Abstract
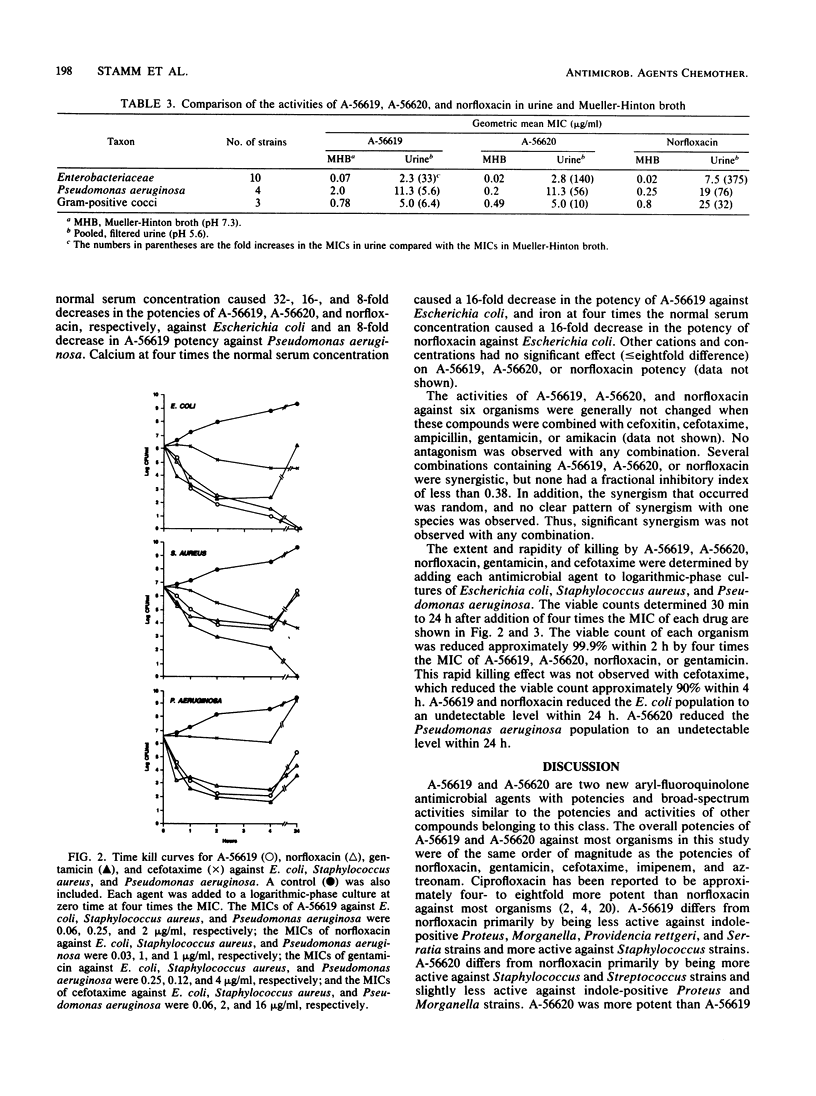
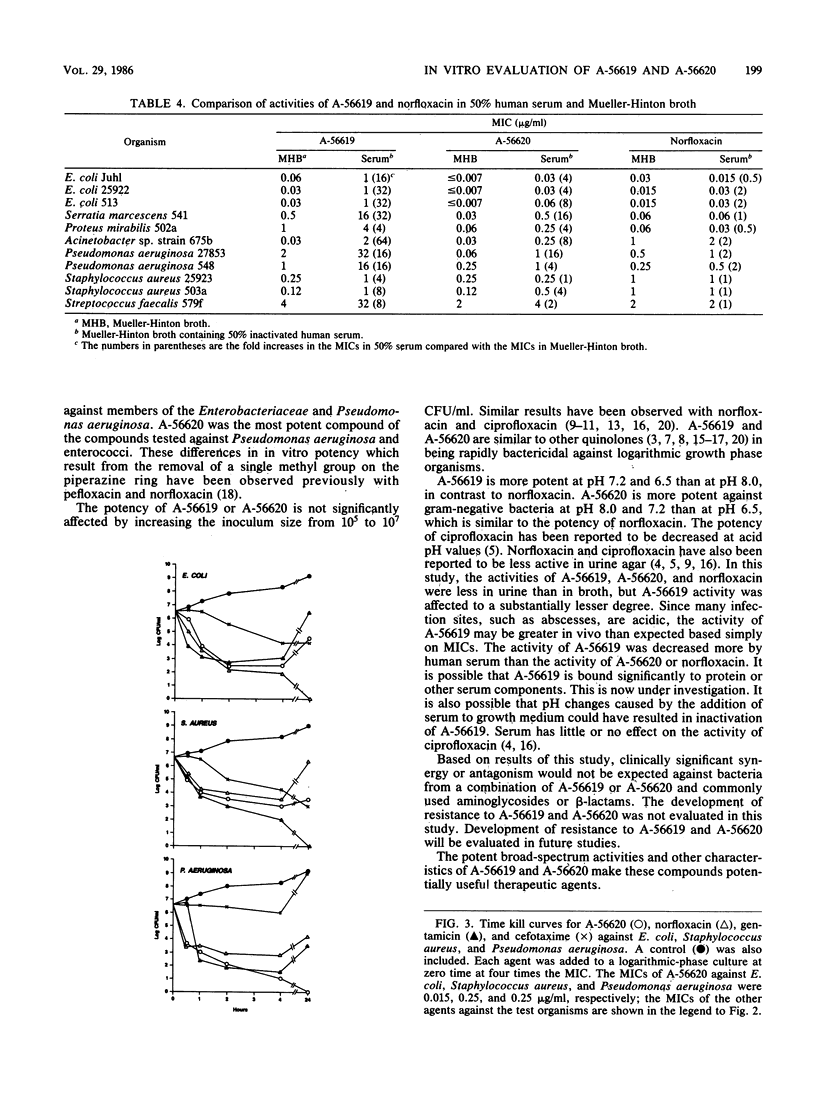
The in vitro antibacterial potencies of A-56619 and A-56620, two new aryl-fluoroquinolones, were compared with the potency of norfloxacin against a broad spectrum of organisms. Cefotaxime, aztreonam, piperacillin, imipenem, penicillin, and gentamicin were also tested for reference purposes. The MICs required to inhibit at least 90% of the strains tested ranged from 0.25 to 4 micrograms/ml for A-56619 and from 0.06 to 0.5 microgram/ml for A-56620 for members of the Enterobacteriaceae. A-56619 was generally twofold less potent and A-56620 was twofold more potent than norfloxacin against most aerobic gram-negative bacilli, including members of the Enterobacteriaceae and Pseudomonas aeruginosa. Against indole-positive Proteus, Morganella, Providencia rettgeri, and Serratia strains, A-56619 was at least 8- to 16-fold less potent than norfloxacin. A-56619 and A-56620 were four- to eightfold more potent than norfloxacin against Staphylococcus aureus and equally potent to fourfold more potent against Streptococcus species, Haemophilus influenzae, and Neisseria gonorrhoeae. The MICs of A-56619 and A-56620 were only slightly affected by increased inoculum size or by the addition of various cations at physiologic concentrations. A-56619 was three- to fivefold less active at pH 8.0 than at pH 6.5 or 7.2. A-56620 was twofold less active at pH 6.5 than at pH 8.0 or 7.2 against members of the Enterobacteriaceae and Pseudomonas aeruginosa; similar pH variations did not affect A-56620 activity against gram-positive cocci. The potencies of A-56619, A-56620, and norfloxacin were less in urine than in Mueller-Hinton broth; however, this effect was more pronounced with norfloxacin. Human serum at a concentration of 50% caused a 4- to 64- fold decrease in the potency of A-56619 and an average 4-fold decrease in the potency of A-56620, compared with no effect on the potency of norfloxacin. A-56619, A-56620, and norfloxacin were bactericidal and, at four times the MIC, reduced the viable cell counts of Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa by approximately 99.9% within 2 h. A-56619, A-56620, and norfloxacin showed no significant synergistic activity and no antagonism when they were aminoglycoside or beta-lactam antimicrobial agents.
Full text
PDF
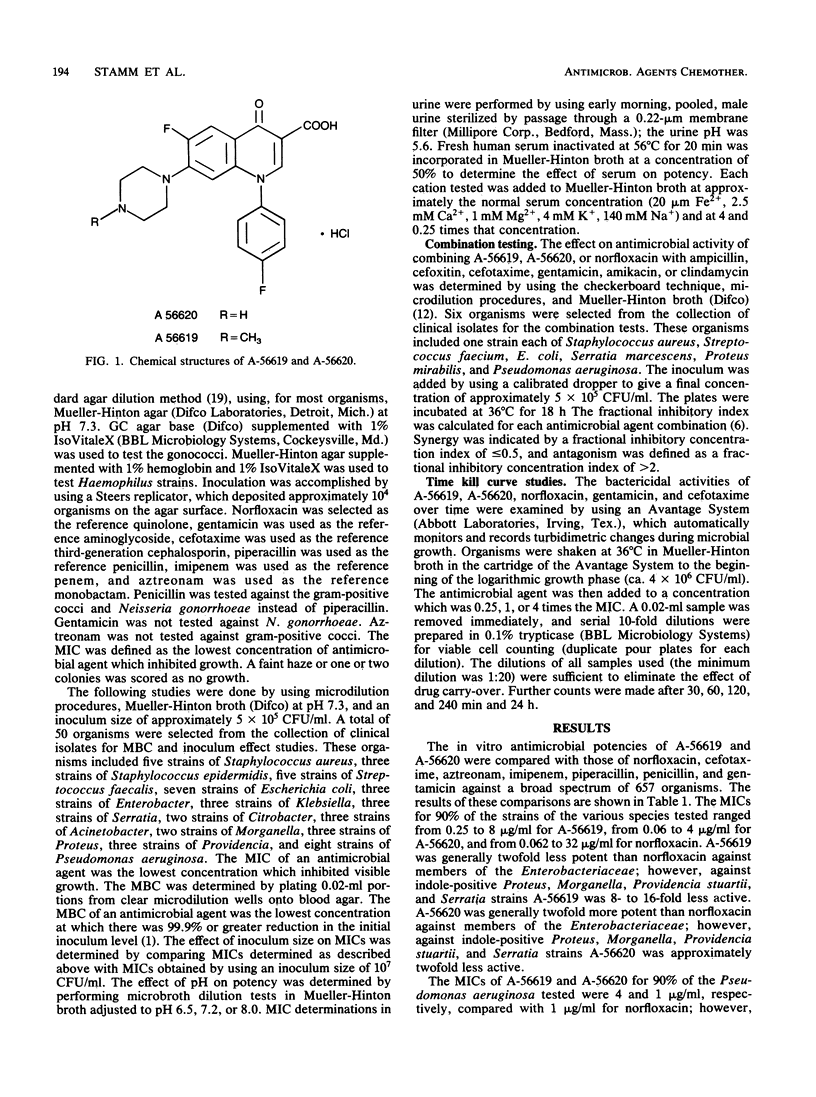
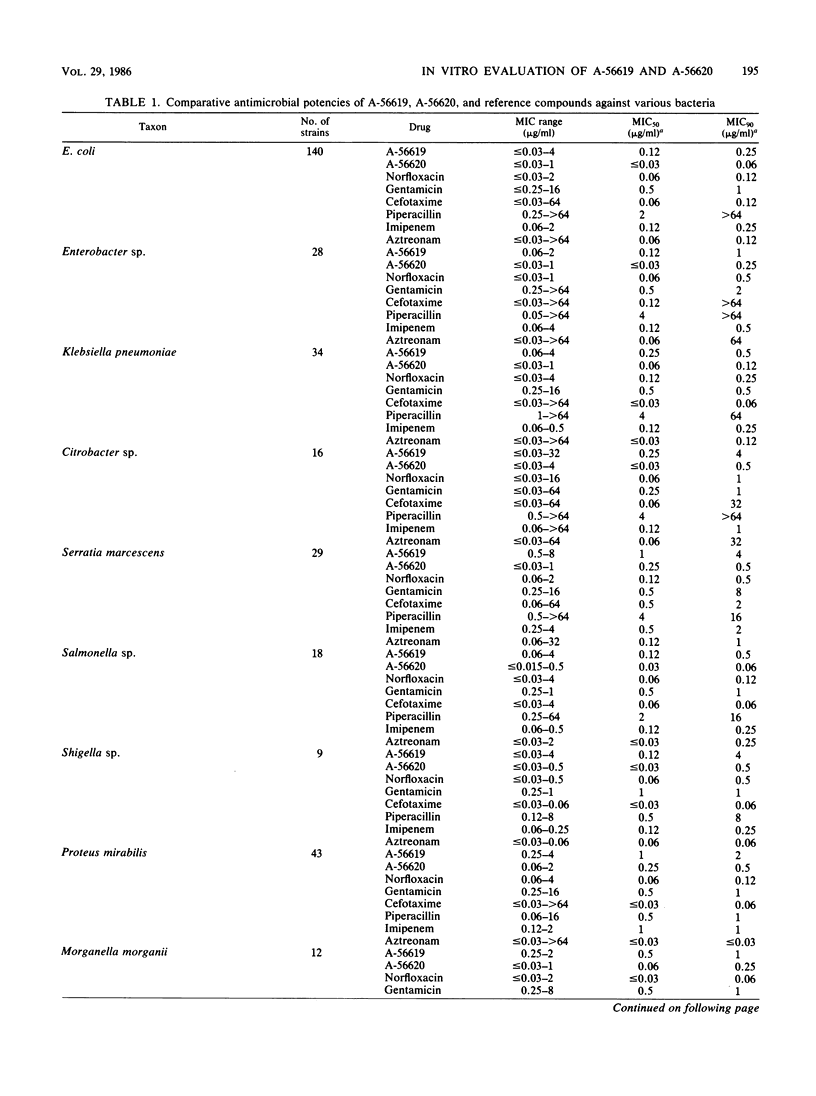
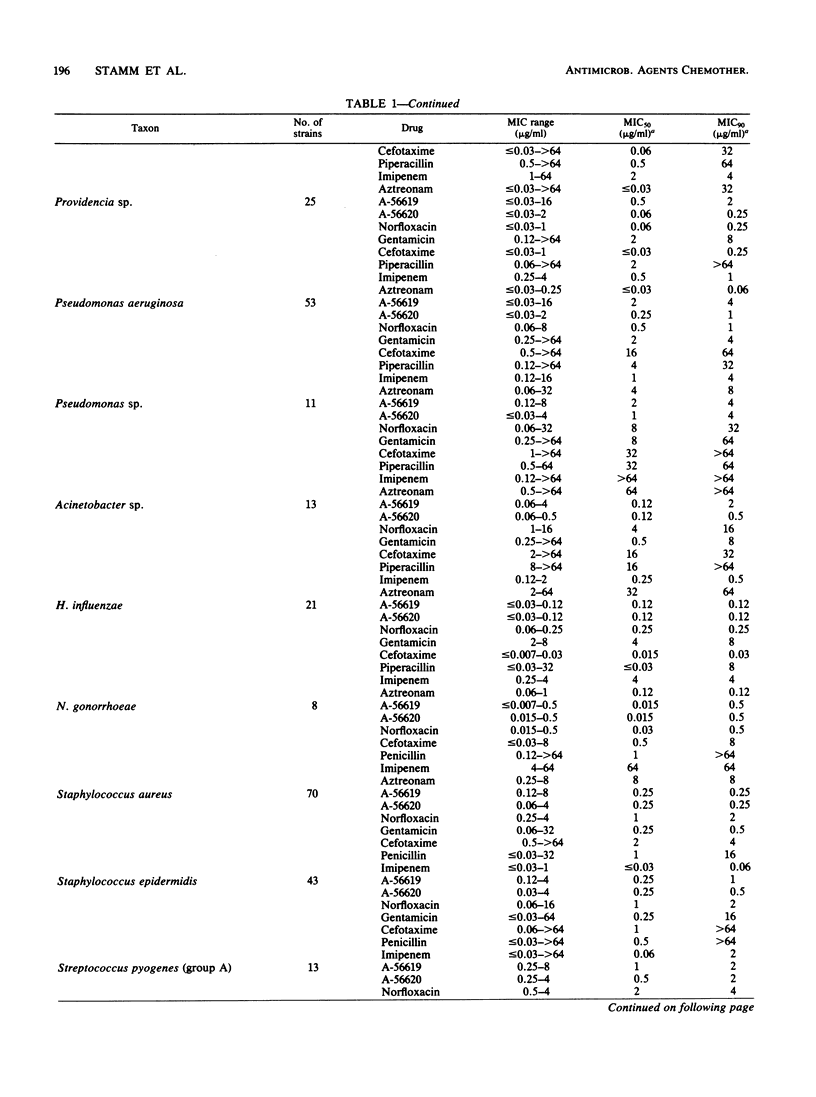
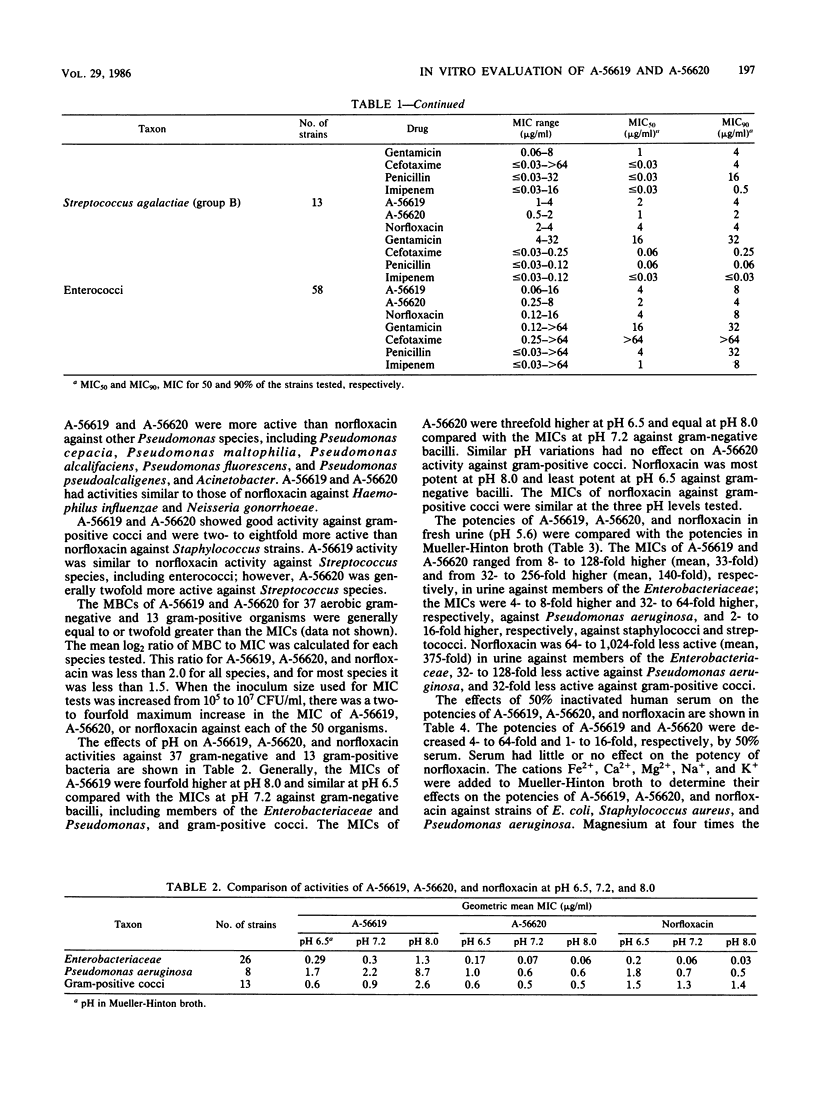

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Jones R. N., Thornsberry C., Ayers L. W., Gerlach E. H., Sommers H. M. Antibacterial activities of ciprofloxacin, norfloxacin, oxolinic acid, cinoxacin, and nalidixic acid. Antimicrob Agents Chemother. 1984 May;25(5):633–637. doi: 10.1128/aac.25.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. Ciprofloxacin, a quinolone carboxylic acid compound active against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1984 Mar;25(3):319–326. doi: 10.1128/aac.25.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. In vitro activity of enoxacin, a quinolone carboxylic acid, compared with those of norfloxacin, new beta-lactams, aminoglycosides, and trimethoprim. Antimicrob Agents Chemother. 1983 Nov;24(5):754–763. doi: 10.1128/aac.24.5.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump B., Wise R., Dent J. Pharmacokinetics and tissue penetration of ciprofloxacin. Antimicrob Agents Chemother. 1983 Nov;24(5):784–786. doi: 10.1128/aac.24.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELION G. B., SINGER S., HITCHINGS G. H. Antagonists of nucleic acid derivatives. VIII. Synergism in combinations of biochemically related antimetabolites. J Biol Chem. 1954 Jun;208(2):477–488. [PubMed] [Google Scholar]
- Goueffon Y., Montay G., Roquet F., Pesson M. Sur un nouvel antibactérien de synthèse : l'acide éthyl-1 fluoro-6 (méthyl-4 pipérazinyl-1)-7 oxo-4 dihydro-1.4 quinoléine-3 carboxylique (1589 R.B.). C R Seances Acad Sci III. 1981 Jan 5;292(1):37–40. [PubMed] [Google Scholar]
- Greenwood D., Osman M., Goodwin J., Cowlishaw W. A., Slack R. Norfloxacin: activity against urinary tract pathogens and factors influencing the emergence of resistance. J Antimicrob Chemother. 1984 Apr;13(4):315–323. doi: 10.1093/jac/13.4.315. [DOI] [PubMed] [Google Scholar]
- Ito A., Hirai K., Inoue M., Koga H., Suzue S., Irikura T., Mitsuhashi S. In vitro antibacterial activity of AM-715, a new nalidixic acid analog. Antimicrob Agents Chemother. 1980 Feb;17(2):103–108. doi: 10.1128/aac.17.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. Norfloxacin (MK-0366, AM-715): in vitro activity and cross-resistance with other organic acids including quality control limits for disk diffusion testing. Diagn Microbiol Infect Dis. 1983 Jun;1(2):165–172. doi: 10.1016/0732-8893(83)90047-0. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. In vitro activity of norfloxacin, a quinolinecarboxylic acid, compared with that of beta-lactams, aminoglycosides, and trimethoprim. Antimicrob Agents Chemother. 1982 Jul;22(1):23–27. doi: 10.1128/aac.22.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby S. R., Jonsson M. Antibacterial activity of norfloxacin. Antimicrob Agents Chemother. 1983 Jan;23(1):15–18. doi: 10.1128/aac.23.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlod D. J., Saravolatz L. D. In vitro susceptibilities of 393 recent clinical isolates to WIN 49375, cefotaxime, tobramycin, and piperacillin. Antimicrob Agents Chemother. 1984 Mar;25(3):377–379. doi: 10.1128/aac.25.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves D. S., Bywater M. J., Holt H. A., White L. O. In-vitro studies with ciprofloxacin, a new 4-quinolone compound. J Antimicrob Chemother. 1984 Apr;13(4):333–346. doi: 10.1093/jac/13.4.333. [DOI] [PubMed] [Google Scholar]
- Sato K., Matsuura Y., Inoue M., Une T., Osada Y., Ogawa H., Mitsuhashi S. In vitro and in vivo activity of DL-8280, a new oxazine derivative. Antimicrob Agents Chemother. 1982 Oct;22(4):548–553. doi: 10.1128/aac.22.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault M., Koumaré B., Soussy C. J., Duval J. Relations structure-activité dans le groupe des quinolones: étude de l'activité antibactérienne de deux nouveaux composés. Ann Microbiol (Paris) 1981 May-Jun;132(3):267–281. [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Edwards L. J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob Agents Chemother. 1983 Apr;23(4):559–564. doi: 10.1128/aac.23.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiler H. J., Grohe K. The in vitro and in vivo activity of ciprofloxacin. Eur J Clin Microbiol. 1984 Aug;3(4):339–343. doi: 10.1007/BF01977490. [DOI] [PubMed] [Google Scholar]



