Abstract
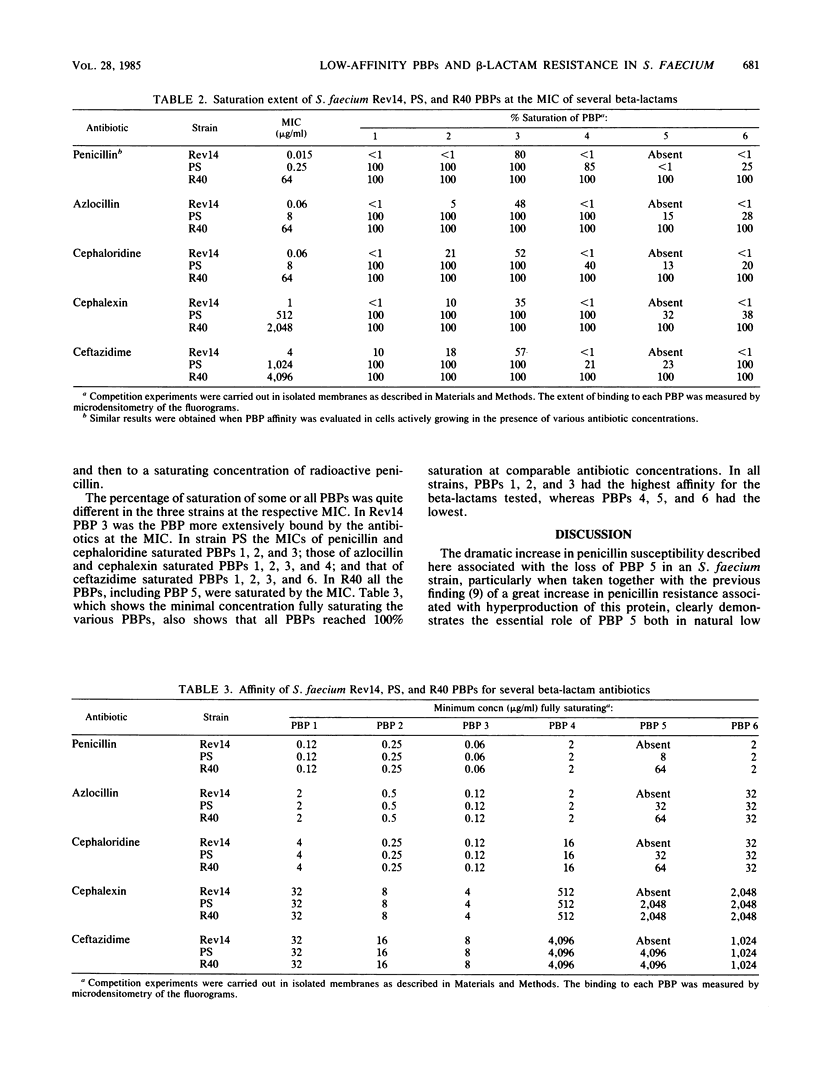
Penicillin-binding protein (PBP) 5 of Streptococcus faecium has been shown to have a very low affinity for penicillin, and this PBP was suggested to be responsible for both the natural low susceptibility and high resistance to the antibiotic in this species (R. Fontana, R. Cerini, P. Longoni, A. Grossato, and P. Canepari, J. Bacteriol. 155:1343-1350, 1983). In this study, an S. faecium mutant (Rev 14) hypersusceptible to penicillin was derived from the highly resistant S. faecium R40 treated with novobiocin, and its properties were compared with those of the parent and S. faecium PS, a relatively susceptible strain from which R40 was isolated. The hypersusceptible strain did not synthesize PBP 5, but it did resemble the parent in cell morphology, growth rate, and autolytic activity. In addition, it was highly susceptible to other beta-lactams but remained as susceptible as R40 and PS to antibiotics of a different mechanisms of action. The affinity of individual PBPs for the beta-lactams tested was the same in all the strains. This finding suggested that Rev 14 hypersusceptibility was due to the lack of PBP 5 and strongly supported the role of this protein in the mechanism of both natural low susceptibility and high-level resistance to beta-lactams in S. faecium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. F., Reynolds P. E. Intrinsic resistance to beta-lactam antibiotics in Staphylococcus aureus. FEBS Lett. 1980 Dec 29;122(2):275–278. doi: 10.1016/0014-5793(80)80455-8. [DOI] [PubMed] [Google Scholar]
- Cornett J. B., Redman B. E., Shockman G. D. Autolytic defective mutant of Streptococcus faecalis. J Bacteriol. 1978 Feb;133(2):631–640. doi: 10.1128/jb.133.2.631-640.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M., Fontana R. The penicillin-binding proteins in Streptococcus faecalis ATCC 9790. Eur J Biochem. 1980 Sep;110(2):445–456. doi: 10.1111/j.1432-1033.1980.tb04886.x. [DOI] [PubMed] [Google Scholar]
- Coyette J., Perkins H. R., Polacheck I., Shockman G. D., Ghuysen J. M. Membrane-bound DD-carboxypeptidase and LD-transpeptidase of Streptococcus faecalis ATCC 9790. Eur J Biochem. 1974 May 15;44(2):459–468. doi: 10.1111/j.1432-1033.1974.tb03504.x. [DOI] [PubMed] [Google Scholar]
- Eliopoulos G. M., Wennersten C., Moellering R. C., Jr Resistance to beta-lactam antibiotics in Streptococcus faecium. Antimicrob Agents Chemother. 1982 Aug;22(2):295–301. doi: 10.1128/aac.22.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana R., Canepari P., Satta G., Coyette J. Identification of the lethal target of benzylpenicillin in Streptococcus faecalis by in vivo penicillin binding studies. Nature. 1980 Sep 4;287(5777):70–72. doi: 10.1038/287070a0. [DOI] [PubMed] [Google Scholar]
- Fontana R., Canepari P., Satta G., Coyette J. Streptococcus faecium ATCC 9790 penicillin-binding proteins and penicillin sensitivity are heavily influenced by growth conditions: proposal for an indirect mechanism of growth inhibition by beta-lactams. J Bacteriol. 1983 May;154(2):916–923. doi: 10.1128/jb.154.2.916-923.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana R., Cerini R., Longoni P., Grossato A., Canepari P. Identification of a streptococcal penicillin-binding protein that reacts very slowly with penicillin. J Bacteriol. 1983 Sep;155(3):1343–1350. doi: 10.1128/jb.155.3.1343-1350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Binding of beta-lactam antibiotics to penicillin-binding proteins of Staphylococcus aureus and Streptococcus faecalis: relation to antibacterial activity. Antimicrob Agents Chemother. 1980 Nov;18(5):834–836. doi: 10.1128/aac.18.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- Williamson R., Calderwood S. B., Moellering R. C., Jr, Tomasz A. Studies on the mechanism of intrinsic resistance to beta-lactam antibiotics in group D streptococci. J Gen Microbiol. 1983 Mar;129(3):813–822. doi: 10.1099/00221287-129-3-813. [DOI] [PubMed] [Google Scholar]