Abstract
A worker ant preserved with microscopic detail has been discovered in Turonian-aged New Jersey amber [ca. 92 mega-annum (Ma)]. The apex of the gaster has an acidopore and, thus, allows definitive assignment of the fossil to the large extant subfamily Formicinae, members of which use a defensive spray of formic acid. This specimen is the only Cretaceous record of the subfamily, and only two other fossil ants are known from the Cretaceous that unequivocally belong to an extant subfamily (Brownimecia and Canapone of the Ponerinae, in New Jersey and Canadian amber, respectively). In lieu of a cladogram of formicine genera, generalized morphology of this fossil suggests a basal position in the subfamily. Formicinae and Ponerinae in the mid Cretaceous indicate divergence of basal lineages of ants near the Albian (ca. 105–110 Ma) when they presumably diverged from the Sphecomyrminae. Sphecomyrmines are the plesiomorphic sister group to all other ants, or they are a paraphyletic stem group ancestral to all other ants—they apparently became extinct in the Late Cretaceous. Ant abundance in major deposits of Cretaceous and Tertiary insects indicates that they did not become common and presumably dominant in terrestrial ecosystems until the Eocene (ca. 45 Ma). It is at this time that modern genera that form very large colonies (at least 10,000 individuals) first appear. During the Cretaceous, eusocial termites, bees, and vespid wasps also first appear—they show a similar pattern of diversification and proliferation in the Tertiary. The Cretaceous ants have further implications for interpreting distributions of modern ants.
Long admired for their industry, the ants (family Formicidae) still capture the popular imagination and scientific attention. All species of Formicidae are eusocial, i.e., their colonies have members that are behaviorally and often highly morphologically specialized for reproduction, foraging, tending larvae, and defense. The only other highly eusocial insects are the termites (order Isoptera), two tribes of corbiculate bees (the Meliponini, or “stingless” bees, and Apini, or “honey” bees) and some social paper wasps in the subfamilies Vespinae and Polistinae (1). Eusociality vastly increases the efficiency of foraging and resource use, as well as defense (2), and it has been commonly invoked as the reason for the ecological dominance of ants, termites, and eusocial bees in terrestrial ecosystems. Ants, however, are far more diverse than these other insects: there are nearly 10,000 described species (perhaps 15,000 total) of ants, but only 3,500 species of other eusocial insects combined. Age of the ants and their niche (they were apparently the first ground-dwelling eusocial predators) have also been implicated as the basis of their remarkable diversity and biomass (2).
A Cretaceous history of ants was first revealed by the discovery of Sphecomyrma freyi in New Jersey amber (3). Sphecomyrmines were subsequently reported in Santonian-aged amber from Taymyr, Siberia (4, 5), Campanian-aged amber from western Canada (6), and Turonian-aged amber from central New Jersey (7). The first Cretaceous record and the oldest known occurrence of the extant subfamily Ponerinae is Brownimecia clavata in New Jersey amber (7); another, younger ponerine, Canapone dentata, occurs in Canadian amber (8). Dlussky (8) also reported the first Cretaceous record of the extant subfamily Dolichoderinae: Eotapinoma macalpinei, also in Canadian amber. Unfortunately, its placement in this subfamily is not definitive, leaving the two ponerines as the only Cretaceous ants definitively attributed to an extant subfamily—until now. Here we describe a recently excavated fossil worker ant in New Jersey amber that is an indisputable member of the extant subfamily Formicinae (Fig. 1).
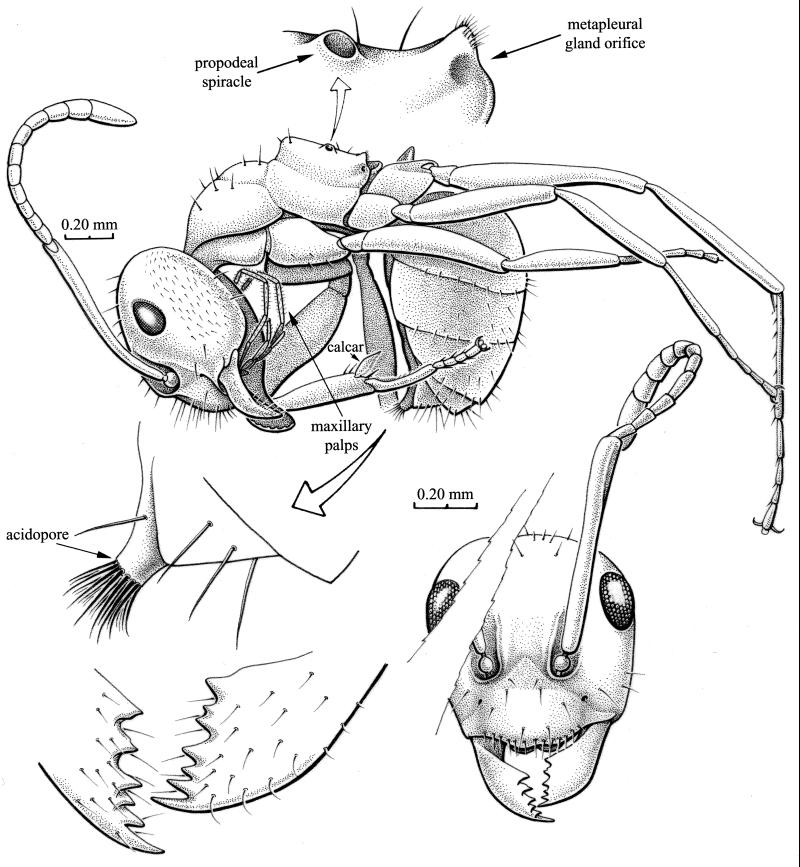
Figure 1.
Kyromyrma neffi holotype (AMNH NJ-1029) in New Jersey Cretaceous amber. Left lateral habitus of specimen in original position, showing propodeal spiracle and metapleural gland area; frontal view of head and dentition of mandibles; and detail of gaster apex, showing acidopore with fringe of hairs.
The Formicinae is one of 16 subfamilies of the family Formicidae and contains some 48 recent genera and approximately 3,000 described species (9). This subfamily contains some very large, ecologically important genera, such as the carpenter ants (Camponotus) and wood ants (Formica). Other genera are renowned for their distinctive biology, such as weaver ants (Oecophylla), slavemaking ants (Polyergus, Rossomyrmex), honeypot ants (Myrmecocystus), and genera living in intimate association with plants (e.g., Cladomyrma and Dendromyrmex; ref. 2). The Formicinae is undoubtedly a monophyletic group, based on the highly modified proventriculus (10) and, more overtly, replacement of the sting with an ability to spray formic acid, a substance unique to this subfamily. All formicines have a poison gland that opens into a fringed, tubular pore at the apex of the gaster, the acidopore.
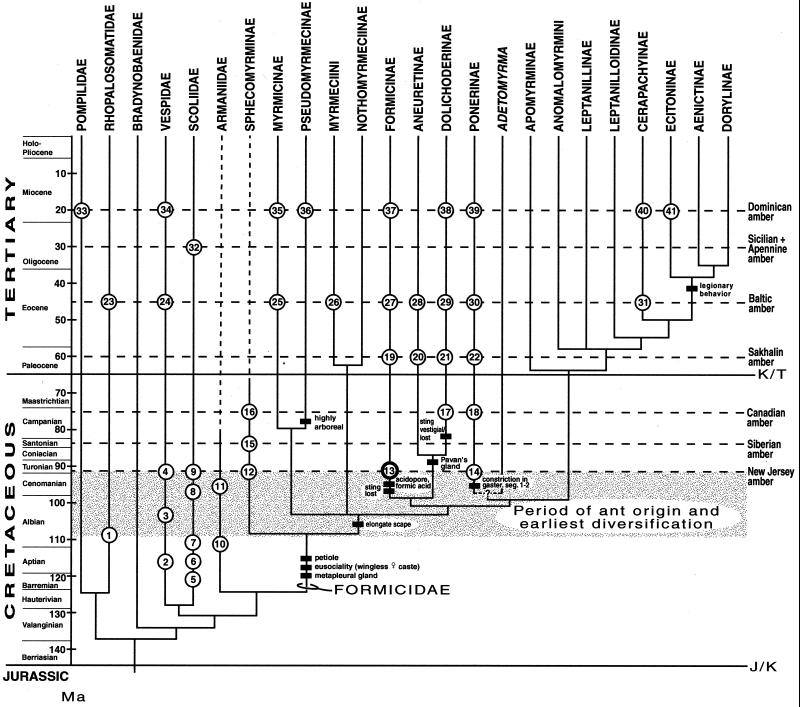
There are only two recent classifications of the Formicinae. Hölldobler and Wilson (2) classified formicine genera into 13 tribes, but without providing diagnostic characteristics. Formicine genera have also been classified into four groups, based on the positions of the coxae, petiole, and helcium (11). The phylogenetic position of the subfamily itself among all ants is somewhat uncertain. The formicines have been placed as the sister group to the rest of the ants based primarily on the putatively primitive absence of a pygidial gland (2), except in Polyergus. This gland, which is found in all other ant subfamilies examined thus far, is the source of alarm and recruitment pheromones and some defensive substances. Absence of the pygidial gland in formicines is arguably a loss related to the dramatic development of the poison gland in these ants. A recent cladistic analysis (7) confirmed the most commonly hypothesized close relationship of the Formicinae to the Aneuretinae and the large subfamily Dolichoderinae, and this lineage as sister group to the Ponerinae and various other ant tribes, including the legionary ants (Ecitoninae and Dorylinae; Fig. 2). Basal to this whole assemblage are the myrmicines (a large assemblage including “gardening” or “leaf-cutting” ants), pseudomyrmecines (“acacia” ants), an Australian lineage comprised of the Myrmeciini (“bulldog” ants), as well as the primitive, monotypic genus Nothomyrmecia and the most primitive lineage of all ants, the Cretaceous Sphecomyrminae. If the Formicinae occupy this phylogenetic position, it suggests a rather incomplete fossil record of ants in the Cretaceous and indicates that the origins of basal ant lineages are even more condensed within the mid Cretaceous than previously hypothesized (7).
Figure 2.
Cladogram of ant subfamilies, some tribes, the Cretaceous genera, and related families of aculeate wasps [from ref. 7 (ants); and ref. 15 for aculeate families]. The cladogram is overlaid on a geological time scale to show ages of fossils (numbered, in circles) and hypothesized dates of divergence. The thick circle (no. 13) is the formicine fossil in the present report. Unlike the prior (7) cladogram, the Sphecomyrminae here are shown as sister group to all other ants, based on the plesiomorphic scape. With the exception of compression fossils of Armaniidae and other families, the only ant fossils included are in amber, because preservation in amber allows accurate phylogenetic placement. Also not shown are several genera of ants in Burmese amber, of undetermined age within the Upper Cretaceous (36). Divergence times for some lineages are largely conjectural because of a paucity of fossils: clade of Apomyrminae and subordinate taxa, and the Myrmicinae + Pseudomyrmecinae clade. The Tertiary divergence time of the Australian clade of Myrmeciini + Nothomyrmeciinae is based on the post-Gondwanan isolation of Australia (35). Significant features of some clades are indicated. Circled numbers refer to the following fossils, deposits, and references. 1, Mesorhopalosoma cearae (Santana Formation, Brazil; ref. 37). 2, 3, Curiosivespa and Priorvespa spp. (Bon-Tsagan Nuur, Mongolia; Zaza Formation of Baissa). 4, Curiosivespa spp. (Kzyl-Zhar, Kazakhstan) and Symmorphus senex (Raritan Formation, New Jersey; nos. 2–4: ref. 30; no. 4, ref. 38). 5, Archaeoscolia hispanica (Lleida, Cuenca, Spain; ref. 39). 6, Archaeoscolia senilis (Bon-Tsagan Nuur, Mongolia; ref. 40). 7, Cretaproscolia josai (Santana Formation, Brazil; ref. 39). 8, Cretoscolia promissiva (Ola' Formation, Magadan). 9, Cretoscolia patiens (Kzyl-Zhar, Kazakhstan; nos. 8 and 9, ref. 40). 10, Armania and Khetania spp. (Emanra Formation, Khabarovsk; ref. 41). 11, Armania (5). 12, Sphecomyrma freyi and sp. (Raritan Formation, New Jersey; refs. 3 and 7). 13, Formicine fossil, this report (12). 14, Brownimecia clavata (Raritan Formation, New Jersey; ref. 7). 15, Cretomyrma spp., Dlusskyidris zherichini (Taimyr, Siberia; Baikura-Neru; ref. 4). 16, Sphecomyrma canadensis (Foremost Formation, Alberta; ref. 6). 17, Eotapinoma macalpini (Foremost Formation, Alberta; ref. 8; placement uncertain). 18, Canapone dentata (Foremost Formation, Alberta; ref. 8). 19, Chimaeromyrma [Paleocene (?), Sakhalin Island]. 20, Aneuretellus [Paleocene (?), Sakhalin Island]. 21, Eotapinoma, Zherichinius [Paleocene (?), Sakhalin Island]. 22, Protopone (nos. 19–22; ref. 42). 23, Propalosoma gutierrezae (Klondike Mountain Formation, Washington; ref. 26). 24, Paleovespa baltica (Baltic amber). 25–31, Various genera of ants in subfamilies indicated (refs. 2, 28, and 43); Arkansas amber (Claiborne Formation; ref. 6). 32, Floriscolia relicta (Florissant, Colorado; ref. 40). 33, Pompilidae, genera indet., Dominican amber (D.G., unpublished observation). 34, Agelaia electra (Dominican amber; ref. 44). 35–41, Various genera of ants in subfamilies indicated (Dominican amber; ref. 45). Not all Tertiary fossils of Pompilidae and Vespidae are indicated; no Cretaceous fossils of Pompilidae or any fossils of Bradynobaenidae are known. Ma, mega-annum; K/T, Cretaceous/Tertiary; J/K, Jurassic/Cretaceous.
Materials and Methods
Amber was collected at a very localized outcrop of lignite and clay of the Raritan Formation (Turonian) in Sayreville, Middlesex County, New Jersey. The amber deposit is the most diverse known thus far from the Cretaceous and was formed in coastal, deltaic swamps by taxodiaceous or pinaceous trees (12).
The amber piece containing the formicine, American Museum of Natural History number AMNH NJ-1029, is clear yellow and also contains wood fragments, suggesting the ant was captured on a tree's trunk. Preparation followed methods described previously (13). The dorsal and right surfaces of the ant are obscured by a semiopaque fracture plane running through the center of the amber. The ventral surface is obscured by the curled position of the specimen, but microscopic details of exposed surfaces are observable under ×150–400 magnification.
The morphological cladogram used here was presented elsewhere (7) and is based largely on the data of Baroni Urbani et al. (14), with the exception of 11 characters that were recoded and several extant and extinct genera that were added to the matrix. Cladistic relationships of closely related aculeate families are based on the observations of Brothers (15)—the ant cladogram is rooted by the closely related families Vespidae and Bradynobaenidae. A matrix of 62 characters and 27 taxa was analyzed with the phylogenetic program HENNIG86; the preferred cladogram (Fig. 2) had a length of 125 steps, consistency index = 0.48, and retention index = 0.65 (for details of the analysis, see ref. 7). The most significant difference between this cladogram and the original (14) is that ants do not fall into two major lineages. Instead, the myrmicine and myrmeciine clades are basal. Also, the Formicinae + Aneuretinae + Dolichoderinae clade (a monophyletic group in both studies) appears as a sister group to the large clade where Ponerinae + Adetomyrma are the basal members (Fig. 2). Of significance is the possibility that the Ponerinae may not be monophyletic, but resolving relationships in this large complex is beyond the scope of this study. If scape length is coded into the matrix (not originally done; ref. 7), the results indicate that Sphecomyrminae is the sister group to all other ants, a hypothesis of relationship we feel will remain stable.
Systematics.
Morphological terminology is used as presented elsewhere (refs. 2 and 9; the former reference also defines standard measurements).
Kyromyrma, New Genus.
Diagnosis.
This genus is unique among formicine genera by the following combination of characters: mandible with six teeth, a row of three decreasing in size proximate to the first intercalary, followed by a distinctly larger median tooth; six maxillary and four labial palps, the former reaching to the occipital concavity; antennae with 12 segments, inserted at anterior part of frontal carina; eyes flat with many small ommatidia, situated laterally behind anterior half of head; ocelli absent; mesonotum separated from propodeum, in lateral view forming a deep furrow; propodeum in lateral view angulate; propodeal spiracle round, at mid height on declivous face; metapleural gland round, without flanges dorsally or ventrally, orifice present; petiolar scale prominent.
Type species.
Kyromyrma neffi, n.sp.
Etymology.
From Greek, kyreo: to “light upon,” i.e., the ant illuminating the early evolution of the Formicidae.
Kyromyrma neffi, New Species.
Description.
Head: length, 0.68 mm; width, 0.66 mm; cephalic index, 97. Integument essentially smooth, without microsculpture; sparse fine setae on vertex; short adpressed setae on genae. Eyes: present, well developed; slightly drop-shaped; of moderate proportions (length, 0.16 mm; width, 0.10 mm); situated far above horizontal mid line of head. Eye index: 24.2. Ocelli: absent or so vestigial as to be undetectable at ×100. Frontal carinae: present, but short and shallow, such that antennal sockets are completely exposed. Postclypeal triangle: present, but very slight. Clypeus: deep, anterior margin with 13 stiff, evenly spaced setae; approximately six other setae on broad surface of clypeus. Antenna: scape long (0.75 mm); funiculus with 11 segments, each gradually increased in width apicad. Funicular segment lengths (basal > apical): 0.08, 0.07, 0.075, 0.08, 0.09, 0.085, 0.09, 0.095, 0.09, 0.095, 0.19 (total length of funiculus 1.04 mm). Scape/funiculus length: 0.72; scape index: 113. Mandibles of generalized structure: subtriangular in shape, dentate, with six teeth on masticatory margin; apical tooth longest; teeth two, four, and six (counting from apex) of approximately equal sizes; tooth three smallest; tooth five slightly shorter and broader than four and six, with two cusps on left mandible. Outer surface of mandible with approximately 20 fine, evenly spaced setae. Masticatory margin with row of six fine, stiff setae. Mandible length: 0.38 mm. Mandible index: 56. Palp formula 6-4, maxillary palps particularly long.
Alitrunk: length, 0.83 mm. Sparse (≈10) fine, erect setae on promesonotum and anterior part of propodeum; otherwise, alitrunk glabrous and without significant microsculpture. Propodeum elongate, anterior edge high and steep. Propodeal spiracle: round, not ovoid or slit-like. Metapleural gland orifice (MGO): well developed, with slight channel along posterolateral margin of propodeum; row of fine setae just posterodorsal to MGO. Petiole and peduncle: mostly hidden from view behind metacoxa, but top of petiole visible enough to discern it is quite narrow in lateral view (not dome shaped). Hind legs: relatively long; trochantellus absent. Lengths of leg segments (in order of trochanter + femur–tibia–basitarsomere–tarsi 2–5 in mm): foreleg, 0.67, 0.48, 0.30, 0.22; midleg, 0.68, 0.55, 0.36, 0.35; hindleg, 0.90, 0.72, 0.53, 0.46.
Gaster: length, ≈0.95 mm. Gastral segment 1 only slight longer than segments 2 and 3. Apex of gaster produced into a small funnel-like opening ringed with approximately 16 stiff setae (the acidopore).
Type.
AMNH NJ-1029, collected by Steven Swolensky (AMNH) at the “White Oaks” (Old Crossman's) clay pits in Sayreville, Middlesex County, NJ (South Amboy Fire Clay: Raritan Formation: Turonian). In Division of Invertebrates, AMNH.
Etymology.
The species name is a patronym, for Mr. Todd Neff, a relative of the collector/donator of the specimen.
Comments.
Structures important for grouping formicine genera (11) require a full ventral view and are largely or entirely obscured in this specimen. These include: the separation of mesal margins of the mid and hind coxae, the shape of the ventral face of the petiole (v- or u-shaped), and the shape and position of the helcium. Tilting of the specimen reveals that the mid coxae are not separated in the ventral view—the hind coxae are completely hidden. The sagitta, helcium, and ventral face of the petiole cannot be observed. The last structure is related to an ability of some formicines to flex the gaster under the alitrunk, thereby pointing the acidopore forward at an enemy. The position in which the fossil is preserved suggests this ability. Kyromyrma can be excluded from the tribe Formicini (sensu; ref. 16) by lack of a slit-shaped propodeal spiracle, and the fact that the spiracle is also not particularly close to the metanotum. It is difficult to discern whether one or two longitudinal rows of setae occur on the ventral surface of the hind tibia—extant Formicini have two such rows. The presence of a metapleural gland (orifice) excludes the fossil from groups of genera where the gland has been lost: Oecophylla (Oecophyllini) and some Camponotini (e.g., most Camponotus, and other genera). Wood fragments in the amber piece suggest the ant was arboreal, like these genera and some other formicines. The fossil bears an overall resemblance to Prolasius, mostly by virtue of the generalized morphology. In lieu of a phylogenetic system for the Formicinae, the plesiomorphic structure of the fossil is highly suggestive of a basal position in the subfamily.
Discussion
Chronology.
Superimposing ant fossils on a cladogram of major ant lineages (Fig. 2) provides a chronology for ant relationships, which allows estimates of dates of divergence. The earliest occurrences of families closely related to ants are from the Barremian to the Turonian, for Rhopalosomatidae, Vespidae, Scoliidae, and the putative sister group to the ants, the extinct Armaniidae. [One hypothesis is that the Armaniidae are Sphecomyrminae on the basis of queen-like proportions of the gaster (17). However, no wingless females (e.g., workers) of armaniids have yet been found, and Armaniidae is plesiomorphic to Sphecomyrminae and other ants based on the wing venation and the barely developed petiole.] The fossil record of various aculeate families extends to the Lower Cretaceous, but only one family is known from the uppermost Jurassic, the extinct Bethylonymidae, which is probably the sister group to all aculeates (18). Thus, the Barremian is probably at or very near the oldest age for the clade of aculeate families that includes the ants. This chronology establishes a lower boundary for the absolute ages of ants.
The most primitive lineage of ants is the Sphecomyrminae, putatively monophyletic on the basis of a long second funicular segment (this segment is short in S. canadensis); in all other respects the Sphecomyrminae are primitive compared with the remaining ants. The group possibly represents a paraphyletic stem-group lineage ancestral to all other ants. Definitive placement of sphecomyrmines in the Formicidae is based on the well developed petiole and metapleural gland. Wingless females in Sphecomyrma, Cretomyrma, and the enigmatic Haidomyrmex (Burmese amber) are known, which are most parsimoniously interpreted as workers. Closely related families of aculeates that have wingless females (all species in the Bradynobaenidae, a few species in Pompilidae and Rhopalosomatidae) are easily distinguished from ants. Eusociality is also suggested by two pieces of New Jersey amber, each containing two wingless females of Sphecomyrma freyi. Cretaceous Sphecomyrmines are exceedingly rare (see below); thus the probability is extremely remote that two individuals were preserved in a piece of amber on the basis of chance alone. Thus far, sphecomyrmines are known from the Turonian to the Campanian and probably became extinct in the latest Cretaceous or earliest Tertiary.
Divergence of basal lineages of ants has been hypothesized in the Campanian to Turonian and origins of modern subfamilies, including the Formicinae, in the Paleocene (19). We hypothesize the basal divergence of the Sphecomyrminae from all other ants 10–20 million years earlier, probably in the Albian, and the divergence of the Formicidae and Armaniidae no earlier than Barremian, ca. 125 Ma. No Cretaceous fossils of the Myrmicinae + Pseudomyrmecinae or the Myrmeciini + Nothomyrmecinae clades have yet been found, although they are expected on the basis of their putatively basal phylogenetic positions. The only other Cretaceous ants besides the formicine that belong to modern subfamilies are Brownimecia and Canapone (Ponerinae), as well as the putative dolichoderine Eotapinoma macalpini. The latter should be carefully reexamined for definitive traits of the Dolichoderinae, because Dlussky (8) could not provide any such traits for E. macalpini. In fact, he stated (8) that “… in the Paleocene… and in the Middle Eocene… the boundaries between these subfamilies [Dolichoderinae and Formicinae] are uncertain.” As we have shown, their definitive separation took place at least 30 million years earlier than supposed.
Ecological Success and Species Diversity.
Ants did not achieve ecological dominance until the Eocene and later. Table 1 shows the proportions of ants preserved in various amber and compression fossil insect Lagerstätte from the Cretaceous and Tertiary. Each deposit is biased by the local paleoenvironment and mode of preservation, but overall the trend is striking. The proportions of ants to all other insects ranges from 0.002 to 0.05% in Cretaceous ambers and 1.2% in Sakhalin amber of probable Paleocene age (20); this proportion gradually increases in the Tertiary to approximately 40% (21–24). Compression remains indicate a similar Tertiary proliferation (refs. 25–27; Table 1). Ecological dominance of ants in the Eocene and later is attributed to the radiations of Myrmicinae, Dolichoderinae, and Formicinae during this time (19, 24), many of which form very large colonies (2), such as Atta, Azteca, and Formica. Many modern genera also appear for the first time in Eocene amber from the Baltic region and Oise, France (23, 28). Kyromyrma indicates that formicines either proliferated much earlier than the Eocene and remained largely unknown in the Paleocene and Cretaceous fossil record or they inexplicably remained minor components of the insect fauna for the first 40–50 million years of their existence.
Table 1.
Proportions of Formicidae in major deposits of Cretaceous and Tertiary insects
| Area | Era | Formation | Fossil type | Percentage of ants | Reference |
|---|---|---|---|---|---|
| Dominican Republic | Miocene | La Toca | A | 24% all insects | Grimaldi* |
| Dominican Republic | Miocene | La Toca | A | 40% Hymenoptera | 24 |
| Florissant, CO | Oligocene | Florissant | C | 20% all insects | 27 |
| Sicily | Oligocene | Ranzano | A | 40% all insects | 22 |
| Baltic | Mid Eocene | Blau Erde | A | 30–80% Hymenoptera | 24 |
| Wyoming | Mid Eocene | Green River | C | 17% Hymenoptera | 26 |
| Washington | Mid Eocene | Klondike Mountain | C | 2% all insects | 25 |
| Arkansas | Mid Eocene | Claiborne | A | 1% all insects | 6, 46 |
| Oise, France | Late Eocene | Soissonais | A | 7% all insects | 23, A. Nel* |
| Sakhalin Island | Paleocene (?) | NA | A | 1.2% all insects | 42 |
| Alberta | Campanian | Foremost | A | 0.002% all insects | 47 |
| Taimyr | Santonian | Kheta | A | 0.001% all insects | Zherikhin* |
| New Jersey | Turonian | Raritan | A | 0.05% all insects | 12 |
Fossil types: A, Amber; C, Compression. NA, not available.
Unpublished figures and personal communications to D.G., May 2000.
Termites and vespid wasps originated in the Lower Cretaceous (Aptian to Hauterivian) (29–31)—only one certain and several possible records of social wasp nests occur in the Upper Cretaceous (32). Termites were apparently eusocial for their entire fossil record beginning in the Lower Cretaceous. The Cretaceous fossil record is largely based on alate (reproductive) specimens belonging to the phylogenetically basal families Mastotermitidae, Hodotermitidae, and Termopsidae (31). The first records of diverse, derived faunas of termites are in the Eocene (31). The age of the only Cretaceous bee (33) is equivocal. However, it is probably uppermost Cretaceous (34) and belongs to the recently derived tribe Meliponini (Apidae sensu lato). The first significant record of corbiculate, social bees is in Baltic amber (Eocene), when an impressive diversity of extinct clades occurred (M. Engel, personal communication). These patterns of diversity and abundance parallel those seen in ants.
Neither age nor eusociality alone, therefore, can account for the unique success of ants—these features plus their predominant and ancestral habits as terrestrial predators must be a significant factor. Another predominant feeding habit of ants, particularly for myrmicines, dolichoderines, and formicines, is the tending and defense of homopterans for their sugary exudates (“honeydew”). These insects particularly include aphids (Aphidoidea), scale insects (Coccoidea), plant lice (Psylloidea), and treehoppers (Membracidae; refs. 1 and 2). Gathering honeydew is a habit clearly derived from predation and, at least for formicines, is probably related to the structure of valves in the proventriculus that allow for storage of large volumes of liquid in the crop. The earliest records of ant–homopteran symbioses are based on two examples involving extant ant genera in Tertiary ambers: Iridomyrmex and aphids in Baltic amber (43) and several pieces of Miocene Dominican amber containing a queen Acropyga ant carrying a pseudococcid mealybug (unpublished data). Iridomyrmex today tend aphids, and Acropyga has an obligatory relationship with root-feeding pseudococcids. Aphids and scale insects are among the most abundant inclusions in Canadian and New Jersey ambers [28% (47) and 10% (12), respectively, among all insects], which are the first truly diverse and abundant fossil records of the Sternorrhyncha. Honeydew was available in abundance to Cretaceous ants but may not have been exploited until the Tertiary.
Biogeography.
Divergence of basal lineages of ants in the mid Cretaceous provides evidence that the distributions of major groups of ants could have been affected by Cretaceous fragmentation of Pangaea (35). Indeed, the Myrmicinae, Formicinae, Dolichoderinae, and Ponerinae are cosmopolitan lineages, whose earliest ancestors were perhaps widespread throughout Pangaea. Some geographically restricted groups, like Myrmeciini in Australia and Aneuretinae in Sri Lanka, have fossils in Baltic amber (28) and thus were previously widespread. With exception of the cosmopolitan Cerapachyinae, continental endemism is largely found in the tribes and subfamilies that represent the sister group to the Anomalomyrmini (Fig. 2). These taxa may have diversified when a widespread ancestor of this group became isolated on major landmasses.
Acknowledgments
The senior author is indebted to Mr. Steven Swolensky, who has generously volunteered many hours in the AMNH amber lab and has donated valuable NJ amber fossils to the AMNH, including the formicine. Drs. Michael Engel, André Nel, and Vladimir Zherikhin contributed unpublished information; Mr. Paul Nascimbene prepared the fossil for observation; AMNH trustees Mr. Robert Goelet and Mr. Henry Walter generously funded research on this and other amber studies; Drs. Amy Berkov, Michael Engel, and Christine Johnson and two anonymous reviewers provided informative commentary.
Abbreviations
- Ma
mega-annum (millions of years ago)
- AMNH
American Museum of Natural History
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240452097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240452097
References
- 1.Wilson E O. The Insect Societies. Cambridge, MA: Harvard Univ. Press; 1971. [Google Scholar]
- 2.Hölldobler B, Wilson E O. The Ants. Cambridge, MA: Belknap; 1990. [Google Scholar]
- 3.Wilson E O, Carpenter F M, Brown W L. Science. 1967;157:1038–1040. doi: 10.1126/science.157.3792.1038. [DOI] [PubMed] [Google Scholar]
- 4.Dlussky G M. In: The Higher Hymenoptera of the Mesozoic. Rasnitsyn A P, editor. Moscow: Acad. Sci. USSR; 1975. pp. 114–122. (in Russian). [Google Scholar]
- 5.Dlussky G M. Paleontol Zhurnal. 1983;3:65–78. (in Russian). [Google Scholar]
- 6.Wilson E O. Psyche. 1985;92:205–216. [Google Scholar]
- 7.Grimaldi D, Agosti D, Carpenter J M. Am Mus Novit. 1997;3208:1–43. [Google Scholar]
- 8.Dlussky G M. Paleontol J. 1999;33:409–412. [Google Scholar]
- 9.Bolton B. Identification Guide to the Ant Genera of the World. Cambridge, MA: Harvard Univ. Press; 1992. [Google Scholar]
- 10.Eisner T. Bull Mus Comp Zool. 1957;116:439–490. [Google Scholar]
- 11.Agosti D. Syst Entomol. 1991;16:293–310. [Google Scholar]
- 12.Grimaldi D, Shedrinsky A, Wampler T. In: Studies on Fossils in Amber, with Particular Reference to the Cretaceous of New Jersey. Grimaldi D, editor. Leiden, The Netherlands: Backhuys; 2000. pp. 1–75. [Google Scholar]
- 13.Nascimbene P, Silverstein H. In: Studies on Fossils in Amber, with Particular Reference to the Cretaceous of New Jersey. Grimaldi D, editor. Leiden, The Netherlands: Backhuys; 2000. pp. 93–102. [Google Scholar]
- 14.Baroni Urbani C, Bolton B, Ward P S. Syst Entomol. 1992;17:301–329. [Google Scholar]
- 15.Brothers D J. Zool Scr. 1999;28:233–249. [Google Scholar]
- 16.Agosti D. Syst Entomol. 1994;19:93–117. [Google Scholar]
- 17.Wilson E O. Paleobiology. 1987;13:44–53. [Google Scholar]
- 18.Ronquist F, Rasnitsyn A, Roy A, Eriksson K, Lindgren M. Zool Scr. 1999;28:13–50. [Google Scholar]
- 19.Dlussky G M, Fedoseeva E B. In: Cretaceous Biocenotic Crisis and Insect Evolution. Zherikhin V V, editor. Moscow: Paleontol. Inst.; 1988. pp. 70–144. (in Russian). [Google Scholar]
- 20.Zherikhin V V, Eskov K Y. Estudios Museo Ciências Naturales de Álva (Spain) 1999;14:119–131. [Google Scholar]
- 21.Larsson S G. Baltic Amber—A Paleobiological Study. Klampenborg, Denmark: Scand. Sci.; 1978. [Google Scholar]
- 22.Skalski A W, Veggiani A. Pr Muz Ziemi. 1990;41:37–49. [Google Scholar]
- 23.Nel A, Plöeg G, DeJax J, Dutheil D, Franceschi D, Gheerbrant E, Godinot M, Hervet S, Menier J-J, Augé M, et al. C R Acad Sci. 1999;329:65–72. [Google Scholar]
- 24.Rasnitysn A P, Kulicka R. Pr Muz Ziemi. 1990;41:53–64. [Google Scholar]
- 25.Lewis S E. Washington Geology. 1992;20:15–19. [Google Scholar]
- 26.Dlussky G M, Rasnitsyn A P. Paleontol J. 1999;33:546–549. [Google Scholar]
- 27.Carpenter F M. Bull Mus Comp Zool. 1930;70:1–66. (+ 11 plates). [Google Scholar]
- 28.Dlussky G M. Paleontol J. 1997;31:616–627. [Google Scholar]
- 29.Carpenter J M, Rasnitsyn A P. Psyche. 1990;97:1–20. [Google Scholar]
- 30.Martínez-Delclòs X, Martinell J. J Paleontol. 1995;69:594–599. [Google Scholar]
- 31.Thorne B L, Grimaldi D, Krishna K. In: Termites: Evolution, Sociality, Symbiosis, and Ecology. Abe T, Bignell D E, Higashi M, editors. Dordrecht, The Netherlands: Kluwer; 2000. pp. 45–60. [Google Scholar]
- 32.Wenzel J W. Psyche. 1990;97:21–29. [Google Scholar]
- 33.Engel M S. Am Mus Novit. 2000;3296:1–11. [Google Scholar]
- 34.Grimaldi D. Ann Mo Bot Gard. 1999;86:373–406. [Google Scholar]
- 35.Smith A G, Smith D G, Funnell B M. Atlas of Mesozoic and Cenozoic Coastlines. Cambridge, U.K.: Cambridge Univ. Press; 1994. [Google Scholar]
- 36.Zherikhin V V, Ross A J. In: The History, Geology, Age and Fauna (Mainly Insects) of Burmese Amber, Myanmar, Bulletin of the Natural History Museum. Ross A J, editor. Vol. 56. London: Nat. Hist. Mus.; 2000. [Google Scholar]
- 37.Darling D C, Sharkey M. In: Insects from the Santana Formation, Lower Cretaceous, of Brazil, Bulletin of the American Museum of Natural History. Grimaldi D A, editor. Vol. 195. New York: Am. Mus. Nat. Hist.; 1990. pp. 123–153. [Google Scholar]
- 38.Carpenter J M. In: Studies on Fossils in Amber, with Particular Reference to the Cretaceous of New Jersey. Grimaldi D, editor. Leiden, The Netherlands: Backhuys; 2000. pp. 333–338. [Google Scholar]
- 39.Rasnitsyn A P, Martínez-Delclòs X. Cretaceous Res. 1999;20:767–772. [Google Scholar]
- 40.Rasnitsyn A P. J Hymenoptera Res. 1993;2:85–96. [Google Scholar]
- 41.Dlussky G M. Paleontol J. 1999;33:274–277. [Google Scholar]
- 42.Dlussky G M. Paleontol J. 1988;22:50–61. [Google Scholar]
- 43.Wheeler W M. Schr Physik-Ökonom Ges Königsb. 1914;55:142. pp. [Google Scholar]
- 44.Carpenter J M, Grimaldi D. Am Mus Novit. 1997;3203:1–7. [Google Scholar]
- 45.Wilson E O. Science. 1985;229:265–267. doi: 10.1126/science.229.4710.265. [DOI] [PubMed] [Google Scholar]
- 46.Saunders W B, Mapes R H, Carpenter F M, Elsik W C. Geol Soc Am Bull. 1974;85:979–984. [Google Scholar]
- 47.Pike E M. Ph.D. thesis. Calgary, Canada: Univ. of Calgary; 1995. [Google Scholar]