Abstract
Phosphatic sedimentary rocks preserve a record of early animal life different from and complementary to that provided by Ediacaran fossils in terminal Proterozoic sandstones and shales. Phosphorites of the Doushantuo Formation, South China, contain eggs, egg cases, and stereoblastulae that document animals of unspecified phylogenetic position; small fossils containing putative spicules may specifically record the presence of sponges. Microfossils recently interpreted as the preserved gastrulae of cnidarian and bilaterian metazoans can alternatively be interpreted as conventional algal cysts and/or egg cases modified by diagenetic processes known to have had a pervasive influence on Doushantuo phosphorites. Regardless of this interpretation, evidence for Doushantuo eumetazoans is provided by millimeter-scale tubes that display tabulation and apical budding characteristic of some Cnidaria, especially the extinct tabulates. Like some Ediacaran remains, these small, benthic, colonial fossils may represent stem-group eumetazoans or stem-group cnidarians that lived in the late Proterozoic ocean.
The Doushantuo Formation, South China, provides an unusually clear window on terminal Proterozoic life, preserving diverse fossils as siliceous and phosphatic permineralizations as well as Burgess Shale-type compressions in shale (1–3). Doushantuo phosphorites, in particular, have generated much interest because of the animal eggs, embryos, and putative sponge body fossils they contain (2, 4–6). To this inventory, Chen et al. (7) recently added microscopic structures interpreted as possible cnidarian and bilaterian gastrulae. In this paper, we evaluate these structures and other evidence for eumetazoan animals in Doushantuo phosphorites.
Doushantuo rocks lie above glaciogenic strata of the Nantuo Tillite and below carbonates of the Dengying Formation that contain rare Ediacaran body fossils in their lower part and basal Cambrian shelly fossils near their top. Chemostratigraphic profiles suggest that Doushantuo fossils predate the last strongly positive C-isotopic excursion of the Proterozoic, dated as 549 ± 1 Ma in Namibia (8). Similarly, Doushantuo microfossils provide biostratigraphic evidence that this formation predates 555 ± 3 Ma (9) sandstones of the Redkino Series, northern Russia, which contain diverse Ediacaran body and trace fossils. Bio- and chemostratigraphic correlations further suggest that Doushantuo fossils are older than diverse Ediacaran assemblages found in Australia, Ukraine, and northern Siberia (10, 11). However, in the absence of direct radiometric constraints, it is uncertain whether Doushantuo fossils predate frondose Ediacaran remains from Newfoundland, dated as 565 ± 3 Ma (12). Thus, Doushantuo rocks were deposited near the beginning of or shortly before the interval of early animal diversification marked by diverse Ediacaran biotas, attaching particular interest to any eumetazoan fossils they may contain.
Reports of Animal Fossils in Doushantuo Rocks.
Tang et al. (13) reported sponge spicules from Doushantuo cherts as early as 1978, but given the similarity of illustrated specimens to elongated crystals, confidence in this interpretation remains low (14). Triaxonic spicules later reported by Zhao et al. (15, 16) are more likely to have been produced by sponges. Sinuous microstructures in Doushantuo cherts originally identified as back-filled microburrows (17) are more parsimoniously explained as oblique slices through large, multilamellate cyanobacterial filaments (3). Animal remains have also been claimed in Burgess-like compressions from uppermost Doushantuo shales (18). In a recent monographic reevaluation of these fossils, Xiao et al. (1) rejected most such interpretations but agreed that at least two conical compression taxa may be peridermal sheaths comparable to those made by some living and Cambrian scyphozoans (19, 20).
Perhaps the best known evidence of Doushantuo animals published to date is the well-preserved animal eggs, egg cases, and early cleavage stage embryos reported from Doushantuo phosphorites at Weng'an (2, 21). These display egg case ornamentation and cleavage geometries consistent with animal stereoblastulae but not with patterns of cell division known to occur in algae (21). Later developmental stages of this population are unknown, however, frustrating systematic attribution.
Li et al. (4) also reported structures interpreted as eggs and embryos and specifically attributed them to sponges, based principally on the presence of small structures interpreted as monoaxonal microscleres. This interpretation may be correct, although the variable morphology of these microstructures—which include very long, curved individuals less than 1 μm thick and occasional twins—suggests that caution is necessary in differentiating spicules from diagenetic minerals in the same bed (22). An argument in favor of the sponge interpretation is that microsclere-like crystals have not been observed in fossils known to have originated as algal thalli.
Collectively, then, previously described fossils indicate that animals lived in the Doushantuo sea, but provide only limited evidence for taxa more derived than sponges.
Cnidarian Gastrulae in Doushantuo Phosphorites?
Chen et al. (7) observed a range of phosphatized structures in Doushantuo rocks and interpreted them as possible cnidarian and bilaterian gastrulae, based on three observations: the relatively invariant dimensions of illustrated specimens, the presence of simple invaginations to complex internal structures interpreted as products of gastrulation, and the regular orientation and thickness of apatite crystals that constitute both external and internal layers (the crystals are interpreted as cell-by-cell replacements of similarly oriented embryonic cells). The authors explicitly acknowledge that alternative explanations are possible, and it is a diagenetic alternative we outline here.
Like all fossils, remains in the Doushantuo Formation have been altered by diagenesis, requiring that postmortem features be identified and removed from consideration before biological interpretation begins. A nearly ubiquitous feature of fossiliferous Doushantuo phosphorites at Weng'an is an isopachous rim of microcrystalline apatite (or, in some instances, silica) that coats fossils and other surfaces (ref. 23; Fig. 1 A–J). Such features are common in phosphatized Phanerozoic fossils, where they record crystal nucleation on an organic substrate, with acicular growth perpendicular to the nucleating surface (24–28). In contrast, the replacement of individual cells by oriented crystals—implicit in the gastrula interpretation—has not, to the best of our knowledge, been recorded in phosphatic fossils of any age.
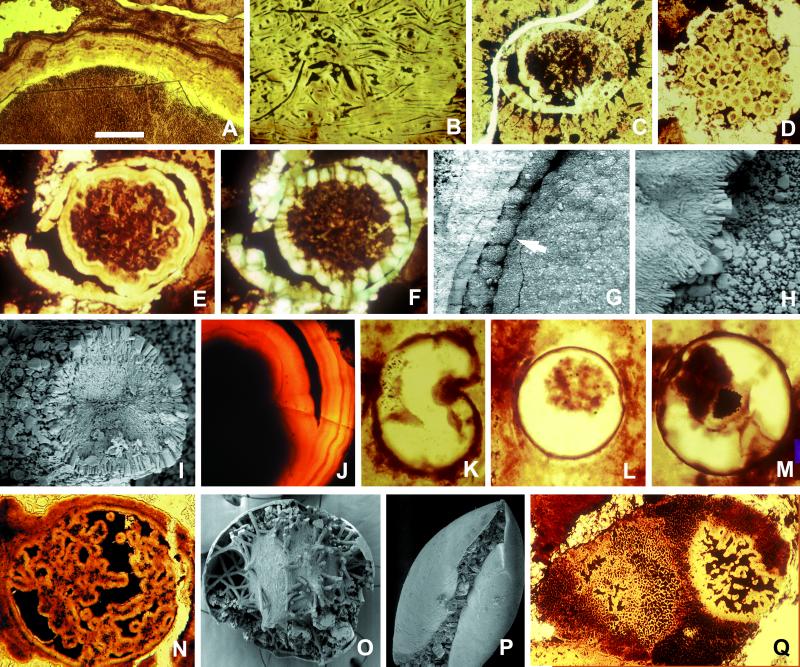
Figure 1.
Diagenetic modifications of microfossils in the Doushantuo Formation. Specimens illustrated in A–J and N–Q are from phosphorites at Weng'an, South China; K-M occur in chert nodules. Phosphatic rims occur on algal thalli (A), cyanobacterial filaments (B), the inner surfaces and collapsed contents of acanthomorphic acritarch vesicles (C), and small coccoidal cells (D). Spheroidal fossil containing diagenetically formed inner and outer rims that display crystal zonation (E) and regular orientation (F, cross-polarization); note how the inner rim is templated on the surface of preserved organic contents. (G) Phosphatic spherulitic coating on the inner surface of a vesicle (arrow), forming bulbous microstructures similar to those interpreted by Chen et al. (7) as large ectodermal cells. (H) The spot marked by an arrow in G is magnified to show crystal forms and orientation. (I) Cross section of phosphatized filament, again illustrating crystal forms and orientation. (J) Bilayered spheroidal structure similar to that in E, showing clear evidence of zoned crystal growth. (K) Silicified organic-walled vesicle with invagination produced by postmortem deformation; if phosphatized, the specimen would resemble forms interpreted as bilaterian gastrulae by Chen et al. (7); viewed in thin section, the phosphatized fossil with postmortem infolding in P would also resemble proposed gastrulae. (L and M) Individual organic-walled algal vesicles with partially collapsed internal contents, drawn from a large population of 90- to 150-μm fossils in Doushantuo cherts; postmortem phosphatization would yield structures with a size range, internal morphology, and crystal orientation comparable to those used by Chen et al. (7) to infer eumetazoan origins. (N) Internally complex spheroid, similar in organization to specimens interpreted as anthozoan planulae by Chen et al. (7), but not interpretable in biologically meaningful terms; the SEM image in O shows diagenetically phosphatized filaments and other internal structures that, viewed in thin section, would resemble N. (Q) Multicellular algal thallus (the small dark structures are cell lumens) containing a decay feature comparable in organization to structures interpreted as anthozoan planulae by Chen et al. (7). (The scale bar in A represents 100 μm for A and B; 60 μm for C, N, and Q; 80 μm for D; 50 μm for E and F; 40 μm for G, K, and L; 4 μm for H and I; 20 μm for J; 30 μm for M; 200 μm for O; and 250 μm for P.)
Because of their mechanism of formation, these isopachous rims display both regular thickness and crystal orientation (the pattern of sweeping extinction noted by Chen et al. is routinely seen in cross-polarized illumination). In the Doushantuo rocks that host the putative gastrulae, isopachous rims 5–18 μm thick coat objects ranging from cyanobacterial filaments and algal cells to small sediment clasts, acritarch walls, and animal egg cases (Fig. 1 A–D; the thicker rind on the algal thallus in Fig. 1A is a composite, reflecting multiple episodes of precipitation). SEM images of these crystals show no evidence of cell replacement (Fig. 1 H and I); indeed, many rims show distinct crystal zonation, precluding their interpretation as single cell layers (Fig. 1 E, F, and J). Similarly, microspherulitic rims, formed when nucleation is patchy and subsequent crystal growth is radial, can be indistinguishable from features interpreted by Chen et al. as large ectodermal cells of possible spiralian gastrulae (compare their figure 3J with Fig. 1 G and H). We do not suggest that Chen et al. would regard the images in Fig. 1 as fossil embryos. Rather, the photographs show that beds that yield proposed gastulae also contain other microstructures in which phosphatic layers of constant thickness and regular crystal orientation are of clear diagenetic origin. By themselves, then, such features cannot provide evidence for eumetazoan or any other type of biology.
What, then, about size and shape? Phosphatic rims faithfully replicate preexisting features, but they do not discriminate between the products of ontogeny and decay. Chen et al. (ref. 7, their figure 2A) illustrate one spheroid, the complex internal structure of which suggests an anthozoan planula with mesenteric folds and immature septa. The specimen could be a planula, but the presence of diagenetically altered microstructures with similar features in the same beds saps confidence in such an interpretation. Spheroidal bodies rimmed by phosphate layers, with broadly lobate internal folds and black organic material in intervening spaces, can be observed in vesicles in which diagenetic phosphate rims filamentous or irregularly collapsed internal contents (Fig. 1 N and O). Such features also occur in spiny acritarchs (3) and in decaying algal thalli (Fig. 1Q). The same structural theme occurs with purported sponge spicules in some specimens, minimally requiring reinterpretation of either spicules or internal morphologies.
Spheroids (100–150 μm) in which a hollow phosphatic surface encloses a smaller spheroidal layer were compared by Chen et al. (ref. 7, their figure 2 C and D) to hydrozoan gastrulae. Alternatively, such structures can be interpreted as phosphatic rims precipitated during early diagenesis on organic walled vesicles and the surfaces of their partially shrunken contents. Fossils capable of producing such structures upon phosphatization are common in Doushantuo rocks (3, 23, 29); they include algal cysts and animal egg cases. The fossils illustrated in Fig. 1 (L and M) are drawn from a large population of 90- to 150-μm algal cysts preserved in Doushantuo cherts, satisfying the criteria of Chen et al. of shape and size distribution but having nothing to do with embryos. In Fig. 1C, inner and outer phosphatic layers occur inside a spiny acritarch (of the same type that envelops an “anthozoan-like” microstructure in ref. 3). The similar crystal orientations of inner and outer layers are the expected product of diagenetic crystal growth.
Chen et al. (ref. 7, their figure 3 A–J) illustrate a third group of structures interpreted as the invaginated gastrulae of bilaterian animals. Most of the illustrated specimens can be interpreted with confidence only as rimmed spheroids with petrographically heterogeneous contents (consider, for example, how phosphatization would affect the organically preserved specimen in Fig. 1M), but several specimens show clear infolding. Again, these could be gastrulae, but postmortem mechanical deformation, commonly observed in Doushantuo fossils (Fig. 1 K and P) provides an alternative explanation.
In summary, Doushantuo structures interpreted by Chen et al. (7) as cnidarian and bilaterian gastrulae can be explained alternatively as populations of algal vesicles and egg cases independently known to occur in Doushantuo rocks, subjected to processes of postmortem deformation and phosphate precipitation known to have had a pervasive influence on Doushantuo sediments. We do not imply that gastrulae are, in principle, absent from or unrecognizable in these rocks but stress the importance of distinguishing taphonomic noise from biological signal when Doushantuo fossils are interpreted. Documentation of cell morphologies by SEM may be necessary to confirm microfossils as embryos.
Phosphatized Eumetazoan Body Fossils?
Regardless of uncertainties surrounding proposed cnidarian and bilaterian gastrulae, we believe that eumetazoan animals may be preserved in Doushantuo phosphorites. The phosphatized embryos described earlier from Doushantuo rocks could have been produced by animals of cnidarian- or bilaterian-grade, although poriferan origins cannot be excluded (21). Also, as noted above, conical compressions in uppermost Doushantuo shales could represent early cnidarians. In the following paragraphs, we discuss another population of candidate eumetazoans—minute tabulate tubes preserved in Doushantuo phosphorite (Figs. 2 and 3 A–F).
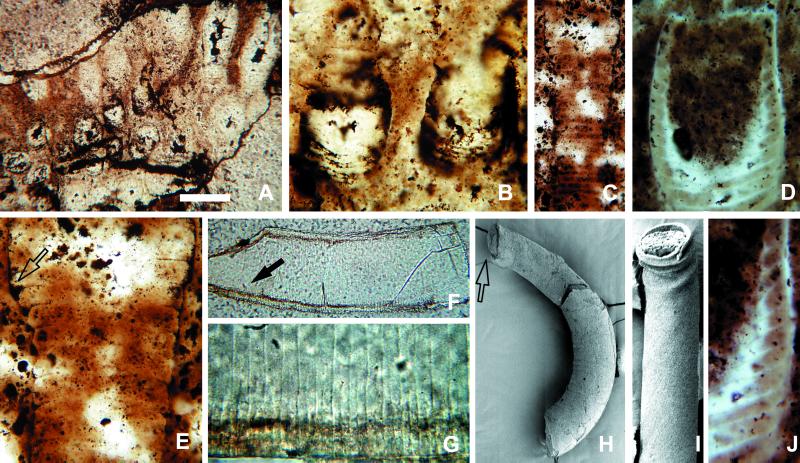
Figure 2.
Sinocyclocyclicus guizhouensis, tabulate fossils interpreted as possible stem cnidarians. (A) Twenty clustered tubes, seen in oblique section. (B) Detail of slightly curved cross-walls. (C) Tube showing expansion at top. (E) The same specimen at higher magnification, illustrating the thickening and curvature of cross-walls where they meet tube walls (arrow). (D and J) Tube with large chamber at upper end; cross-walls are incomplete and curve downward to make side walls of the chamber—cross-walls beneath the chamber are complete. (F) Tube with phosphatic rim along inner surface of tube walls and both complete and incomplete cross walls, preserved as boundaries between the phosphatic infillings of adjacent chambers. (G) Detail of complete and incomplete cross-walls (arrow in F). (H) SEM of tube with a bulbous structure at the end (arrow), as well as a laminated phosphatic rim on the tube wall. (I) Folded tube, demonstrating original flexibility of wall. (The scale bar in A represents 100 μm for A and F; 25 μm for B, D, and E; 60 μm for C; 20 μm for G and J; 200 μm for H; and 150 μm for I.)
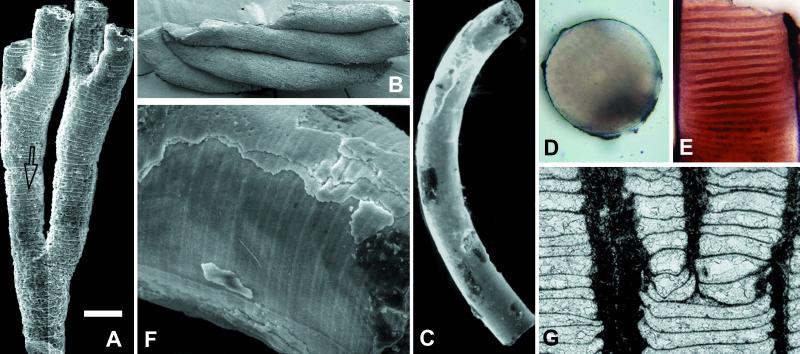
Figure 3.
Sinocyclocyclicus guizhouensis, tabulate fossils interpreted as possible stem cnidarians. (A) SEM of branched tube preserved as phosphatic internal molds of tube chambers; note branching pattern as well as wedge-shaped chamber formed where an incomplete and complete cross-wall meet (arrow). (B) SEM of four clustered tubes. (C) SEM of curved tube. (D and E) Cross and longitudinal sections through this specimen. (F) An enlarged SEM view of the surface, showing cross-walls, phosphatic laminae on the wall, and a longitudinal ridge on the concave side. (G) Saffordophyllum newcombae, an Ordovician tabulate showing bending and thickening of cross-walls where they meet side walls, as well as apical budding (reproduced with permission from Ref. 36); compare with Figs. 2E and 3A. (The scale bar in A represents 140 μm for A; 200 μm for B; 150 μm for C; 80 μm for D and E; 30 μm for F; and 1 mm for G.)
The fossils in question were originally described as Sinocyclocyclicus guizhouensis by Xue et al. (30), who illustrated millimeter-scale phosphatic cylinders composed of stacked tablets 150–240 μm wide and 13–60 μm thick. Xue et al. (30) interpreted these fossils as miniature crinoid stems, but Li et al. (31) later recognized their tubular nature and compared them with Lower Cambrian small shelly fossils. Zhang et al. (3) observed similar fossils in thin section and demonstrated that they are cylindrical tubes with closely spaced cross-walls; the tablets interpreted as tiny columnals by Xue et al. (30) are internal molds of tube chambers. Based on thin-section and SEM studies of more than 40 specimens, we propose that certain Paleozoic cnidarians provide the best available guides to the paleobiology of S. guizhouensis.
Morphology.
Tubes are circular in cross section, with a diameter of 0.1–0.3 mm; the diameter can vary markedly along the length of a single individual (31). No demonstrably complete tubes are known, but incomplete specimens can be more than 1 mm long. Specimens occur both as gregarious clusters with individuals oriented subperpendicular to bedding (Fig. 2 A and B) and as dispersed specimens without preferred orientation. Some tubes have thick (ca. 10–15 μm), even multilamellate outer walls (Figs. 2H and 3F), likely formed or modified by diagenetic phosphatization. Others are preserved as internal molds without enveloping walls (Fig. 3A). Bent specimens show folds on the compressional but not the extensional side (Fig. 2I), indicating that walls were originally flexible.
Cross-walls oriented perpendicular to the main axis divide tubes into a more or less regular series of chambers 6–12 μm thick. Most cross-walls are complete, but some tabulae extend only part of the way across the tube (Fig. 2 F and G); they may intersect with adjacent cross-walls to form wedge-like chambers (Fig. 3A, arrow). Limited observations suggest that cross-walls are not perforated by through-going internal structures such as siphuncles (Fig. 3D).
Well-preserved specimens show that tabulae may curve slightly where they intersect with the tube wall. Indeed, well-preserved walls in Fig. 2 C and E (arrow) show that the point of insertion is thickened and wedge-like in cross section, manifested in internal molds as distinct eaves at tablet boundaries (Fig. 3A). In a few tubes, cross-walls are absent or only vaguely visible; without exception, such specimens are poorly preserved, with interiors filled by secondary silica, dolomite, or phosphatic filaments and spherules.
Terminal ends are poorly known; however, a few specimens taper to a blunt conical termination. One tube contains what appears to be a distinct terminal chamber (Fig. 2 D and H), defined by a complete but strongly concave cross-wall (Fig. 2D). Abutting tabulations are incomplete and curved at their intersection with the chamber floor (Fig. 2J).
A few specimens show a distinctive pattern of dichotomous branching (Fig. 3A); tubes expand gradually along one axis to the point of dichotomy and then split into two branches, the combined circular cross sections of which are equal in area to the elliptical section below the branch point. Finally, a thin (1-μm) ridge has been observed running along the concave side of a single curved internal mold (Fig. 3F); its structural or systematic importance is unclear.
Morphological Interpretation.
Its gregarious habit, orientation, and branching morphology suggest that S. guizhouensis was a benthic and, likely, colonial organism. Taphonomic considerations suggest that tube walls were originally organic, although we cannot rule out light biomineralization of side walls. Closely spaced, straight or curved cross-walls are consistent with accretional growth in which the constructing organism occupied only the top portion of the tube, episodically moving upward as the tube lengthened and secreting new chamber floors as it went. Incomplete cross walls are consistent with such a growth pattern.
Systematic Interpretation.
Several observations cast doubt on the interpretation of as S. guizhouensis as algal: its branching mode is distinct from patterns seen in uniseriate algal filaments; its mode of preservation is distinct from that of unambiguous multicellular algae in the same beds (which routinely preserve cell walls in addition to phosphatic molds of cell lumens); and details of cell size (large), morphology (strongly tabular), and cross-walls (curved and sometimes incomplete) find no collective match in known algal filaments.
Among animals, a few groups of bilaterians (including annelids, pogonophorans, tentaculitids, hyoliths, and pterobranchs) construct tubular structures with external annulations, and some may branch dichotomously (for example, colonial pterobranchs). Some of these tubes may be as small as 100–200 μm in diameter (32). None of these bilaterians, however, produce regular transverse walls comparable to those of S. guizhouensis. Bilaterian conchs that do have cross-walls (including cephalopods and some Cambrian shelly fossils) rarely branch. Therefore, we doubt that the Doushantuo tubes are products of bilaterian biology.
In contrast, the general morphology of S. guizhouensis compares favorably with those displayed by a number of cnidarians or interpreted cnidarians, particularly the extinct tabulates. Tabulates were benthic, colonial, skeleton-forming organisms that were widely distributed in Paleozoic oceans before their late Permian extinction. Individual corallites were tubular and grew by accretion. Regularly distributed tabulae can be complete or incomplete and may show marginal bending and thickening comparable to that observed in the Doushantuo tubes. Asexual reproduction by budding was common (33), and in forms with terminal budding, parent corallites split axially into two or more smaller daughters. Thus, both the tabulation and branching patterns seen in S. guizhouensis find a counterpart among tabulates (figure 3G from ref. 34). The possible end chambers in some Doushantuo tubes can be compared with tabulate calices. Prominent differences between S. guizhouensis and tabulates include the larger size, effusively colonial habit, and CaCO3 biomineralization of the latter. Therefore, we view tabulates as architectural guides to the interpretation of Doushantuo tubes, not as direct descendants.
Some organisms previously interpreted as tabulates have been reclassified as sclerosponges (35). Chaetetids (which lack well-defined tabulae) and stromatoporoid and other “coralline” sponges (which did construct cross-walls) provide a possible alternative model for interpretation. However, these younger fossils tend to form massive colonies and display irregular “tabulation” (36). Moreover, the walls of the Doushantuo fossils appear to be products of well-defined epithelia, suggesting a level of tissue organization not found in sponges.
Other fossil and extant cnidarians also share some features with the Doushantuo tubes. For example, the stolons and hydrocauli of some colonial hydrozoans are surrounded by chitinous thecae that are the right size, can branch dichotomously, and may have external annulations and transverse diaphragms (37). However, hydrozoan diaphragms are perforated and do not occur throughout the tubular stolons. The coenenchyme of gorgonian octocorals also bears mention because of its rod-like internal skeleton, which is partially mineralized and has closely spaced, curved transverse walls (38); however, the axial skeletons of gorgonians are internal, and their transverse walls are more or less irregular.
A number of Paleozoic fossils interpreted as cnidarians also have one or more characters in common with Doushantuo tubes. Of these, Septodaeum siluricum, found in Silurian and Devonian rocks in Australia and Sweden (39), is of particular interest. Tubes of S. siluricum are a few hundred micrometers in diameter, are colonial, and show evidence of axial budding. Moreover, thecae of S. siluricum are partitioned by regularly spaced tabulae. However, these tubes differ from the Doushantuo fossils in the presence of a stomodaeum (pharynx), vertically oriented mesenteries, and stomodaeal mesenteries. Bischoff (39) interpreted S. siluricum as an anthozoan, despite significant differences from extant members of this group. Some Paleozoic coralomorphs (40), such as Moorowipora and Flindersipora, also share certain features (e.g., axial budding and transverse walls) with the Doushantuo tubes, despite obvious differences from them. Paleozoic conularids and Stephanothallus, interpreted as fossil scyphozoans (41–43), have one or more transverse walls (“schotts”; refs. 42 and 43), but their tubes are tetraradially or biradially symmetrical.
In summary, Doushantuo tubes display a suite of characters found elsewhere in cnidarians and fossils interpreted as cnidarians. Nonetheless, these characters do not relate the Doushantuo tubes unambiguously to crown group anthozoans, hydrozoans, or scyphozoans. Given molecular phylogenies that unite hydrozoans and scyphozoans into a clade nested within a paraphyletic Anthozoa (44), it is not unreasonable to interpret the small, benthic, unmineralized S. guizhouensis as a stem cnidarian or, for that matter, a stem eumetazoan.
Conclusions
Ediacaran biotas contain relatively large organisms preserved at a fairly coarse scale of morphological resolution. In contrast, phosphorites of the terminal Proterozoic Doushantuo Formation preserve exquisite miniatures that provide a different and complementary perspective on early animal evolution. Uncertainty attends possible eumetazoan gastrulae in Doushantuo rocks, but small body fossils in the same beds provide independent evidence of possible eumetazoan biology. Eumetazoans would be consistent with the presence of purported scyphozoan compressions in Doushantuo shales, as well as the interpretation of broadly contemporaneous frondose fossils from Newfoundland as colonial diploblastic organisms sister to either cnidarians or the Eumetazoa as a whole (45). Still older candidate cnidarians occur in northwestern Canada, where discs that bear radial marking suggestive of septa occur in preglacial rocks (46). If any one of these fossils is correctly interpreted as a stem- or crown-group cnidarian, phylogenetic logic demands that stem triploblasts existed by Doushantuo time, as well. Whether molecular clock arguments in favor of deep bilaterian divergence will be confirmed by recognizably bilaterian fossils in pre-Ediacaran rocks remains to be seen.
Acknowledgments
We thank L. Yin and the late Y. Zhang for valuable discussions, D.-J. Lee and R. Elias for permission to use Fig. 3G, and C. R. Scrutton for advice on cnidarian fossils. R. Elias and J. Farmer critically read the manuscript. This research was supported in part by the National Science Foundation (EAR-9805032), NSFC (49972006), MSTC-973 (G2000077700), CAS, and the Nanjing Institute of Geology and Paleontology, Academia Sinica.
Abbreviation
- Ma
megaannum (1 million years ago)
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250491697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250491697
References
- 1.Xiao, S., Yuan, X., Steiner, M. & Knoll, A. H. (2000) J. Paleontol., in press.
- 2.Xiao S, Zhang Y, Knoll A H. Nature (London) 1998;391:553–558. [Google Scholar]
- 3.Zhang Y, Yin L, Xiao S, Knoll A H. Paleontological Soc Memoir. 1998;50:1–52. [Google Scholar]
- 4.Li C-W, Chen J-Y, Hua T-E. Science. 1998;279:879–882. doi: 10.1126/science.279.5352.879. [DOI] [PubMed] [Google Scholar]
- 5.Xue Y, Zhou C, Tang T. Acta Micropalaeontologica Sinica. 1999;16:1–4. [Google Scholar]
- 6.Xiao S, Knoll A H. Acta Micropalaeontologica Sinica. 1999;16:313–323. [Google Scholar]
- 7.Chen J-Y, Oliveri P, Li C-W, Zhou G-Q, Gao F, Hagadorn J W, Peterson K J, Davidson E H. Proc Natl Acad Sci USA. 2000;97:4457–4462. doi: 10.1073/pnas.97.9.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grotzinger J P, Bowring S A, Saylor B Z, Kaufman A J. Science. 1995;270:598–604. [Google Scholar]
- 9.Martin M W, Grazhdankin D V, Bowring S A, Evans D A D, Fedonkin M A, Kirschvink J L. Science. 2000;288:841–845. doi: 10.1126/science.288.5467.841. [DOI] [PubMed] [Google Scholar]
- 10.Walter M R, Veevers J J, Calver C R, Gorjan P, Hill A C. Precambrian Res. 2000;100:371–433. [Google Scholar]
- 11.Knoll A H, Grotzinger J P, Kaufman A J, Kolosov P. Precambrian Res. 1995;73:251–270. doi: 10.1016/0301-9268(94)00081-2. [DOI] [PubMed] [Google Scholar]
- 12.Benus A P. In: Trace Fossils, Small Shelly Fossils and the Precambrian-Cambrian Boundary. Landing E, Narbonne G M, Myrow P, editors. New York: Bulletin of the New York State Museum; 1988. pp. 8–9. [Google Scholar]
- 13.Tang T, Zhang J, Jiang X. Acta Stratigraphica Sinica. 1978;2:32–45. [Google Scholar]
- 14.Brasier M, Green O, Shields G. Geology. 1997;25:303–306. [Google Scholar]
- 15.Zhao Z, Xing Y, Ding Q, Liu G, Zhao Y, Zhang S, Meng X, Yin C, Ning B, Han P. The Sinian System of Hubei. Wuhan: China University of Geosciences Press; 1988. [Google Scholar]
- 16.Zhao Z, Xing Y, Ma G, Chen Y. Biostratigraphy of the Yangtze Gorge Area, (1) Sinian. Beijing: Geological Publishing House; 1985. [Google Scholar]
- 17.Awramik S M, McMenamin D S, Yin C, Zhao Z, Ding Q, Zhang S. Nature (London) 1985;315:655–658. [Google Scholar]
- 18.Chen M, Xiao Z. Acta Palaeontologica Sinica. 1992;31:513–529. [Google Scholar]
- 19.Conway Morris S, Robison R A. The University of Kansas Paleontological Contributions. 1988;122:23–84. [Google Scholar]
- 20.Werner B. Helgoländer wissenschaftliche Meeresuntersuchungen. 1966;13:317–347. [Google Scholar]
- 21.Xiao S, Knoll A H. J Paleontol. 2000;74:767–788. [Google Scholar]
- 22.Zhang Y, Yuan X, Yin L. Science. 1998;282:1783. [Google Scholar]
- 23.Xiao S, Knoll A H. Lethaia. 1999;32:219–240. doi: 10.1111/j.1502-3931.1999.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhu M, Qian Y, Jiang Z, He T. Acta Micropalaeontologica Sinica. 1996;13:241–254. [Google Scholar]
- 25.Müller K J. Philos Trans R Soc London Ser B Biol Sci. 1985;311:67–73. [Google Scholar]
- 26.Bengtson S. Lethaia. 1976;9:185–206. [Google Scholar]
- 27.Huesken T-C, Eiserhardt K-H. Acta Universitatis Carolinae. 1996;40:445–455. [Google Scholar]
- 28.Martill D M, Wilby P R. Kaupia Darmstädter Beiträger Naturgeschichte. 1994;4:71–77. [Google Scholar]
- 29.Yuan X, Hofmann H J. Alcheringa. 1998;22:189–222. [Google Scholar]
- 30.Xue Y-s, Tang T-f, Yu C-l. Acta Palaeontologica Sinica. 1992;31:530–539. [Google Scholar]
- 31.Li G, Xue Y, Zhou C. PalaeoWorld. 1997;7:29–37. [Google Scholar]
- 32.Barnes R D. Bull Mar Sci. 1977;27:340–343. [Google Scholar]
- 33.Hill D. In: Treatise on Invertebrate Paleontology, Part F, Coelenterata, Supplement 1, Rugosa and Tabulata, 2. Teichert C, editor. Lawrence, KS: Geological Society of America and University of Kansas; 1981. pp. F430–F669. [Google Scholar]
- 34.Lee D-J, Elias R J. J Paleontol. 2000;74:404–425. [Google Scholar]
- 35.Hartman W D, Goreau T F. Zoological Soc London Symposia. 1970;25:205–243. [Google Scholar]
- 36.Reitner J. Berliner Geowissenshaftliche Abhandlungen. 1992;1:1–352. [Google Scholar]
- 37.Cornelius P F S. North-west European Thecate Hydroids and Their Medusae: Keys and Notes for Identification of the Species, Part 2. Shrewsbury, England: Field Studies Council; 1995. [Google Scholar]
- 38.Szmant-Froelich A. Marine Biol. 1974;27:299–306. [Google Scholar]
- 39.Bischoff G C O. Senckenbergiana Lethaea. 1978;59:229–273. [Google Scholar]
- 40.Scrutton C T. Proc Yorkshire Geol Soc. 1997;51:177–208. [Google Scholar]
- 41.van Iten H. In: The Early Evolution of Metazoa and the Significance of Problematic Taxa. Simonetta A M, Conway Morris S, editors. Cambridge, U.K.: Cambridge University Press; 1991. pp. 145–155. [Google Scholar]
- 42.van Iten H. Palaeontology. 1991;34:939–954. [Google Scholar]
- 43.van Iten H, Cox R S, Mapes R H. Lethaia. 1992;25:135–144. [Google Scholar]
- 44.Bridge D, Cunningham C W, DeSalle R, Buss L. Mol Biol Evolution. 1995;12:679–689. doi: 10.1093/oxfordjournals.molbev.a040246. [DOI] [PubMed] [Google Scholar]
- 45.Buss L, Seilacher A. Paleobiology. 1994;20:1–4. [Google Scholar]
- 46.Hofmann H J, Narbonne G M, Aitken J D. Geology. 1990;18:1199–1202. [Google Scholar]