Abstract
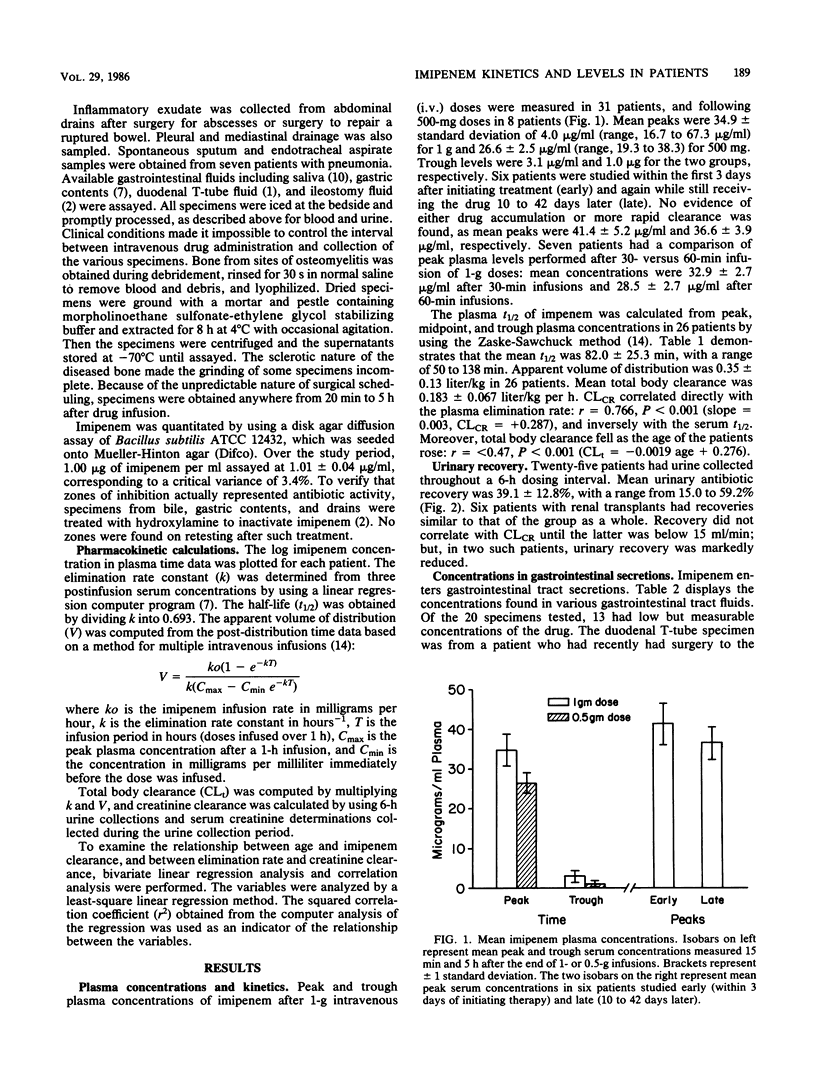
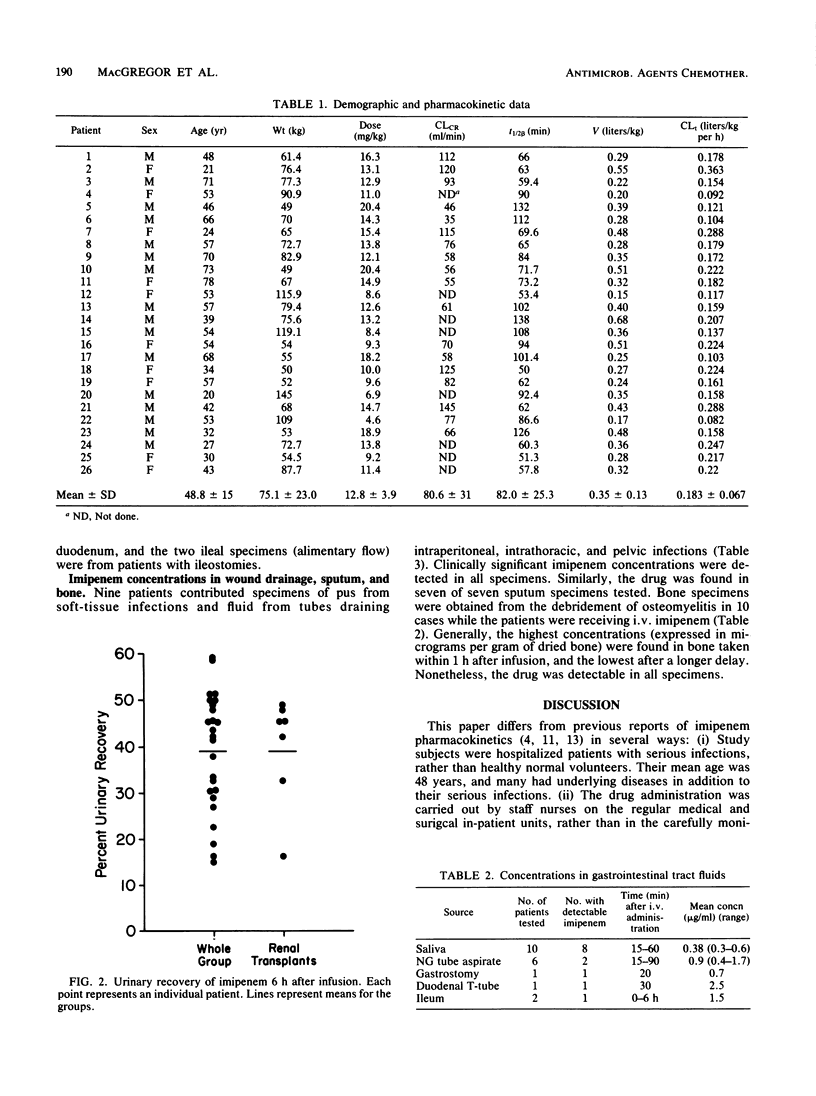
Serum, urine, tissue, and body fluids were collected from 40 adult patients who were receiving imipenem/cilastatin treatment for serious infections. Thirty-two patients were given 1 g every 6 h (4 g/day), and eight received 500 mg (2 g/day). Mean peak concentrations in serum were 34.9 +/- 4.0 micrograms/ml for the 1-g dose and 26.6 +/- 2.5 micrograms/ml for the 500-mg dose. Trough levels were 3.1 and 1.0 micrograms/ml, respectively. No evidence of drug accumulation was found after comparing peaks measured early in the treatment with those measured late. Peak levels were only marginally increased when infusions were given over 30 versus 60 min. The mean serum half-life was 82.0 +/- 25.3 min, with a range of 50 to 138 min. The apparent volume of distribution was 0.35 +/- 0.13 liter/kg, and the mean total body clearance was 0.183 +/- 0.067 liter/kg per h. Creatinine clearance correlated directly with the plasma elimination rate and inversely with the serum half-life. Moreover, total body clearance fell as the age of the patient rose. The mean urinary recovery was 39.1 +/- 12.8% (range, 15.0 to 59.2%) and did not correlate with creatinine clearance until it was below 15 ml/min. Of 20 specimens of various gastrointestinal secretions, 13 had imipenem concentrations that were low, but above the MIC for most resident flora. Pus, sputum, and bone all had concentrations of the drug sufficient to inhibit the infecting organisms, and these levels reflected generally excellent clinical responses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alestig K., Carlberg H., Nord C. E., Trollfors B. Effect of cefoperazone on faecal flora. J Antimicrob Chemother. 1983 Aug;12(2):163–167. doi: 10.1093/jac/12.2.163. [DOI] [PubMed] [Google Scholar]
- Calandra G. B., Hesney M., Grad C. A multiclinic randomized study of the comparative efficacy, safety and tolerance of imipenem/cilastatin and moxalactam. Eur J Clin Microbiol. 1984 Oct;3(5):478–487. doi: 10.1007/BF02017380. [DOI] [PubMed] [Google Scholar]
- Drusano G. L., Standiford H. C., Bustamante C., Forrest A., Rivera G., Leslie J., Tatem B., Delaportas D., MacGregor R. R., Schimpff S. C. Multiple-dose pharmacokinetics of imipenem-cilastatin. Antimicrob Agents Chemother. 1984 Nov;26(5):715–721. doi: 10.1128/aac.26.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L. J., Hixon D. L., Park C. H., Goldenberg R. I., Poretz D. M. Imipenem versus moxalactam in the treatment of serious infections. Antimicrob Agents Chemother. 1983 Dec;24(6):841–846. doi: 10.1128/aac.24.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesado T., Hashizume T., Asahi Y. Antibacterial activities of a new stabilized thienamycin, N-formimidoyl thienamycin, in comparison with other antibiotics. Antimicrob Agents Chemother. 1980 Jun;17(6):912–917. doi: 10.1128/aac.17.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppe P., Hamann C. A program for non-linear regression analysis to be used on desk-top computers. Comput Programs Biomed. 1980 Dec;12(2-3):121–128. doi: 10.1016/0010-468x(80)90058-6. [DOI] [PubMed] [Google Scholar]
- Kropp H., Sundelof J. G., Kahan J. S., Kahan F. M., Birnbaum J. MK0787 (N-formimidoyl thienamycin): evaluation of in vitro and in vivo activities. Antimicrob Agents Chemother. 1980 Jun;17(6):993–1000. doi: 10.1128/aac.17.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. Comparative in vitro activity of N-formimidoyl thienamycin against gram-positive and gram-negative aerobic and anaerobic species and its beta-lactamase stability. Antimicrob Agents Chemother. 1982 Jan;21(1):180–187. doi: 10.1128/aac.21.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord C. E., Kager L., Philipson A., Stiernstedt G. Impact of imipenem/cilastatin therapy on faecal flora. Eur J Clin Microbiol. 1984 Oct;3(5):475–477. doi: 10.1007/BF02017379. [DOI] [PubMed] [Google Scholar]
- Norrby S. R., Alestig K., Björnegård B., Burman L. A., Ferber F., Huber J. L., Jones K. H., Kahan F. M., Kahan J. S., Kropp H. Urinary recovery of N-formimidoyl thienamycin (MK0787) as affected by coadministration of N-formimidoyl thienamycin dehydropeptidase inhibitors. Antimicrob Agents Chemother. 1983 Feb;23(2):300–307. doi: 10.1128/aac.23.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby S. R., Björnegård B., Ferber F., Jones K. H. Pharmacokinetics of imipenem in healthy volunteers. J Antimicrob Chemother. 1983 Dec;12 (Suppl 500):109–124. doi: 10.1093/jac/12.suppl_d.109. [DOI] [PubMed] [Google Scholar]
- Sawchuk R. J., Zaske D. E. Pharmacokinetics of dosing regimens which utilize multiple intravenous infusions: gentamicin in burn patients. J Pharmacokinet Biopharm. 1976 Apr;4(2):183–195. doi: 10.1007/BF01086153. [DOI] [PubMed] [Google Scholar]
- Tally F. P., Jacobus N. V., Gorbach S. L. In vitro activity of N-formimidoyl thienamycin (MK0787). Antimicrob Agents Chemother. 1980 Oct;18(4):642–644. doi: 10.1128/aac.18.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac B. A., Fisher M. A., Gibson G. A., MacGregor R. R. Safety and efficacy of high-dose treatment with imipenem-cilastatin in seriously ill patients. Antimicrob Agents Chemother. 1985 May;27(5):745–748. doi: 10.1128/aac.27.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]



