Abstract
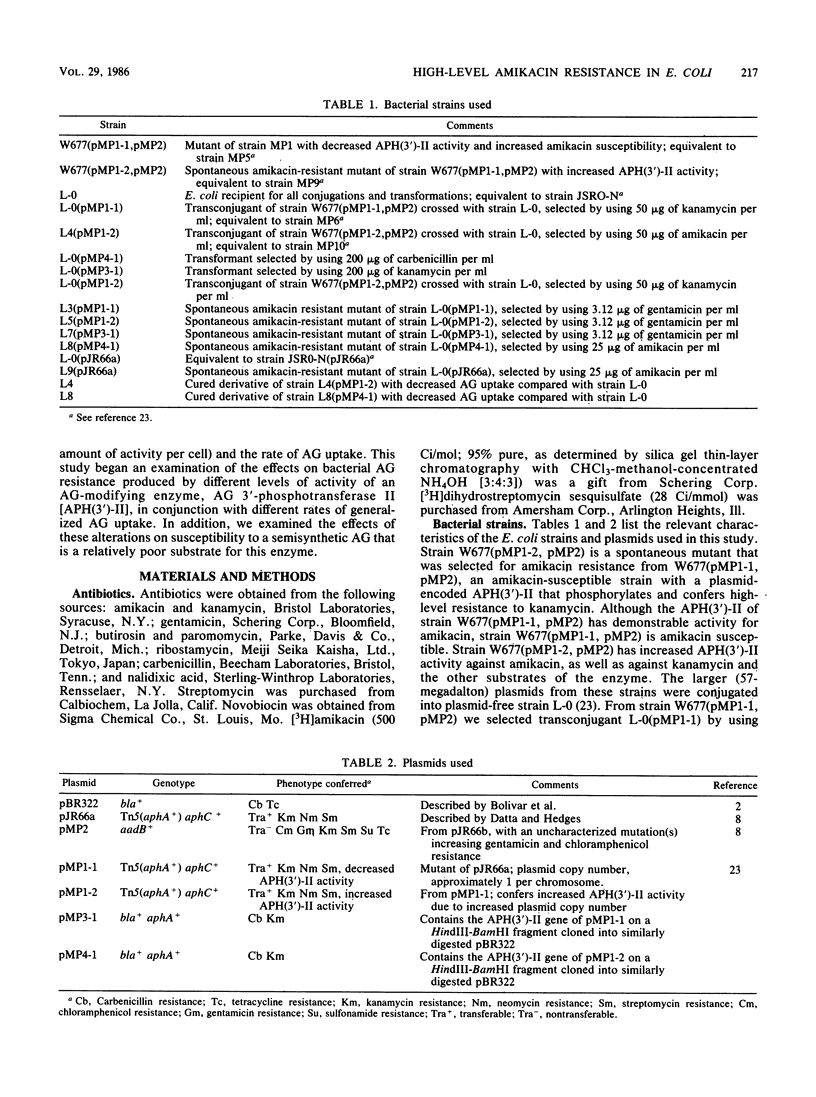
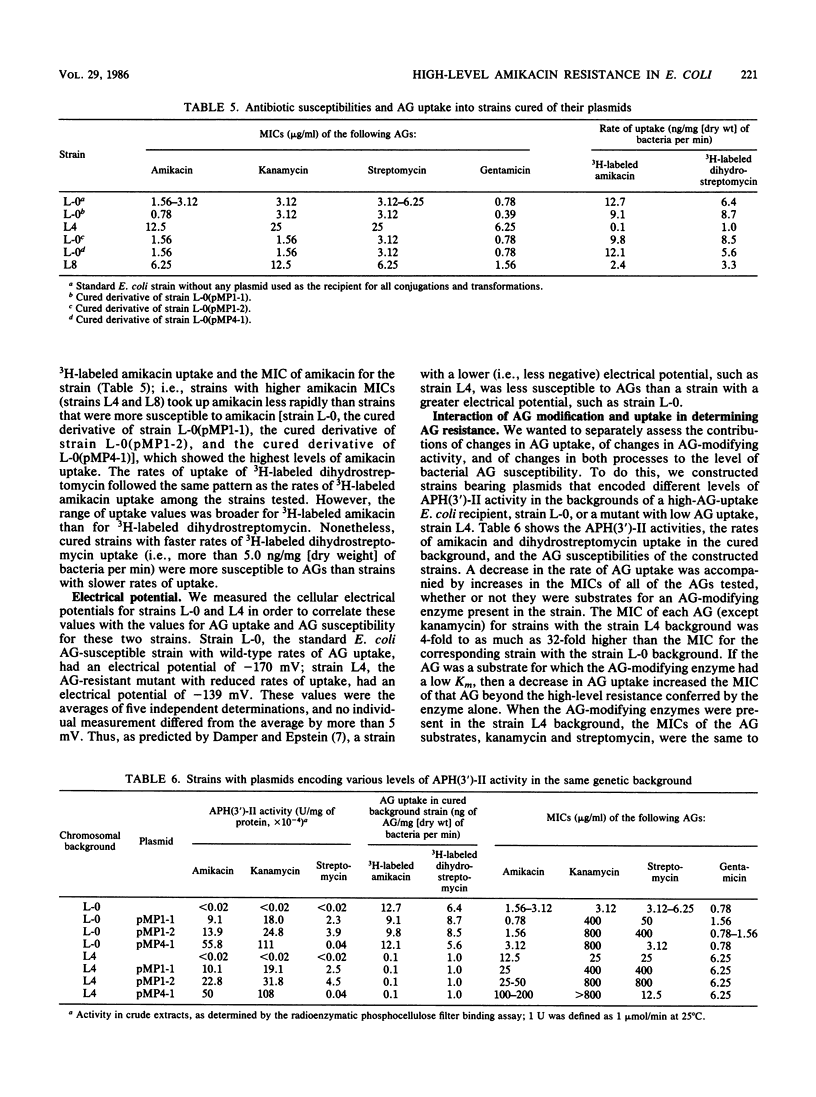
Plasmid pMP1-1 in Escherichia coli L-0 encodes aminoglycoside (AG) 3'-phosphotransferase II [APH(3')-II]. This enzyme modifies and confers high-level resistance to kanamycin. Although amikacin is a substrate for APH(3')-II, strain L-0(pMP1-1) is susceptible to amikacin. Plasmid pMP1-2 is a spontaneous mutant of pMP1-1 which determines increased APH(3')-II activity for amikacin, apparently as a result of an increase in the copy number of the plasmid. From amikacin-susceptible, gentamicin-susceptible transformants and transconjugants that bear the APH(3')-II gene on plasmid pMP1-1 or pMP1-2 or cloned into multicopy plasmid pBR322, we selected spontaneous mutants at concentrations of amikacin or gentamicin that were two to four times higher than the MICs of these antibiotics. In each case, whether they were selected by using amikacin or gentamicin, the mutants exhibited modest (two- to eightfold) increases in the MIC of gentamicin and major (64- to 128-fold) increases in the MIC of amikacin. Using these laboratory strains of E. coli, we examined the effects on AG susceptibility of the interaction of AG-modifying enzyme activity and generalized AG uptake. Increasing the level of activity of an AG phosphotransferase in these strains lowered their susceptibility to AGs which were substrates for which the enzyme had low Kms. However, an increase in AG-modifying activity alone did not result in large increases in the MICs for poor substrates of the enzyme. In strains which lacked AG-modifying enzymes, a decrease in the rate of AG uptake increased the MICs modestly for a broad spectrum of AGs. When a strain bore the phosphotransferase, a decrease in generalized AG uptake could raise the MIC further, not only for low-Km substrates, but even for AG substrates for which the enzyme had high Kms. Thus, increased modifying activity, together with a diminished rate of uptake, could produce even higher MICs for poor AG substrates.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bryan L. E., Kwan S. Roles of ribosomal binding, membrane potential, and electron transport in bacterial uptake of streptomycin and gentamicin. Antimicrob Agents Chemother. 1983 Jun;23(6):835–845. doi: 10.1128/aac.23.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M. Effects of membrane-energy mutations and cations on streptomycin and gentamicin accumulation by bacteria: a model for entry of streptomycin and gentamicin in susceptible and resistant bacteria. Antimicrob Agents Chemother. 1977 Aug;12(2):163–177. doi: 10.1128/aac.12.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Davies J. Plasmid-medicated aminoglycoside phosphotransferase of broad substrate range that phosphorylates amikacin. Antimicrob Agents Chemother. 1977 Apr;11(4):619–624. doi: 10.1128/aac.11.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damper P. D., Epstein W. Role of the membrane potential in bacterial resistance to aminoglycoside antibiotics. Antimicrob Agents Chemother. 1981 Dec;20(6):803–808. doi: 10.1128/aac.20.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. An I pilus-determining R factor with anomalous compatibility properties, mobilizing a gentamicin-resistance plasmid. J Gen Microbiol. 1973 Jul;77(1):11–17. doi: 10.1099/00221287-77-1-11. [DOI] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- DeHertogh D. A., Lerner S. A. Correlation of aminoglycoside resistance with the KmS and Vmax/Km ratios of enzymatic modification of aminoglycosides by 2''-O-nucleotidyltransferase. Antimicrob Agents Chemother. 1985 Apr;27(4):670–671. doi: 10.1128/aac.27.4.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie P., Bryan L. E., Pickard M. A. Effect of enzymatic adenylylation on dihydrostreptomycin accumulation in Escherichia coli carrying an R-factor: model explaining aminoglycoside resistance by inactivating mechanisms. Antimicrob Agents Chemother. 1978 Oct;14(4):569–580. doi: 10.1128/aac.14.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Guerry P., Hedges R. W., Datta N. Polynucleotide sequence relationships among plasmids of the I compatibility complex. J Gen Microbiol. 1974 Nov;85(1):65–76. doi: 10.1099/00221287-85-1-65. [DOI] [PubMed] [Google Scholar]
- Foster T. J. Plasmid-determined resistance to antimicrobial drugs and toxic metal ions in bacteria. Microbiol Rev. 1983 Sep;47(3):361–409. doi: 10.1128/mr.47.3.361-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Riestra C., Perlin M. H., Lerner S. A. Lack of accumulation of exogenous adenylyl dihydrostreptomycin by whole cells or spheroplasts of Escherichia coli. Antimicrob Agents Chemother. 1985 Jan;27(1):114–119. doi: 10.1128/aac.27.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand D. H., Shepard H. M., O'Farrell P. H., Polisky B. Isolation and characterization of ColE1-derived plasmid copy-number mutant. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5869–5873. doi: 10.1073/pnas.75.12.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman P. R., Northrop D. B. Purification and spectrophotometric assay of neomycin phosphotransferase II1;. Biochem Biophys Res Commun. 1976 Mar 8;69(1):230–236. doi: 10.1016/s0006-291x(76)80297-5. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Perlin M. H., Lerner S. A. Amikacin resistance associated with a plasmid-borne aminoglycoside phosphotransferase in Escherichia coli. Antimicrob Agents Chemother. 1979 Nov;16(5):598–604. doi: 10.1128/aac.16.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin M. H., Lerner S. A. Decreased susceptibility to 4'-deoxy-6'-N-methylamikacin (BB-K311) conferred by a mutant plasmid in Escherichia coli. Antimicrob Agents Chemother. 1982 Jul;22(1):78–82. doi: 10.1128/aac.22.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin M. H., Lerner S. A. Localization of an amikacin 3'-phosphotransferase in Escherichia coli. J Bacteriol. 1981 Aug;147(2):320–325. doi: 10.1128/jb.147.2.320-325.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R. N., Rogers S. G. Plasmid pKC7: a vector containing ten restriction endonuclease sites suitable for cloning DNA segments. Gene. 1979 Sep;7(1):79–82. doi: 10.1016/0378-1119(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Robison L. R., Seligsohn R., Lerner S. A. Simplified radioenzymatic assay for chloramphenicol. Antimicrob Agents Chemother. 1978 Jan;13(1):25–29. doi: 10.1128/aac.13.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]



