Asp substitution at 187 in secreted gelsolin.
Aim—To undertake a closer examination of the Danish subtype of FAF and report immunohistochemical and biochemical findings.
Results—Immunostaining of plasma gelsolin isolated from heterozygous FAF of the Danish subtype revealed a pattern similar to that found in FAF-Asn 187. The > 60 kDa gelsolin species contain an epitope characteristic of the amyloid forming region as revealed by an amyloid specific antibody, whereas the ∼50 kDa fragments are devoid of it. Compared with the wild-type gelsolin peptide (Asp-187), the corresponding mutant peptide (Tyr-187) showed dramatically increased fibrillogenicity as revealed by quantitative thioflavine-T based fluorimetry; ultrastructurally, amyloid-like fibrils were formed by the mutant peptide. Immunohistochemistry showed that antibodies directed against residues 231–242 of secreted gelsolin, representing the carboxy terminus of the sequence forming the amyloid protein (residues 173–243) laid down in the tissues in a fibrillar form in FAF, specifically labelled the amyloid deposited in rectum and skin in the Danish (654G-T) subtype.
Conclusions—The 654G-T mutation in the gelsolin gene gives rise to an amyloid disease clinically and pathogenetically similar to that caused by the 654G-A mutation.
Key Words: amyloidosis • Finnish familial amyloidosis • gelsolin mutation 654G-T • fibrillogenesis
Full Text
The Full Text of this article is available as a PDF (214.6 KB).
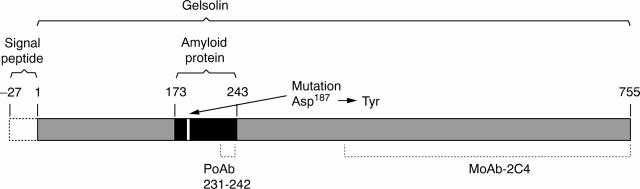
Figure 1 Schematic presentation of the amyloid forming region (residues 173–243) and mutation site in secreted gelsolin (residues 1–755) in Danish-type FAF, as well as the regions of the gelsolin molecule to which the amyloid specific polyclonal antibody (PoAb 231–242) and the monoclonal antibody MoAb-2C4 are directed.
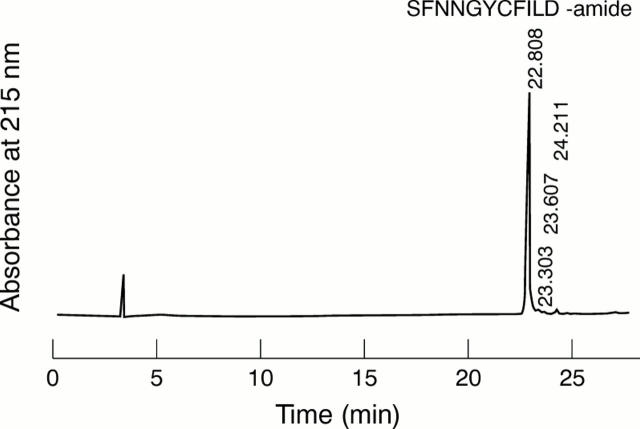
Figure 2 Reverse phase high pressure liquid chromatography on a Vydac C18 column of synthetic mutant 182–192 gelsolin peptide (Tyr-187).
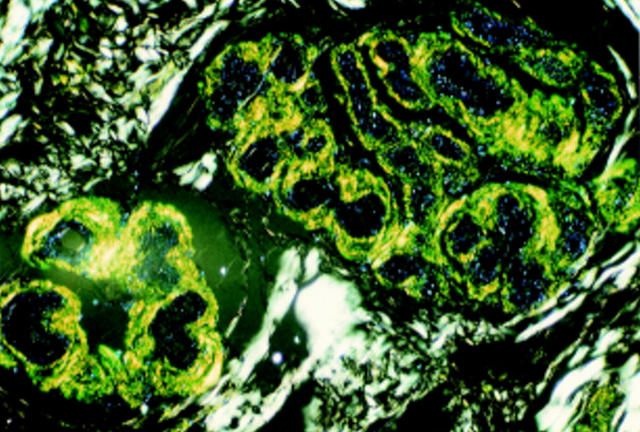
Figure 3 Dermal eccrine glands in Danish subtype (Tyr-187) familial amyloidosis showing prominent amyloid deposits in thickened basement membranes. Congo red staining, polarised light, magnification x140.
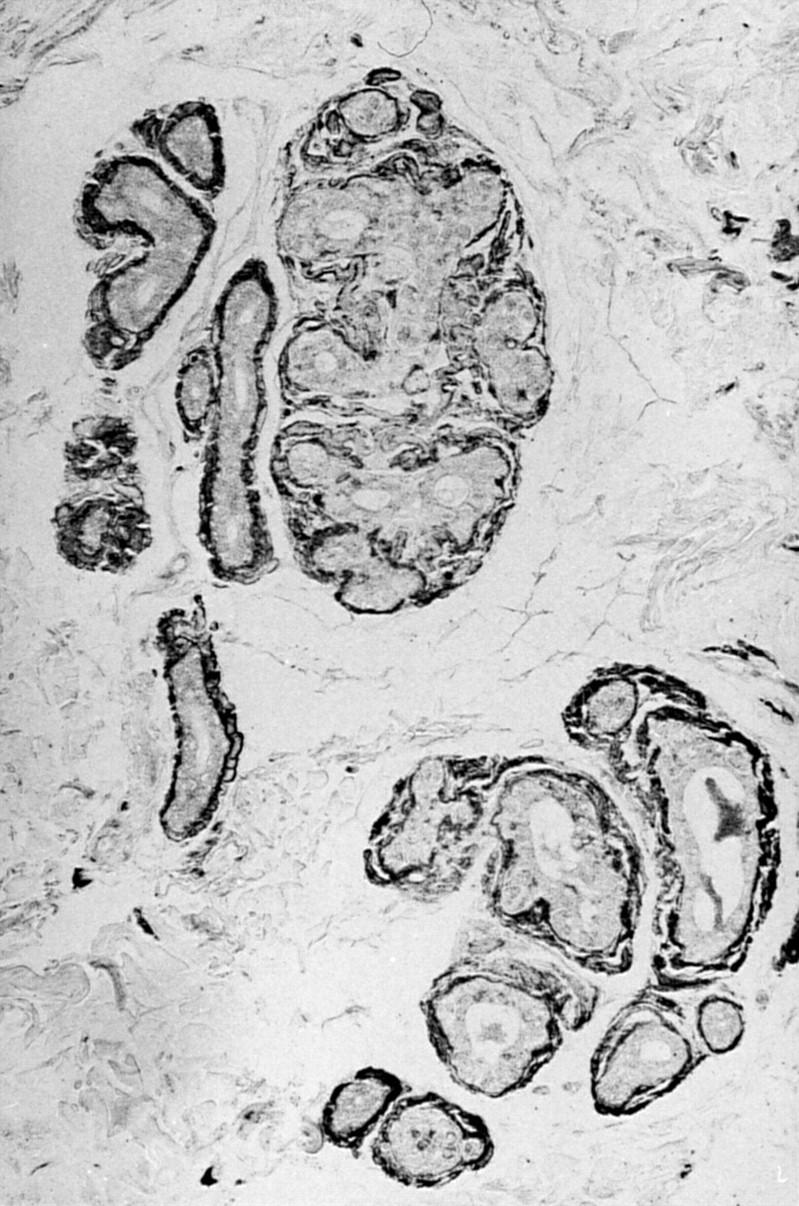
Figure 4 Immunogold-silver staining with amyloid specific antibody PoAb 231–242 of a skin biopsy in Danish subtype (Tyr-187) amyloidosis. Amyloid located in basement membranes is specifically stained. Magnification x134.
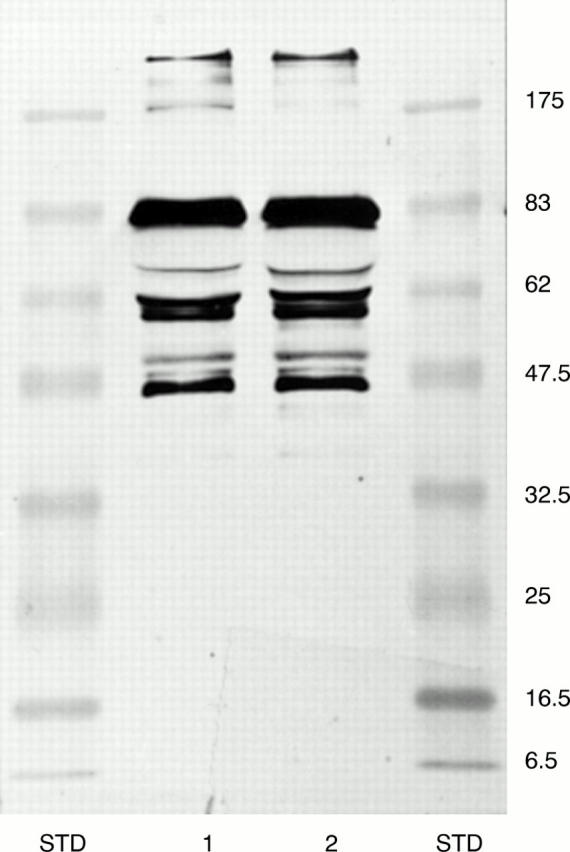
Figure 5 Comparison of heterozygous Danish subtype FAF (Tyr-187) and heterozygous Asn-187 FAF by immunoblotting by the monoconal MoAb-2C4 antibody. Lane 1, FAF Asn-187; lane 2, Danish FAF (Tyr-187); STD, prestained molecular weight markers (kDa).
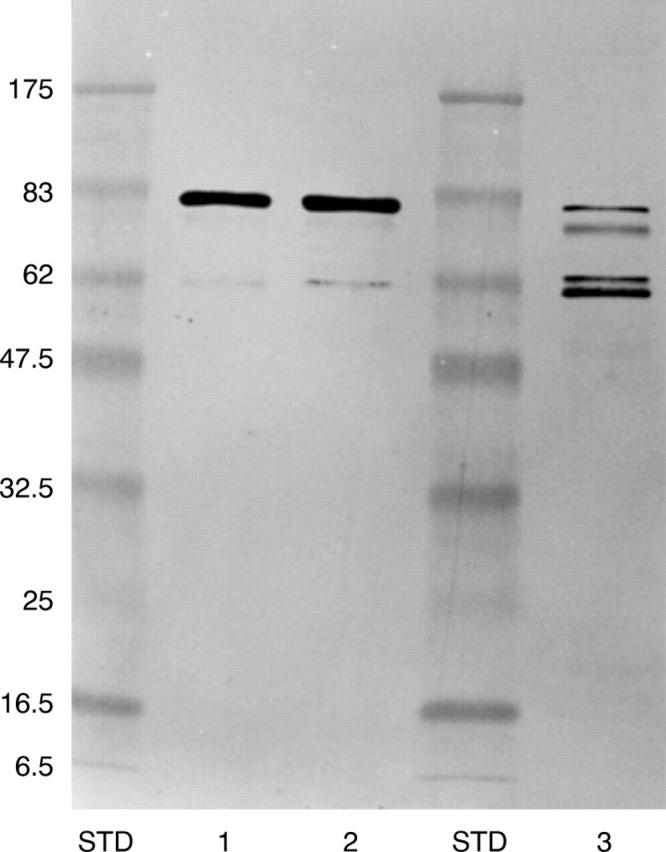
Figure 6 Immunostaining of the gelsolin fraction isolated from FAF plasma by affinity chromatography on an MoAb-2C4 Sepharose column by polyclonal antiamyloid antibodies directed against the C terminus of the amyloid region (231–242). Lane 1, heterozygous FAF Tyr-187 (Danish); lane 2, heterozygous FAF Asn-187; lane 3, homozygous FAF Asn-187; STD, prestained molecular weight markers (kDa). Note that the ∼50 kDa bands (fig 5) are not stained by the amyloid specific antibody as they are devoid of the amyloid forming sequence.
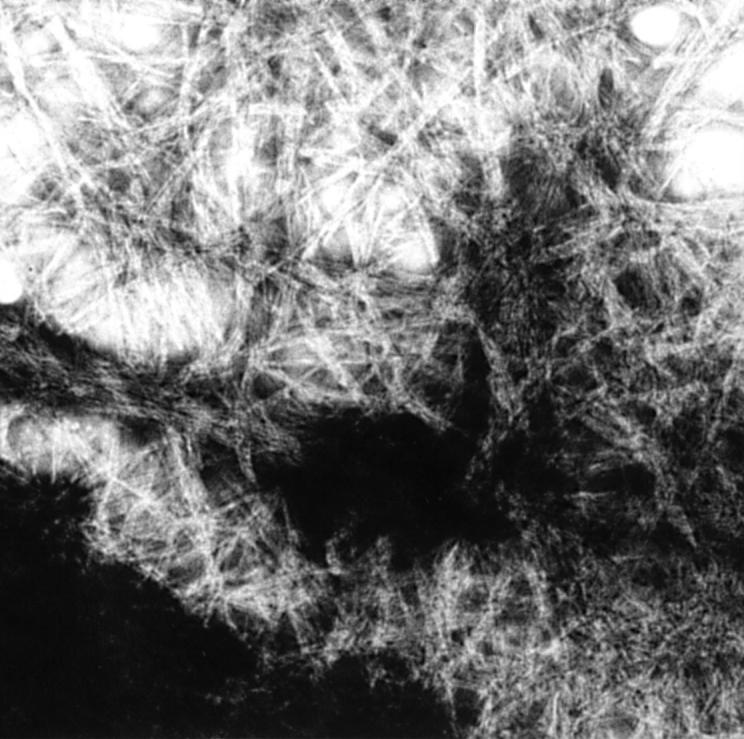
Figure 7 Electron micrograph of negatively stained protein assemblies formed by the mutant (Tyr-187) peptide, corresponding to residues 182–192 in gelsolin. Magnification x59 595.
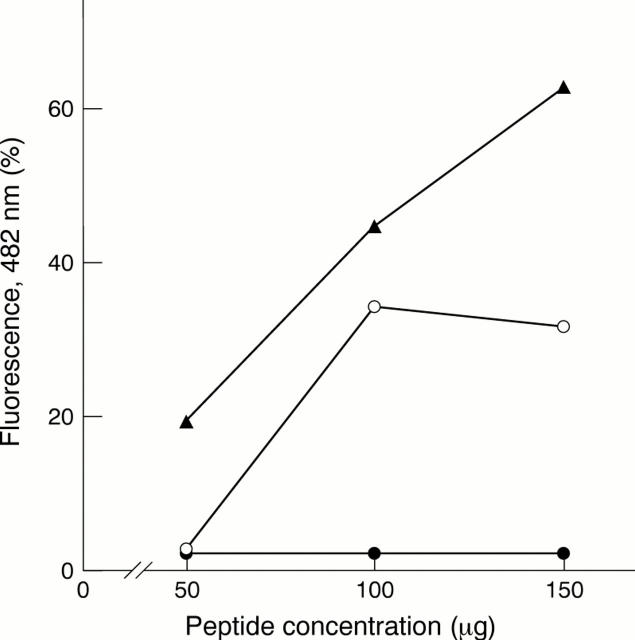
Figure 8 Comparison of amyloid fibril formation at different peptide concentrations by mutant (Tyr-187) gelsolin 182–192 peptide corresponding to Danish type amyloidosis (filled triangles), by mutant (Asn-187) gelsolin peptide 182–192 corresponding to Finnish amyloidosis (empty circles), and wild-type (Asp-187) gelsolin 182–192 peptide (filled circles), as measured by quantitative thioflavine-T based fluorimetry.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boysen G., Galassi G., Kamieniecka Z., Schlaeger J., Trojaborg W. Familial amyloidosis with cranial neuropathy and corneal lattice dystrophy. J Neurol Neurosurg Psychiatry. 1979 Nov;42(11):1020–1030. doi: 10.1136/jnnp.42.11.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas H., Paunio T., Kalkkinen N., Jalanko A., Peltonen L. In vitro expression analysis shows that the secretory form of gelsolin is the sole source of amyloid in gelsolin-related amyloidosis. Hum Mol Genet. 1996 Sep;5(9):1237–1243. doi: 10.1093/hmg/5.9.1237. [DOI] [PubMed] [Google Scholar]
- Kiuru S., Matikainen E., Kupari M., Haltia M., Palo J. Autonomic nervous system and cardiac involvement in familial amyloidosis, Finnish type (FAF). J Neurol Sci. 1994 Oct;126(1):40–48. doi: 10.1016/0022-510x(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Levy E., Haltia M., Fernandez-Madrid I., Koivunen O., Ghiso J., Prelli F., Frangione B. Mutation in gelsolin gene in Finnish hereditary amyloidosis. J Exp Med. 1990 Dec 1;172(6):1865–1867. doi: 10.1084/jem.172.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury C. P., Alli K., Baumann M. Finnish hereditary amyloidosis. Amino acid sequence homology between the amyloid fibril protein and human plasma gelsoline. FEBS Lett. 1990 Jan 15;260(1):85–87. doi: 10.1016/0014-5793(90)80072-q. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Baumann M. Isolation and characterization of cardiac amyloid in familial amyloid polyneuropathy type IV (Finnish): relation of the amyloid protein to variant gelsolin. Biochim Biophys Acta. 1990 Nov 14;1096(1):84–86. doi: 10.1016/0925-4439(90)90016-i. [DOI] [PubMed] [Google Scholar]
- Maury C. P. Gelsolin-related amyloidosis. Identification of the amyloid protein in Finnish hereditary amyloidosis as a fragment of variant gelsolin. J Clin Invest. 1991 Apr;87(4):1195–1199. doi: 10.1172/JCI115118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury C. P. Homozygous familial amyloidosis, Finnish type: demonstration of glomerular gelsolin-derived amyloid and non-amyloid tubular gelsolin. Clin Nephrol. 1993 Jul;40(1):53–56. [PubMed] [Google Scholar]
- Maury C. P. Immunohistochemical localization of amyloid in Finnish hereditary amyloidosis with antibodies to gelsolin peptides. Lab Invest. 1991 Mar;64(3):400–404. [PubMed] [Google Scholar]
- Maury C. P., Kere J., Tolvanen R., de la Chapelle A. Finnish hereditary amyloidosis is caused by a single nucleotide substitution in the gelsolin gene. FEBS Lett. 1990 Dec 10;276(1-2):75–77. doi: 10.1016/0014-5793(90)80510-p. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Kere J., Tolvanen R., de la Chapelle A. Homozygosity for the Asn187 gelsolin mutation in Finnish-type familial amyloidosis is associated with severe renal disease. Genomics. 1992 Jul;13(3):902–903. doi: 10.1016/0888-7543(92)90183-s. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Nurmiaho-Lassila E. L. Creation of amyloid fibrils from mutant Asn187 gelsolin peptides. Biochem Biophys Res Commun. 1992 Feb 28;183(1):227–231. doi: 10.1016/0006-291x(92)91632-z. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Nurmiaho-Lassila E. L., Rossi H. Amyloid fibril formation in gelsolin-derived amyloidosis. Definition of the amyloidogenic region and evidence of accelerated amyloid formation of mutant Asn-187 and Tyr-187 gelsolin peptides. Lab Invest. 1994 Apr;70(4):558–564. [PubMed] [Google Scholar]
- Maury C. P., Rossi H. Demonstration of a circulating 65K gelsolin variant specific for familial amyloidosis, Finnish type. Biochem Biophys Res Commun. 1993 Feb 26;191(1):41–44. doi: 10.1006/bbrc.1993.1181. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Sletten K., Totty N., Kangas H., Liljeström M. Identification of the circulating amyloid precursor and other gelsolin metabolites in patients with G654A mutation in the gelsolin gene (Finnish familial amyloidosis): pathogenetic and diagnostic implications. Lab Invest. 1997 Oct;77(4):299–304. [PubMed] [Google Scholar]
- McLaughlin P. J., Gooch J. T., Mannherz H. G., Weeds A. G. Structure of gelsolin segment 1-actin complex and the mechanism of filament severing. Nature. 1993 Aug 19;364(6439):685–692. doi: 10.1038/364685a0. [DOI] [PubMed] [Google Scholar]
- Meretoja J. Familial systemic paramyloidosis with lattice dystrophy of the cornea, progressive cranial neuropathy, skin changes and various internal symptoms. A previously unrecognized heritable syndrome. Ann Clin Res. 1969 Dec;1(4):314–324. [PubMed] [Google Scholar]
- Naiki H., Higuchi K., Hosokawa M., Takeda T. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal Biochem. 1989 Mar;177(2):244–249. doi: 10.1016/0003-2697(89)90046-8. [DOI] [PubMed] [Google Scholar]
- Paunio T., Sunada Y., Kiuru S., Makishita H., Ikeda S., Weissenbach J., Palo J., Peltonen L. Haplotype analysis in gelsolin-related amyloidosis reveals independent origin of identical mutation (G654A) of gelsolin in Finland and Japan. Hum Mutat. 1995;6(1):60–65. doi: 10.1002/humu.1380060112. [DOI] [PubMed] [Google Scholar]
- Steiner R. D., Paunio T., Uemichi T., Evans J. P., Benson M. D. Asp187Asn mutation of gelsolin in an American kindred with familial amyloidosis, Finnish type (FAP IV). Hum Genet. 1995 Mar;95(3):327–330. doi: 10.1007/BF00225202. [DOI] [PubMed] [Google Scholar]
- Westermark P., Engström U., Johnson K. H., Westermark G. T., Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Terabayashi M., Egawa T., Hayashi E., Nakamura H., Kishimoto S. Affinity separation of human plasma gelsolin on Affi-Gel Blue. J Biochem. 1989 May;105(5):799–802. doi: 10.1093/oxfordjournals.jbchem.a122748. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A., Kere J., Sack G. H., Jr, Tolvanen R., Maury C. P. Familial amyloidosis, Finnish type: G654----a mutation of the gelsolin gene in Finnish families and an unrelated American family. Genomics. 1992 Jul;13(3):898–901. doi: 10.1016/0888-7543(92)90182-r. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A., Tolvanen R., Boysen G., Santavy J., Bleeker-Wagemakers L., Maury C. P., Kere J. Gelsolin-derived familial amyloidosis caused by asparagine or tyrosine substitution for aspartic acid at residue 187. Nat Genet. 1992 Oct;2(2):157–160. doi: 10.1038/ng1092-157. [DOI] [PubMed] [Google Scholar]