Abstract
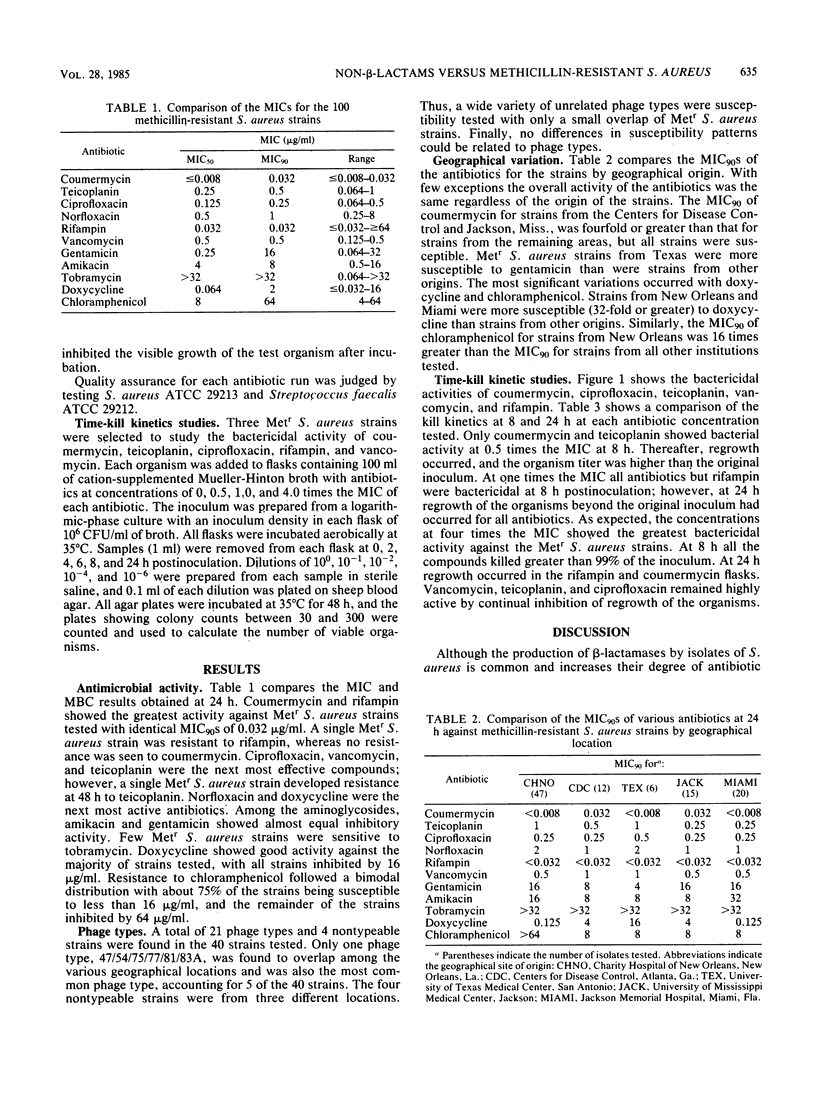
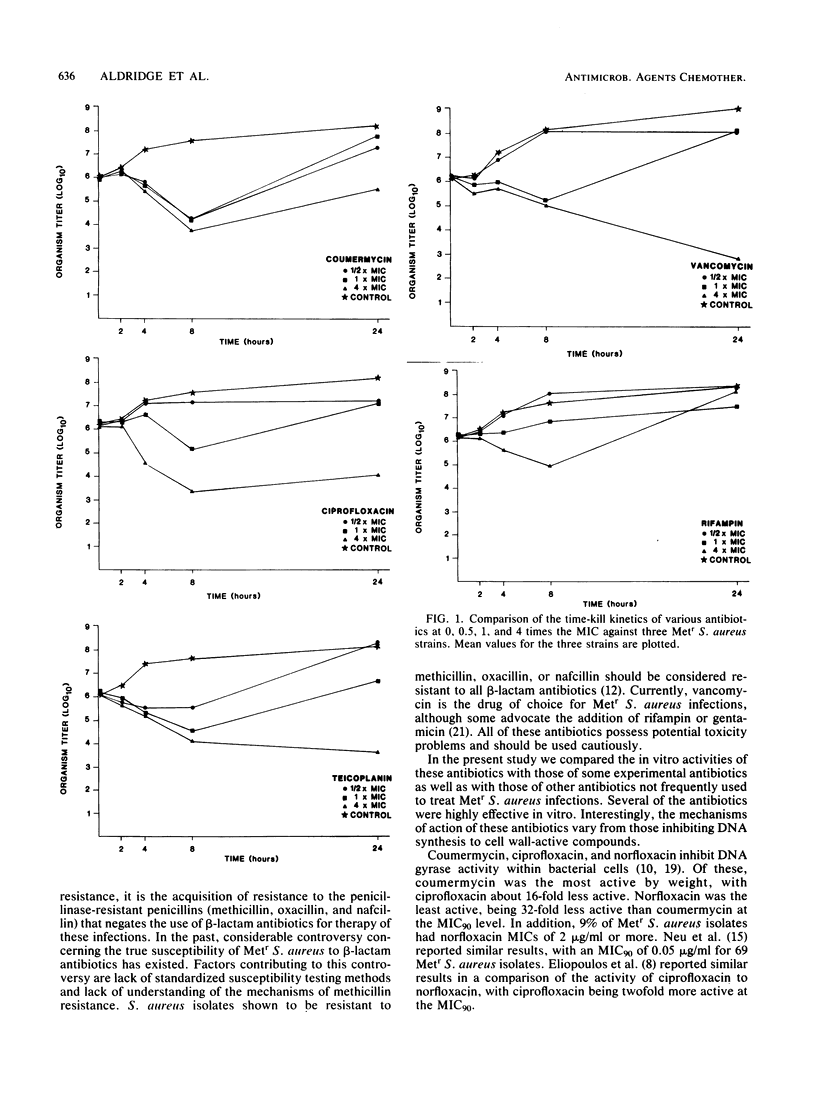
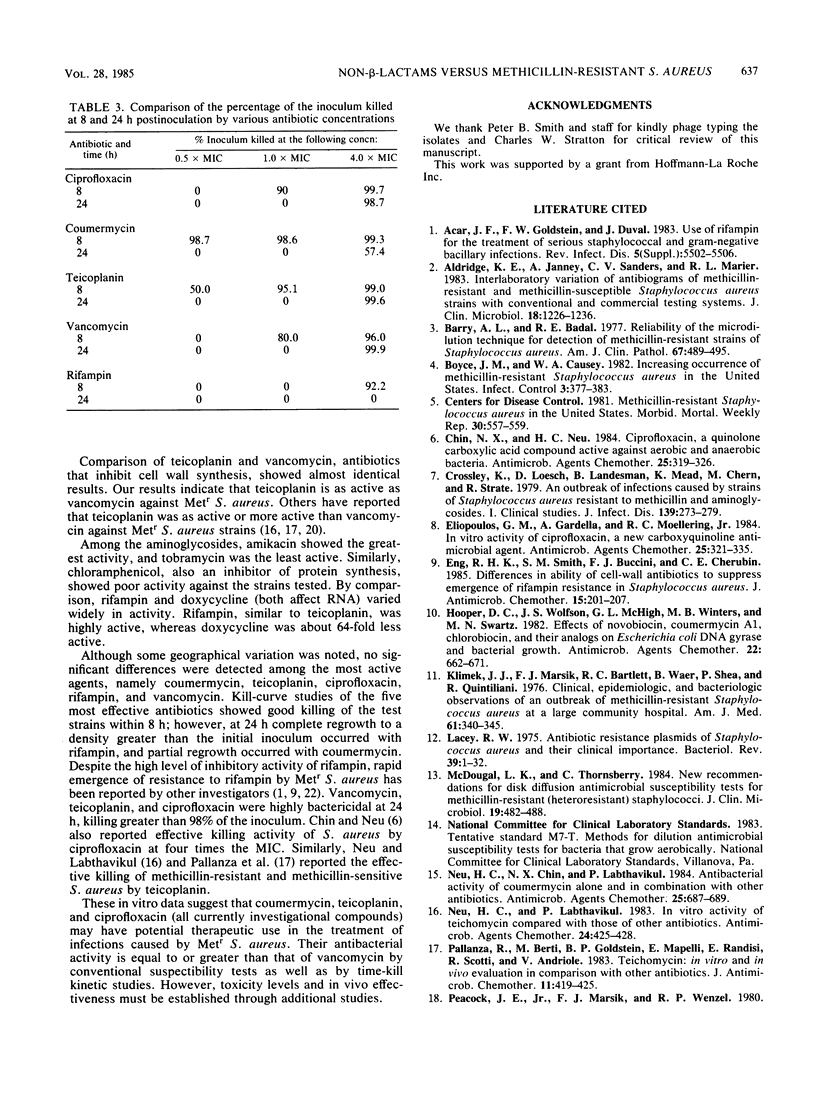
One hundred clinical isolates of methicillin-resistant (Metr) Staphylococcus aureus from five different geographical origins were tested for their susceptibility to 12 non-beta-lactam antibiotics by the broth microdilution technique recommended by the National Committee for Clinical Laboratory Standards. Coumermycin and rifampin showed the highest activity with of MIC90s of 0.032 micrograms/ml for each compound. No resistance was found to coumermycin, whereas a single Metr S. aureus strain was found to be resistant to rifampin. Ciprofloxacin, vancomycin, and teicoplanin were the next most active compounds with MIC90s of 0.25, 0.5, and 0.5 micrograms/ml, respectively. Norfloxacin was less active, with an MIC90 of 1.0 micrograms/ml. Amikacin was the most active aminoglycoside tested, whereas high levels of resistance were found to tobramycin and gentamicin. Doxycycline and chloramphenicol showed variable results. Geographical variations in results were seen with doxycycline, chloramphenicol, and aminoglycosides. Kill-curve studies showed that coumermycin, ciprofloxacin, teicoplanin, vancomycin, and rifampin were highly bactericidal; more than 90% of the inoculum was killed within 8 h.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge K. E., Janney A., Sanders C. V., Marier R. L. Interlaboratory variation of antibiograms of methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains with conventional and commercial testing systems. J Clin Microbiol. 1983 Nov;18(5):1226–1236. doi: 10.1128/jcm.18.5.1226-1236.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Badal R. E. Reliability of the microdilution technic for detection of methicillin-resistant strains of staphylococcus aureus. Am J Clin Pathol. 1977 May;67(5):489–495. doi: 10.1093/ajcp/67.5.489. [DOI] [PubMed] [Google Scholar]
- Boyce J. M., Causey W. A. Increasing occurrence of methicillin-resistant Staphylococcus aureus in the United States. Infect Control. 1982 Sep-Oct;3(5):377–383. doi: 10.1017/s0195941700057337. [DOI] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. Ciprofloxacin, a quinolone carboxylic acid compound active against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1984 Mar;25(3):319–326. doi: 10.1128/aac.25.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley K., Loesch D., Landesman B., Mead K., Chern M., Strate R. An outbreak of infections caused by strains of Staphylococcus aureus resistant to methicillin and aminoglycosides. I. Clinical studies. J Infect Dis. 1979 Mar;139(3):273–279. doi: 10.1093/infdis/139.3.273. [DOI] [PubMed] [Google Scholar]
- Eliopoulos G. M., Gardella A., Moellering R. C., Jr In vitro activity of ciprofloxacin, a new carboxyquinoline antimicrobial agent. Antimicrob Agents Chemother. 1984 Mar;25(3):331–335. doi: 10.1128/aac.25.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng R. H., Smith S. M., Buccini F. J., Cherubin C. E. Differences in ability of cell-wall antibiotics to suppress emergence of rifampicin resistance in Staphylococcus aureus. J Antimicrob Chemother. 1985 Feb;15(2):201–207. doi: 10.1093/jac/15.2.201. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., McHugh G. L., Winters M. B., Swartz M. N. Effects of novobiocin, coumermycin A1, clorobiocin, and their analogs on Escherichia coli DNA gyrase and bacterial growth. Antimicrob Agents Chemother. 1982 Oct;22(4):662–671. doi: 10.1128/aac.22.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimek J. J., Marsik F. J., Bartlett R. C., Weir B., Shea P., Quintiliani R. Clinical, epidemiologic and bacteriologic observations of an outbreak of methicillin-resistant Staphylococcus aureus at a large community hospital. Am J Med. 1976 Sep;61(3):340–345. doi: 10.1016/0002-9343(76)90370-3. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Antibiotic resistance plasmids of Staphylococcus aureus and their clinical importance. Bacteriol Rev. 1975 Mar;39(1):1–32. doi: 10.1128/br.39.1.1-32.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal L. K., Thornsberry C. New recommendations for disk diffusion antimicrobial susceptibility tests for methicillin-resistant (heteroresistant) staphylococci. J Clin Microbiol. 1984 Apr;19(4):482–488. doi: 10.1128/jcm.19.4.482-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Chin N. X., Labthavikul P. Antibacterial activity of coumermycin alone and in combination with other antibiotics. Antimicrob Agents Chemother. 1984 Jun;25(6):687–689. doi: 10.1128/aac.25.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. In vitro activity of teichomycin compared with those of other antibiotics. Antimicrob Agents Chemother. 1983 Sep;24(3):425–428. doi: 10.1128/aac.24.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallanza R., Berti M., Goldstein B. P., Mapelli E., Randisi E., Scotti R., Arioli V. Teichomycin: in-vitro and in-vivo evaluation in comparison with other antibiotics. J Antimicrob Chemother. 1983 May;11(5):419–425. doi: 10.1093/jac/11.5.419. [DOI] [PubMed] [Google Scholar]
- Tuazon C. U., Miller H. Comparative in vitro activities of teichomycin and vancomycin alone and in combination with rifampin and aminoglycosides against staphylococci and enterococci. Antimicrob Agents Chemother. 1984 Apr;25(4):411–412. doi: 10.1128/aac.25.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanakunakorn C. Treatment of infections due to methicillin-resistant Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):376–378. doi: 10.7326/0003-4819-97-3-376. [DOI] [PubMed] [Google Scholar]



