Abstract
Rivers have been suggested to have played an important role in shaping present-day patterns of ecological and genetic variation among Amazonian species and communities. Recent molecular studies have provided mixed support for the hypothesis that large lowland Amazonian rivers have functioned as significant impediments to gene flow among populations of neotropical species. To date, no study has systematically evaluated the impact that riverine barriers might have on structuring whole Amazonian communities. Our analyses of the phylogeography of frogs and small mammals indicate that a putative riverine barrier (the Juruá River) does not relate to present-day patterns of community similarity and species richness. Rather, our results imply a significant impact of the Andean orogenic axis and associated thrust-and-fold lowland dynamics in shaping patterns of biotic diversity along the Juruá. Combined results of this and other studies significantly weaken the postulated role of rivers as major drivers of Amazonian diversification.
The Amazonian tropical rainforest harbors a species diversity that is vastly disproportionate to its geographic area (1, 2). Numerous hypotheses have been proposed to account for this, tending to emphasize aspects of the maintenance or origins of this megadiversity (3–8). The oldest such hypothesis has its roots in the works of Alfred Russel Wallace, who observed that the ranges of some closely related neotropical vertebrate species (primates, birds) abut at major rivers (9, 10). Indeed, Wallace defined distinct areas within South America, bounded by major Amazonian rivers like the Negro, Madeira, and Amazon, which differed in species composition of communities (10). These and similar observations (e.g., refs. 11–13) have prompted the suggestion that lowland Amazonian rivers, of which there are many, may function as effective barriers to the dispersal of organisms. This may have a variety of consequences for patterns of species diversity on the Amazonian landscape. First, major Amazonian rivers may have played a significant role in species generation by impeding gene flow between populations with the eventual evolution of sister species on opposite banks (14, 15). Second, the expansion of species from their centers of origin may be halted by the presence of large watercourses; therefore, they may be restricted to only one bank (13). Finally, compared with a species distributed across landscapes without barriers, the probability of subsequent recolonization of a species that has gone locally extinct on one bank will be lower because immigration from the opposite bank is less likely (for a general discussion of local extinction/colonization dynamics, see ref. 16). All of these factors might be expected to accentuate differences in species composition of opposite-bank communities.
To date this so-called riverine barrier hypothesis largely has been tested by examining patterns of genetic differentiation among populations with mixed support across locales, studies, and taxa (15, 17–23). These studies have emphasized the possible historical role of rivers in generating species diversity. However, if rivers have played significant roles in speciation and continue to function as impediments to movement of organisms, then there are explicit, testable community-level predictions. First, because the distance of separation and putative barrier strength are larger between opposing bank upland sites (terra firme), which is the intervening river width plus the width of the flooded forest planes (várzea), than for flooded forest sites (river width only), faunal differences are expected to be pronounced. In addition, meander cutoffs physically transfer blocks of várzea, but not terra firme, from one riverbank to the other over time. Second, similarity in species composition between opposing-bank paired sites should decrease with increasing barrier strength (i.e., with river width or water flow). Third, after controlling for the effect of distance, species composition among adjacent sites on the same bank should be more similar than in sites on opposing banks. Finally, boundaries of species distributions should coincide more frequently with major rivers than expected by chance. Herein, we provide a test of these community-level predictions of the riverine barrier hypothesis using patterns of species diversity in frog and small mammal (rodent and marsupial) communities of a major tributary of the Amazon River, the Juruá River, in western Amazonia (Fig. 1). The geographic context and sampling design of this study further allow us to test for the existence of a west–east decreasing trend in species richness that has been suggested for both frogs (24) and mammals (25).
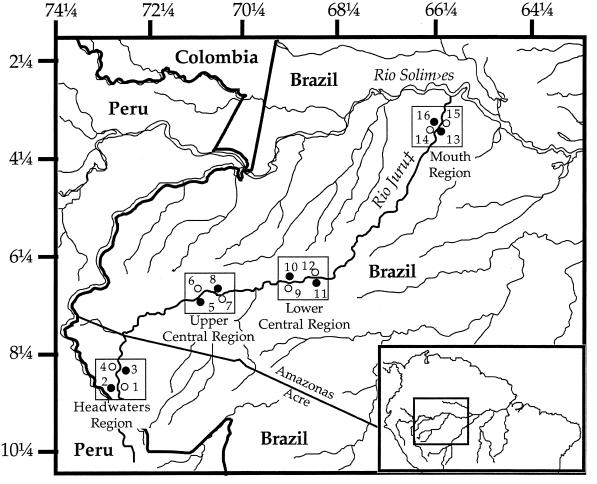
Figure 1.
Map of the study area (Inset showing relative location within South America). The Juruá River extends from its headwaters in Peru to the Amazon River just outside of Tefé. Numbers correspond to sampling sites within four regions (headwaters, upper central, lower central, and mouth). 1, Igarapé Porongaba (8°40′S, 72°47′W); 2, opposite Igarapé Porongaba (8°40′S, 72°47′W); 3, Nova Vida (8°22′S, 72°49′W); 4, Sobral (8°22′S, 72°49′W); 5, Sacado (6°45′S, 70°51′W); 6, Seringal Condor (6°45′S, 70°51′W); 7, Penedo (6°50′S, 70°45′W); 8, Igarapé Nova Empresa (6°48′S, 70°44′W); 9, Altamira (6°35′S, 68°54′W); 10, opposite Altamira (6°35′S, 68°56′W); 11, Jainu (6°28′S, 68°46′W); 12, Barro Vermelho (6°28′S, 68°46′W); 13, Ilha Paxiuba (3°19′S, 66°00′W); 14, Colocação Vira-Volta (3°17′S, 66°14′W); 15, Lago Vai-Quem-Quer (3°19′S, 66°01′W); 16, Ilhazinha (3°17′S, 66°14′W). Terra firme sites are indicated by open circles; várzea sites are indicated by closed circles. See ref. 30 for more details.
Methods
Study Area and Sampling Design.
The Juruá River is a long watercourse that spans a straight-line distance of over 1200 km from the Peru–Brazil border near Pulcallpa northeast to its junction with the Amazon, just west of the Brazilian city of Tefé. It is a dynamic river system (26), with a width that varies from several tens of meters at its headwaters to more than 500 m at its mouth. The width of flooded forest is much greater, from 6 km at the headwater sites to approximately 25 km at the mouth. Reasons for choosing the Juruá River to examine the riverine barrier hypothesis include the following: (i) it is the third-largest whitewater river in Amazonia (excluding the Amazon–Solimões proper), (ii) the Juruá River basin has been identified as an important contact zone, at least for birds (27), and (iii) this region of Western Amazonia has been identified as potentially an area of historically rapid environmental transitions (28, 29).
From July 1991 to June 1992, we sampled paired sites on opposing banks (both terra firme and várzea habitats) in each of four regions along the Juruá River from the headwaters to the mouth (Fig. 1). Mean distance between sites in adjacent regions ranged from approximately 220 (upper and lower central) to 450 km (lower central and mouth). Within each region, a pair of opposing-bank sites in terra firme and várzea was selected from maps and local information, with the primary criterion being that sites contained undisturbed, old growth forest. The sinuous nature of the river, where bends abut with terra firme forest with the immediate opposite bank always with várzea forest (30), meant a staggered sampling of sites of each forest type within regions (Fig. 1). For conformity with other publications, we designate the north bank as left (even-numbered sites in Fig. 1) and the south bank as right (odd-numbered sites). Upland forest sites were situated on dissected terrain near the edge of the floodplain. In the floodplain, we sampled areas of mature várzea forest that contained large-diameter trees and were structurally similar to upland forests. Based on descriptions of floodplain forests along the Rio Manú, we estimate these forests to be >300 years old (31).
Mammal Surveys.
Small mammals were sampled using four to six terrestrial trap lines (five trap lines at 14 of 16 sites) and two to four (three trap lines at 12 of 16 sites) canopy trap lines per site. Trap lines were established along parallel trails spaced at least 100 m apart and consisted of 15 trap stations at 20-m intervals. Terrestrial trap stations consisted of a 14 × 14 × 40-cm wire mesh (Tomahawk) trap and a 8 × 8 × 23-cm folding aluminum (Sherman) trap placed 2–4 m apart (two trap sizes were used to lessen biases to particular size classes of small mammals). Canopy stations consisted of a single Sherman placed on top of a Tomahawk trap. Canopy trap height, measured with a range finder to the nearest 0.1 m, averaged 11.9 m in upland forest (n = 345, SE = 0.13, range = 6.6–19.2 m) and 10.3 m in floodplain forest (n = 375, SE = 0.13, range = 2.5–20.7 m). Terrestrial and canopy traps were left open for 7 and 12 consecutive nights, respectively. We left canopy traps open longer than terrestrial traps to partly compensate for fewer trap stations and lower capture success in the canopy. Bait consisted of plantain and a mixture of ground peanuts, vegetable oil, and honey. Because canopy traps were baited only when they were first set or after a capture, they also were set with a long-term bait source not easily removed by insects (a small cloth sack containing raisins and the ground peanut mixture). Complete sampling details may be found elsewhere (30).
Frog Surveys.
At each site, precut trails of equal length (approximately 5 km) were walked for 4–12 nights to determine frog species presence. Trails were walked at an approximately constant rate of 1.5 km/h, and all calling or observed frogs were collected and identified. Some animals were brought back to camp for tissue collection and preservation, whereas others were released after standard field data were collected. Potential aquatic breeding habitats (ponds, pools, streams) located close to the trails were surveyed more extensively for breeding individuals. At these sites, all calling and observed frogs were also identified and collected or released. As the presence of many species of tropical frogs depends on the presence and availability of suitable breeding habitat (32), the number of ponds, pools, and small and large streams was counted at each site. Sites only differed with respect to the number of large streams (some sites had no such habitats present in the survey region). Species that are known to only breed in these habitats were excluded from analyses: Cochranella sp., Hyla granosa, Hyla boans, and Osteocephalus buckleyi. This controlled for possible spurious differences in community composition or species richness among sites due simply to differences in proportion of available breeding habitats. Further, we excluded juvenile individuals of Colostethus sp. and Eleutherodactylus sp. that could not be reliably identified. Paired opposing-bank sites were sampled concurrently to avoid confounding effects due to differences in rainfall during the sampling year.
Statistical Analyses.
For all pairwise comparisons of species composition between sites, we used the Jaccard's similarity index (33). For frogs and mammals considered separately, we tested for higher community similarity between várzea compared with terra firme paired sites using a one-tailed t test. The relationship between barrier strength and community similarity was accomplished by visual inspection of rank order of paired Jaccard's values from the headwaters region (weakest) to the mouth region (strongest) for both habitat types for frogs and mammals.
Evaluation of opposing-bank vs. same-bank similarities and boundaries of species distributions were restricted to mammals because rainfall and other seasonal factors may influence them less than frogs. For the comparison of opposing- and same-bank community similarity in mammals, we controlled for geographic distance in two ways. In the first method, we used only those Jaccard's indices calculated between sites from different regions (which resulted in equal distributions of intersite distances between the opposing- and same-bank pairs) (34). For várzea and terra firme forest considered separately, a Mantel's nonparametric test (35, 36) with 9,999 permutations was used to test for correlation between the matrix of all possible pairwise Jaccard's indices and a design matrix in which opposing-bank pairs were coded as one and same-bank pairs as zero. The influence of same-region pairs (4 of the 28 pairs) was excluded by setting their Jaccard's to the mean of the remaining pairs. In the second method, we controlled for the effect of distance by conducting Mantel's tests (again with 9,999 permutations) on the residuals from a regression of community similarity on intersite geographic distance.
To evaluate the relationship between the river and the distributions of mammalian species, we compared the actual number of recorded occurrences on both riverbanks against expectation if occurrence was independent of bank. Specifically, a species i present at Li of the eight sites on the left bank and Ri of the eight sites on the right bank was classified as “evenly” distributed across the river if Li − Ri = 0 (Li + Ri even) or | Li − Ri | = 1 (Li + Ri odd). Otherwise, it was classified as “unevenly” distributed. Given Li + Ri sites in total, the probability of an “even” classification under random expectation was obtained from the binomial distribution (with the restriction that a species could be present at a maximum of eight sites on one side of the river). For example, a species caught at 10 sites would be evenly distributed (five sites per river bank) with probability 0.251 (in the same manner, the probability of five heads in ten flips of a fair coin is 0.251, given that at most eight heads or tails can occur). Calling this expected probability pi for species i, the expected number of “even” species under the null hypothesis was as follows:
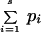 |
1 |
where s is the total number of species (e.g., if the expected probability of an even distribution for each of four species is 0.25, then we would predict that 4 × 0.25 = 1 would have such a distribution). Observed and expected frequencies of “even” species were compared using a χ2 test. Species recorded at only 1 or at 15 or more sites could be classified only as unevenly or evenly distributed; hence, they were excluded from the calculations. More generally, our mammal surveys allow us to qualitatively evaluate the distributions of species as to whether they were confined to specific regions or locales, riverbanks or forest types within the Juruá basin.
As the Juruá River runs in an approximately east–northeast direction (Fig. 1), our sampling regions approximate a rough west–east transect (from headwaters to mouth). If the general pattern of decreasing richness west–east holds over this geographic scale, we would expect highest values for headwaters with a diminution to the lowest in mouth. Further, insofar as the Juruá River represents a natural and long-term impediment to movement of western and eastern faunal elements, we might expect there to be differences between banks in species richness. To estimate species richness at each site for frogs, we used the nonparametric Chao2 estimator of species richness (37). For mammals, we used the Michaelis–Menten equation to estimate the predicted asymptotes of species-sampling effort (38). This equation provided smaller residual sums of squares on average than the negative exponential equation; hence, it was deemed more appropriate for estimating mammalian species richness. To examine west–east patterns of species richness, we visually inspected rank-order of richness values for mammals and frogs separately, for each of terra firme and várzea. For both frogs and mammals, two-tailed paired t tests were used to test for differences in species richness across the river for each of terra firme and várzea. For mammals, we subdivided the tests to consider terrestrial and canopy samples separately. We further tested to see which habitat contained greater species richness (t test pairing same-bank várzea and terra firme sites) for frogs and mammals.
Results and Discussion
Patterns of Species Composition.
Community similarity was not higher between várzea paired sites than between terra firme sites, either for frogs (t = 1.695, P = 0.189) or for small mammals (t = 0.166, P = 0.439). For neither frogs nor small mammals was a clear gradient of decreasing community similarity from the headwaters to mouth evident within either terra firme or várzea habitats (Table 1). For both terra firme and várzea, there was a significant decrease in mammalian community similarity with increasing distance between sites (Mantel r = −0.679, two-tailed P < 0.001; Mantel r = −0.379, P = 0.049, respectively). In neither case was opposing-bank similarity less than same-bank similarity, either for várzea or terra firme forest (P always >0.694). For the test using Jaccard's indices between regions, neither forest type showed significant Mantel correlations [r = 0.084, P = 0.695 (terra firme); r = 0.2499, P = 0.943 (várzea)]. The same result was obtained for the Mantel's test on residuals [r = 0.1246, P = 0.844 (terra firme); r = 0.299, P = 0.992 (várzea)]. Concerning the role of the river in bounding small mammal species distributions, of the 29 species caught at more than 1 and at less than 15 sites, 8 were classified as “unevenly” and 21 as “evenly” distributed across the river. These counts differed significantly from the null expectations of 16 and 13, respectively (P < 0.005, two-tailed χ2 test), but in a direction opposite to that predicted by the riverine barrier hypothesis. More generally for mammals, distribution patterns related more to forest type and sampling region than to riverbank. Eighteen species were restricted to terra firme forest, seven to várzea forest, with nine species found in both forest types. Six species were exclusive to the headwaters, with only one unique to the mouth region. Five species were detected in the two central regions, although three of these have broad Amazonian distributions and may well occur in the other two sampling regions.
Table 1.
Species richness estimates using Chao2 (frogs) and the Michaelis–Menten equation (small mammals) and Jaccard's community similarity indices for paired sites within each sampled region along the Juruá River in Amazonia
| Region | Predicted asymptote left-bank site | Predicted asymptote right-bank site | Jaccard's similarity index | ||
|---|---|---|---|---|---|
| Frogs | |||||
| Terra firme | |||||
| Headwaters | 39.5 | 30.1 | 0.356 | ||
| Upper central | 87.3 | 49.1 | 0.429 | ||
| Lower central | 60.7 | 43.6 | 0.533 | ||
| Mouth | 49.2 | 48.4 | 0.474 | ||
| Mean (SD) | 59.2 (20.7) | 42.8 (8.8) | |||
| Várzea | |||||
| Headwaters | 22.2 | 27.8 | 0.316 | ||
| Upper central | 26.3 | 28.3 | 0.278 | ||
| Lower central | 27.4 | 20.1 | 0.552 | ||
| Mouth | 25.0 | 21.0 | 0.182 | ||
| Mean (SD) | 25.2 (2.2) | 24.3 (4.3) | |||
| Small mammals* | |||||
| Terra firme | |||||
| Headwaters | 14.1 | —† | 14.2 | — | 0.611 |
| Upper central | 14.8 | 5.7 | 14.7 | 9.1 | 0.571 |
| Lower central | — | 3.5 | 13.9 | 2.2 | 0.316 |
| Mouth | 7.9 | 9.7 | 8.9 | — | 0.500 |
| Mean (SD) | 12.3 (3.8) | 6.3 (3.1) | 12.9 (2.7) | 5.6 (4.9) | |
| Várzea | |||||
| Headwaters | 11.2 | — | 18.4 | 5.4 | 0.591 |
| Upper central | 8.1 | 8.8 | 6.7 | 7.8 | 0.643 |
| Lower central | 6.5 | 6.2 | 6.7 | 3.8 | 0.357 |
| Mouth | 7.6 | 4.6 | 5.9 | 6.4 | 0.429 |
| Mean (SD) | 8.4 (2.0) | 6.5 (2.2) | 9.4 (6.0) | 5.9 (1.7) | |
Predicted asymptote, terrestrial canopy.
Dashes indicate that richness estimates were not obtained because the nonlinear regressions failed to converge or because, relative to other regressions, residual sums of squares were inordinately large.
Patterns of Species Richness.
For frogs, there was not a clearly defined pattern of decreased richness from headwaters to mouth (Table 1). For example, the headwaters had the lowest richness values for terra firme samples, whereas differences across regions for várzea samples were slight. However, for mammals the two upstream regions tended to have higher predicted asymptotic richness than the two downstream regions, at least for terrestrial samples (Table 1). Such a relationship is less clear for canopy samples, although missing values make definitive statements difficult.
We detected no differences in frog species richness between left and right river banks (Table 1) for either terra firme (t = 2.047, P = 0.133) or várzea (t = 0.319, P = 0.771). Overall, however, terra firme sites had much higher predicted species richness than várzea sites (mean for terra firme = 59.9, mean for várzea = 24.5, paired same bank comparison, t = 4.448, P = 0.003). Similarly, differences in small mammal richness between the two riverbanks were not significant, either for terra firme (terrestrial t =1.058, P = 0.401; canopy t = −0.431; P = 0.741) or várzea (terrestrial t = −0.507; P = 0.647; canopy t = 0.405, P = 0.725). This same result was obtained when data from the two habitats were combined (terrestrial t = −0.660, P = 0.534; canopy t = −0.090, P = 0.933). Compared with várzea, terra firme forest had greater mammalian terrestrial species richness (mean for terra firme = 12.6, mean for várzea = 8.9, same-bank paired t = 2.064, P = 0.085) but not greater canopy richness (mean for terra firme = 6.1, mean for várzea = 6.2, t = −0.127, P = 0.905). These patterns of small mammal species richness were evident even after observed species richness was linearly adjusted for number of individuals captured and tested using two-way analysis of covariance (ANCOVA). Examination of residuals revealed that the ANCOVA assumptions of normality, homogeneity of variances, and equality of slopes were justified. Adjusted richness did not differ between river banks (terrestrial F1,1 = 0.04, P = 0.848; canopy F1,1 = 0.69, P = 0.423). Adjusted terrestrial richness was significantly greater in terra firme than várzea forest (F1,1 = 5.74, P = 0.04) but not adjusted canopy richness (F1,1 = 1.95, P = 0.19).
These results imply that the Juruá River does not represent a general barrier to the movement of species. Importantly, this observation extends to upland forest species that could not have been moved from one bank to the other by dynamic meander cutoffs. However, before further exploring the implications of these results, we should discuss other factors that may explain or confound our interpretations. First, one might argue that species could have moved eastward from the headwaters spreading down both sides of the Juruá River. If this supposition is correct, the river is indeed a barrier, but this fact is obscured by historical patterns of colonization. However, our genetic studies of evolutionary relationships among populations of mammals and frogs (18–23) reveal patterns that are inconsistent with this possibility, at least for the taxa examined (see below).
A second possible confounding factor centers on our molecular studies indicating that many traditionally regarded Amazonian “species” contain divergences that may predate the Pleistocene (e.g., refs. 18 and 22). The apparent lack of major morphological discontinuities within “species” suggests that substantial time may be required for diagnosable morphological differences to develop between distinct evolutionary lineages. Thus, we may not find phenotypically distinguishable sister taxa on opposite banks simply because insufficient time has elapsed since the formation of major east-flowing drainages in Amazonia in the late Tertiary (39). We can address this possibility as follows. (i) Other mechanisms (e.g., local extinction and expansion from a center of origin) may result in compositional differences between opposite-bank communities irrespective of a role for riverine barriers in speciation. (ii) Even if riverine speciation were the sole driver of community differentiation between banks, the possibility that development of phenotypic differences requires millions of years suggests that existing rivers have not played a significant role in shaping recent patterns of ecological diversity. (iii) The major phylogeographic divisions in taxa with significant genetic population structure correspond to the headwaters and mouth regions rather than to opposite banks (18, 21, 22). Insofar as morphological differences reflect genetic distinctiveness among populations (e.g., ref. 40), in these examined taxa we would expect an east–west axis of differentiation contra the riverine barrier hypothesis.
Finally, in our statistical analyses, we have treated species as independent entities, whereas one species may be influenced by the presence of other species or by local environmental conditions. We cannot discount this possibility completely, and this would diminish our ability to detect a riverine effect on community composition. Certainly, edaphic and vegetation characteristics can be important predictors of patterns of local diversity and differences in community composition (e.g., ref. 41). However, insofar as we were able, we minimized the impact of these factors by sampling small mammals in only one type of forest (large-stature primary forest) and sampling frogs across an array of habitats and excluding species known to inhabit specific habitats not found across sites (e.g., streams).
We believe that, together with other genetic and ecological data (18–22, 42, 43), our present results weaken the hypothesis that riverine barriers have played a geographically pervasive role in species diversification and shaping of patterns of species diversity across lowland Amazonia. That being said, there is some provisional evidence to suggest that the largest river channels may act as impediments to gene flow for some taxa (15, 17, 43). For example, one of the most geographically comprehensive molecular phylogeographic studies to date (43) found deep phylogenetic divisions throughout Amazonia for studied small mammals (usually above 7–10% for assayed mtDNA cytochrome b sequence). These phylogeographic breaks were geographically coincident with rivers in two areas: the lower Rio Negro and the Upper Amazon. Obviously, more definitive statements of the role of major river channels as historical barriers to gene flow await more intensive geographic sampling from an array of taxa.
In summary, our results indicate the following: (i) the most significant predictors of community similarity in species composition for both frogs and mammals were geographic distance and habitat type (flooded vs. upland forest) rather than river bank affiliation; (ii) species richness did not differ across river for either mammals or frogs; (iii) a diminution of species richness from west to east is evident for terrestrial, small-mammal samples but is not clear for frogs over the geographic scale considered; and (iv) small-mammal distributions tend to terminate perpendicularly to the river (parallel to the Andes Mountains). Collectively, these data suggest a significant impact of the Andean orogenic axis and associated thrust-and-fold morphology of the lowlands in generating patterns of diversity along the Juruá River (30, 44).
In the present study, we examined only a single, albeit large, Amazonian river. Whereas only additional work will allow us to test the generality of present results, we believe that they can be legitimately extended as a working hypothesis to all large, meandering rivers (originating in the Andean slopes of Western Amazonia and together, which comprise the largest section of Amazonia). Whether similar results would be obtained for the more channeled and older, clear-water rivers flowing from the Brazilian Shield remains to be tested. We suggest that the chief role of rivers in lowland Amazonia may be in generating significant possibilities for β-diversification through the generation of floodplain and successional habitats (26, 45, 46).
Acknowledgments
We thank O. Pereira and P. Narvaes for field assistance and extend special thanks to J. Robinson and M. Ayres for their support. We also thank three anonymous reviewers for constructive criticisms. We gratefully acknowledge the financial and logistical support of the Wildlife Conservation Society, the National Geographic Society, the Natural Sciences and Engineering Research Council of Canada, the University of Guelph, the University of California at Berkeley, the Instituto Nacional de Pesquisas da Amazonia, and the Ministry of Science and Technology of Brazil (CNPq).
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 13470.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.230136397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.230136397
References
- 1.Wilson E O. The Diversity of Life. New York: Norton; 1992. [Google Scholar]
- 2.Gaston K J, Williams P H. In: Biodiversity: A Biology of Numbers and Difference. Gaston K J, editor. Cambridge, MA: Blackwell Science; 1996. pp. 202–229. [Google Scholar]
- 3.Pianka E R. Am Nat. 1966;100:33–46. [Google Scholar]
- 4.Terborgh J. Am Nat. 1973;107:481–501. [Google Scholar]
- 5.Rhohde K. Oikos. 1992;65:514–527. [Google Scholar]
- 6.Rosenzweig M L. Species Diversity in Space and Time. Cambridge, UK: Cambridge Univ. Press; 1995. [Google Scholar]
- 7.Bush M B. Journal of Biogeography. 1994;21:1–5. [Google Scholar]
- 8.Haffer J. Biodiversity and Conservation. 1997;6:451–476. [Google Scholar]
- 9.Wallace A R. Proc Zool Soc (London) 1852;20:107–110. [Google Scholar]
- 10.Wallace A R. The Geographical Distribution of Animals. Vol. 1. New York: Harper and Brothers; 1876. [Google Scholar]
- 11.Remsen J V, Jr, Parker T A., III Biotropica. 1983;15:223–231. [Google Scholar]
- 12.Ayres J M, Clutton-Brock T H. Am Nat. 1992;140:531–535. doi: 10.1086/285427. [DOI] [PubMed] [Google Scholar]
- 13.Hershkovitz P. Living New World Monkeys (Platyrrhini) with an Introduction to Primates. Vol. 1. Chicago: Univ. Chicago Press; 1977. [Google Scholar]
- 14.Sick H. Atas Simposia Biota Amazônica (Zool) 1967;5:495–520. [Google Scholar]
- 15.Capparella A P. Acta Congr Intern Ornithol. 1988;19:1658–1664. [Google Scholar]
- 16.Hanski I, Gilpin M E, editors. Metapopulation Biology: Ecology, Genetics, and Evolution. New York: Academic; 1997. [Google Scholar]
- 17.Capparella A P. Acta Congr Intern Ornithol. 1990;20:307–316. [Google Scholar]
- 18.Patton J L, da Silva M N F, Malcolm J R. Evolution. 1994;48:1314–1323. doi: 10.1111/j.1558-5646.1994.tb05315.x. [DOI] [PubMed] [Google Scholar]
- 19.Gascon C, Lougheed S C, Bogart J P. Biotropica. 1996;29:376–387. [Google Scholar]
- 20.Gascon C, Lougheed S C, Bogart J P. Biotropica. 1998;30:104–119. [Google Scholar]
- 21.Patton J L, da Silva M N F, Malcolm J R. Mol Ecol. 1996;5:229–238. doi: 10.1111/j.1365-294x.1996.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 22.Lougheed S C, Gascon C, Jones D A, Bogart J P, Boag P T. Proc R Soc London Ser B. 1999;266:1829–1835. doi: 10.1098/rspb.1999.0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peres C A, Patton J L, da Silva M N F. Folia Primatol. 1996;67:113–124. doi: 10.1159/000157213. [DOI] [PubMed] [Google Scholar]
- 24.Duellman W E. Ann Mo Bot Gard. 1988;75:79–104. [Google Scholar]
- 25.Voss R S, Emmons L H. Bull Am Mus Nat Hist. 1996;230:1–115. [Google Scholar]
- 26.Salo J, Kalliola R, Hakkinen I, Makinen Y, Puhalla M, Coley P. Nature (London) 1986;322:254–258. [Google Scholar]
- 27.Haffer J. Publ Nuttall Ornithol Club. 1974;14:1–390. [Google Scholar]
- 28.Brown K S. Am Zool. 1982;22:453–471. [Google Scholar]
- 29.Endler J. In: Biological Diversification in the Tropics. Prance G T, editor. New York: Columbia Univ. Press; 1982. pp. 641–657. [Google Scholar]
- 30.Patton J L, da Silva M N F, Malcolm J R. Bull Am Mus Nat Hist. 1999;244:1–306. [Google Scholar]
- 31.Foster R B. In: Four Neotropical Rainforests. Gentry A H, editor. New Haven, CT: Yale Univ. Press; 1990. pp. 565–572. [Google Scholar]
- 32.Zimmerman B L. In: Measuring and Monitoring Biological Diversity, Standard Methods for Amphibians. Heyer W R, Donnelly M A, McDiarmid R, Hayek L C, Foster M S, editors. Washington DC: Smithsonian Inst. Press; 1994. pp. 146–150. [Google Scholar]
- 33.Ludwig J A, Reynolds J F. Statistical Ecology: A Primer on Methods and Computing. New York: Wiley; 1988. [Google Scholar]
- 34.Fortin M, Gurevitch J. In: Design and Analysis of Ecological Experiments. Scheiner S M, Gurevitch J, editors. New York: Chapman and Hall; 1993. [Google Scholar]
- 35.Mantel N. Cancer Res. 1967;27:209–220. [PubMed] [Google Scholar]
- 36.Douglas M E, Endler J A. J Theor Biol. 1982;99:777–795. [Google Scholar]
- 37.Chao A. Scand J Stat. 1984;11:265–270. [Google Scholar]
- 38.Colwell R K, Coddington J A. Philos Trans R Soc London B. 1994;345:101–118. doi: 10.1098/rstb.1994.0091. [DOI] [PubMed] [Google Scholar]
- 39.Putzer H. In: The Amazon: Limnology and Landscape Ecology of a Mighty Tropical River and Its Basin. Sioli H, editor. Dordrecht, The Netherlands: Junk; 1984. pp. 15–46. [Google Scholar]
- 40.Johns G C, Avise J C. Mol Biol Evol. 1998;15:1481–1490. doi: 10.1093/oxfordjournals.molbev.a025875. [DOI] [PubMed] [Google Scholar]
- 41.Emmons L H. Biotropica. 1984;16:210–222. [Google Scholar]
- 42.Gascon C. Biotropica. 1996;28:136–140. [Google Scholar]
- 43.da Silva M N F, Patton J L. Mol Ecol. 1998;7:475–486. doi: 10.1046/j.1365-294x.1998.00276.x. [DOI] [PubMed] [Google Scholar]
- 44.Räsänen M, Salo J S, Jungner H, Pittman L R. Terra Nova. 1990;2:320–332. [Google Scholar]
- 45.Salo J R. Rainforest Diversification in the Western Amazon Basin: The Role of River Dynamics. 1988. , Report No. 16 (Dept. Biol., Univ. of Turku, Finland). [Google Scholar]
- 46.Gentry A H. Ann Mo Bot Gard. 1988;75:1–34. [Google Scholar]