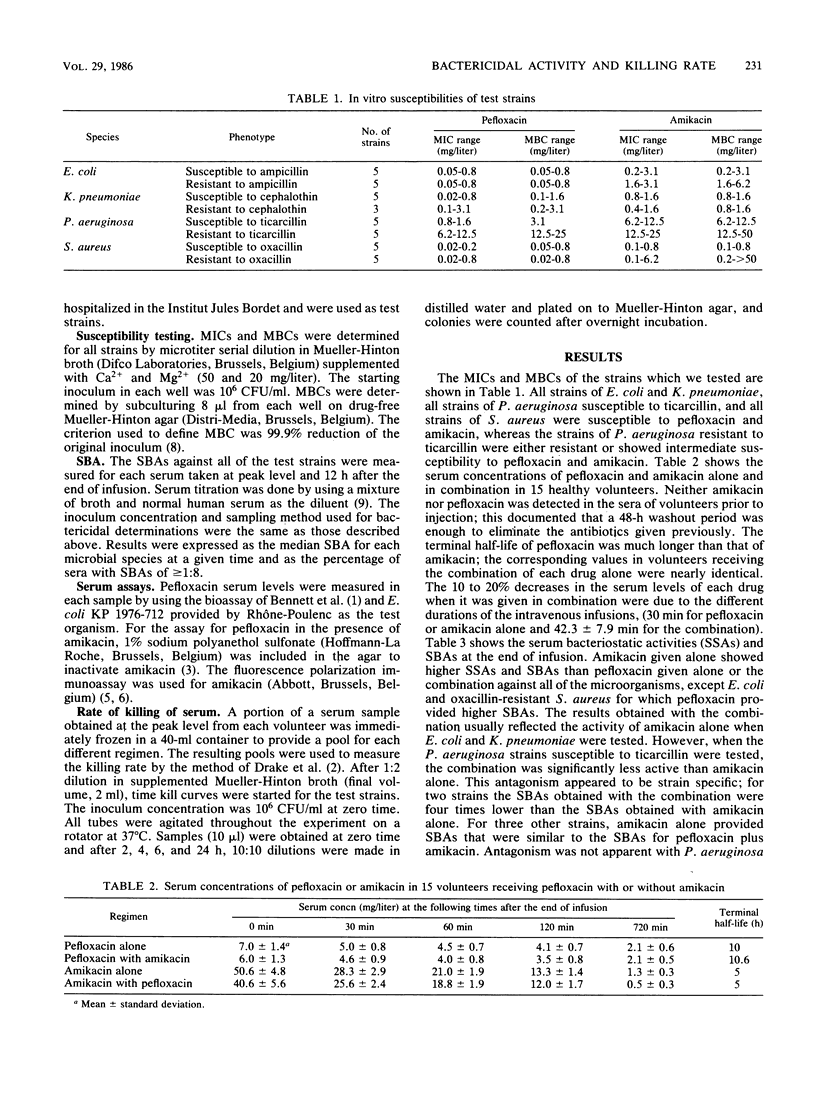
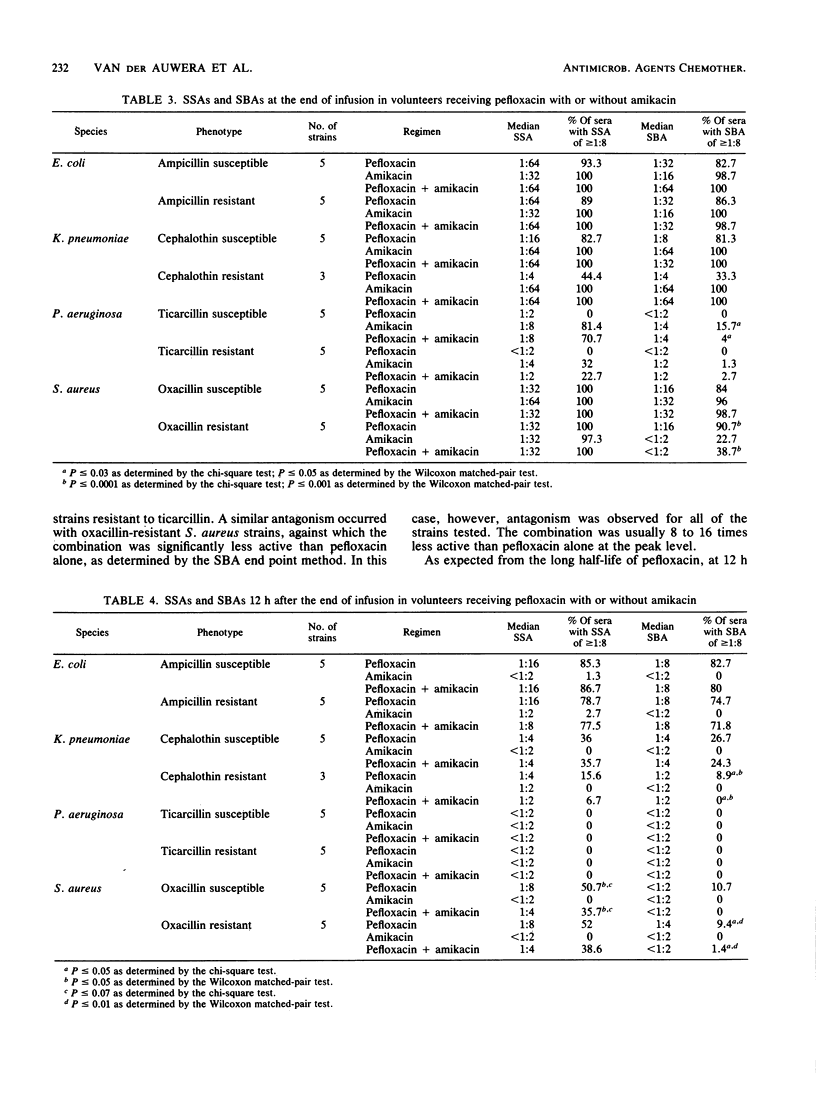
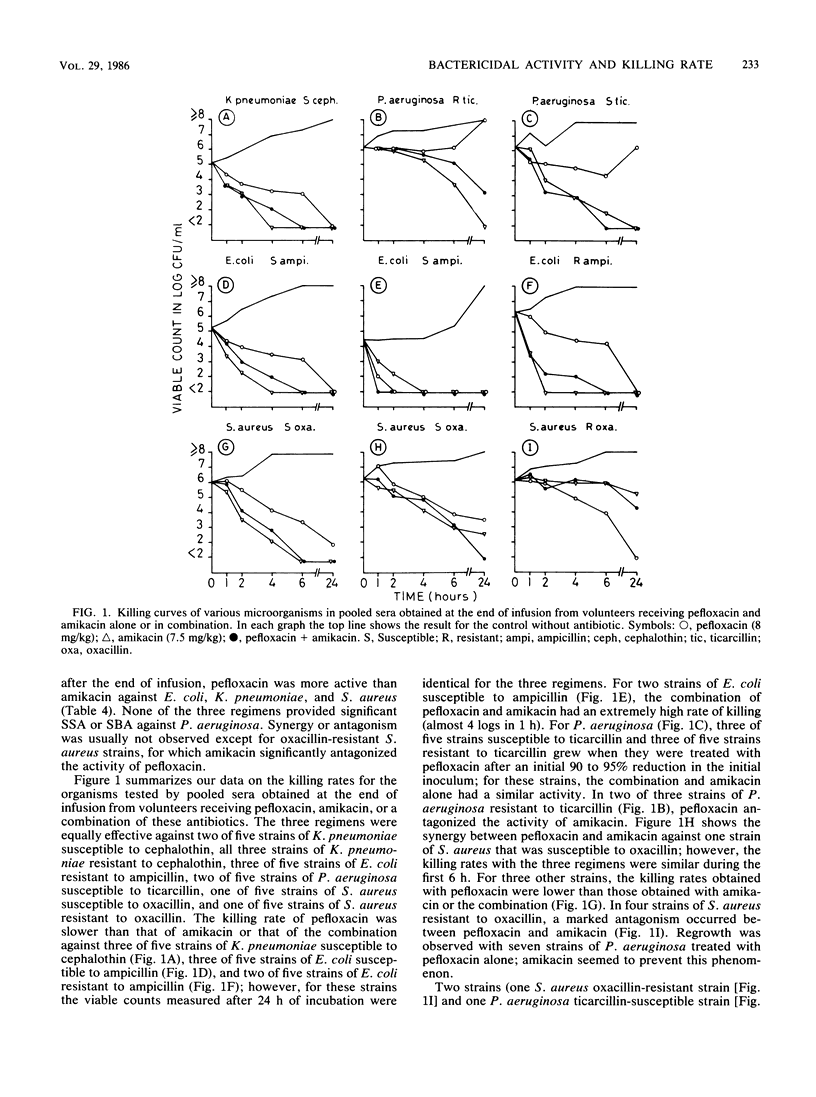
Abstract
Serum bactericidal activities (SBAs) were studied after intravenous administration of pefloxacin (8 mg/kg) and amikacin (7.5 mg/kg) alone or in combination to 15 human volunteers. About 10 strains each of Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus were tested. The serum levels of pefloxacin were measured microbiologically by using E. coli KP 1976-712 as the test organism at 0, 30, 60, 120, and 720 min after infusion; at 0, 30, 60, and 720 min these levels were 7 +/- 1.4, 5 +/- 0.8, 4.5 +/- 0.7, and 2.1 +/- 0.6 mg/liter (mean +/- standard deviation), respectively, with a terminal half-life of 10 h. The serum levels of pefloxacin in the presence of amikacin were measured similarly; 1% sodium polyanethol sulfonate was added to the agar to inactivate amikacin. Treatment with pefloxacin alone resulted in high SBAs against E. coli, K. pneumoniae strains susceptible to cephalothin, and Staphylococcus aureus at the peak concentration; 81 to 100% of the sera had SBAs of greater than or equal to 1:8. However, treatment with pefloxacin alone resulted in low SBAs against K. pneumoniae strains resistant to cephalothin and P. aeruginosa; only 34% of the sera had SBAs of greater than or equal to 1:8. At trough concentrations the percentages of sera with SBAs greater than or equal to 1:8 were 75 to 83% (E. coli), 9 to 27% (K. pneumoniae), 0% (P. aeruginosa), and 10% (S. aureus). The combination of pefloxacin plus amikacin was most often additive; the peak activity was due to amikacin, and the trough activity was due to pefloxacin. Occasionally antagonism occurred with P. aeruginosa, K. pneumoniae, and S. aureus strains. These observations were confirmed by the killing curves in pooled serum obtained at peak and trough levels. Regrowth was observed for seven strains of P. aeruginosa treated with pefloxacin alone; amikacin seemed to prevent this phenomenon.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake T. A., Hackbarth C. J., Sande M. A. Value of serum tests in combined drug therapy of endocarditis. Antimicrob Agents Chemother. 1983 Nov;24(5):653–657. doi: 10.1128/aac.24.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S. C., Bottenbley C. J., Gam K. Use of sodium polyanethol sulfonate to selectively inhibit aminoglycoside and polymyxin antibiotics in a rapid blood level antibiotic assay. Antimicrob Agents Chemother. 1976 Mar;9(3):414–417. doi: 10.1128/aac.9.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley M. E. Fluorescence polarization immunoassay for the determination of therapeutic drug levels in human plasma. J Anal Toxicol. 1981 Sep-Oct;5(5):236–240. doi: 10.1093/jat/5.5.236. [DOI] [PubMed] [Google Scholar]
- Jolley M. E., Stroupe S. D., Schwenzer K. S., Wang C. J., Lu-Steffes M., Hill H. D., Popelka S. R., Holen J. T., Kelso D. M. Fluorescence polarization immunoassay. iii. an automated system for therapeutic drug determination. Clin Chem. 1981 Sep;27(9):1575–1579. [PubMed] [Google Scholar]
- Montay G., Goueffon Y., Roquet F. Absorption, distribution, metabolic fate, and elimination of pefloxacin mesylate in mice, rats, dogs, monkeys, and humans. Antimicrob Agents Chemother. 1984 Apr;25(4):463–472. doi: 10.1128/aac.25.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reller L. B., Stratton C. W. Serum dilution test for bactericidal activity. II. Standardization and correlation with antimicrobial assays and susceptibility tests. J Infect Dis. 1977 Aug;136(2):196–204. doi: 10.1093/infdis/136.2.196. [DOI] [PubMed] [Google Scholar]
- Sculier J. P., Klastersky J. Significance of serum bactericidal activity in gram-negative bacillary bacteremia in patients with and without granulocytopenia. Am J Med. 1984 Mar;76(3):429–435. doi: 10.1016/0002-9343(84)90662-4. [DOI] [PubMed] [Google Scholar]
- Sculier J. P., Weerts D., Klastersky J. Causes of death in febrile granulocytopenic cancer patients receiving empiric antibiotic therapy. Eur J Cancer Clin Oncol. 1984 Jan;20(1):55–60. doi: 10.1016/0277-5379(84)90034-8. [DOI] [PubMed] [Google Scholar]
- Thabaut A., Durosoir J. L. Activité antibactérienne comparée in vitro de la péfloxacine (1589 RB), de l'acide nalidixique, de l'acide pipémidique et de la fluméquine. Pathol Biol (Paris) 1982 Jun;30(6):394–397. [PubMed] [Google Scholar]
- Wise R., Lockley R., Dent J., Webberly M. Pharmacokinetics and tissue penetration of enoxacin. Antimicrob Agents Chemother. 1984 Jul;26(1):17–19. doi: 10.1128/aac.26.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M., Regnier B., Daldoss C., Nkam M., Vachon F. Penetration of pefloxacin into cerebrospinal fluid of patients with meningitis. Antimicrob Agents Chemother. 1984 Sep;26(3):289–291. doi: 10.1128/aac.26.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Auwera P., de Moor G., Lacroix G., Mambour A., Rossion N., Schuyteneer F. In vitro activity of enoxacin compared with norfloxacin and amikacin. Eur J Clin Microbiol. 1985 Feb;4(1):55–58. doi: 10.1007/BF02148662. [DOI] [PubMed] [Google Scholar]



