Abstract
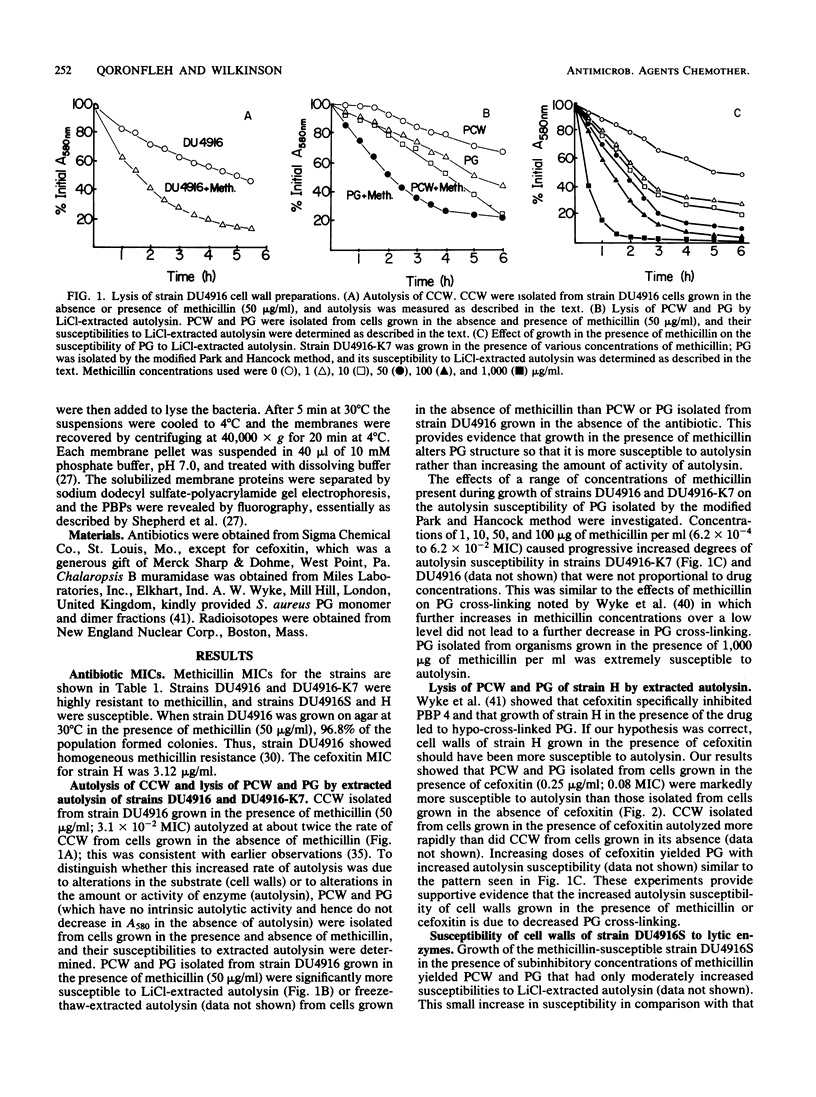
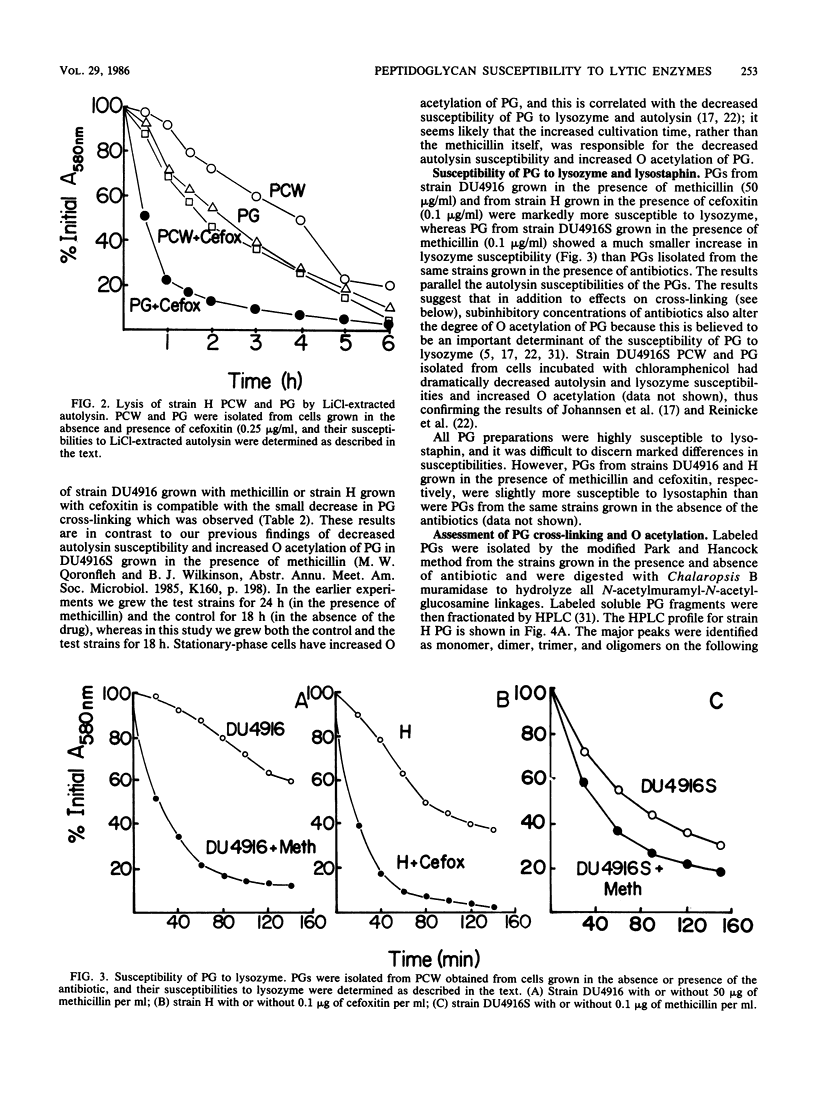
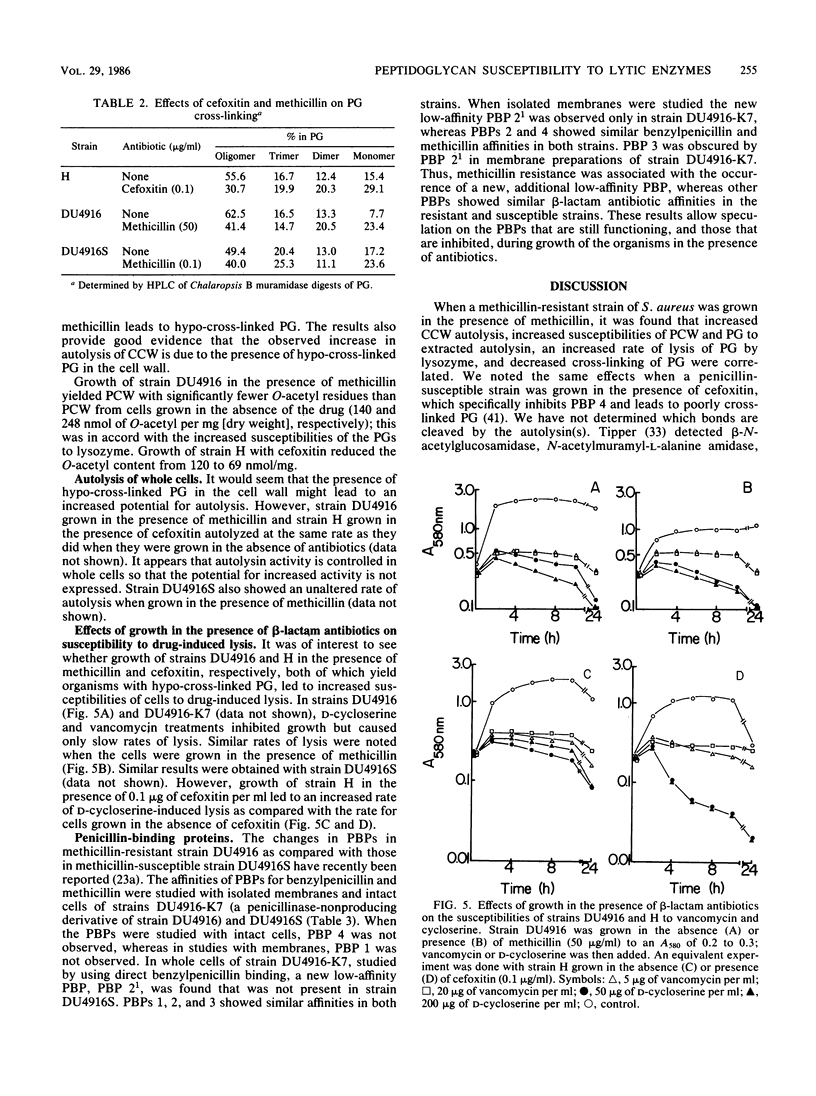
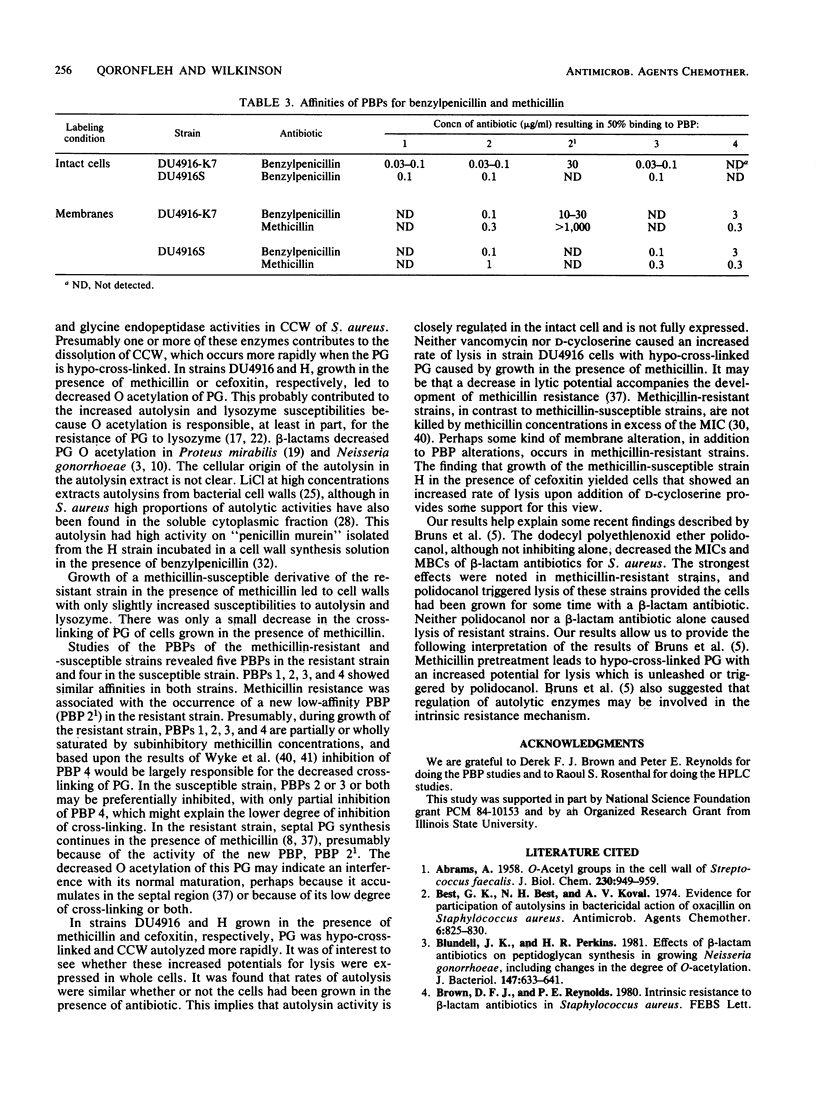
Growth of methicillin-resistant Staphylococcus aureus DU4916 in the presence of methicillin yielded crude cell walls that showed an increased rate of autolysis and purified cell walls (PCW) and peptidoglycan (PG) that had increased susceptibilities to autolysin extracted with LiCl and to lysozyme. The PG of cells grown in the presence of methicillin had markedly decreased cross-linking and O acetylation. Growth of the methicillin-susceptible strain H in the presence of subinhibitory concentrations of cefoxitin, a specific inhibitor of penicillin-binding protein (PBP) 4, caused a substantial decrease in PG cross-linking and O acetylation and increased susceptibilities of PCW and PG to LiCl-extracted autolysin and to lysozyme. Strain DU4916 cells grown in the presence of methicillin did not show an increased rate of autolysis or an increased susceptibility to vancomycin- or D-cycloserine-induced lysis, even though their PG was hypo-cross-linked. This implies that the potential for increased autolysis is controlled in intact cells and that this regulation may be involved in the methicillin resistance phenomenon. Growth of the methicillin-susceptible strain DU4916S in the presence of methicillin yielded PCW and PG that showed small increases in susceptibilities to LiCl-extracted autolysin and to lysozyme and a small decrease in PG cross-linking. Comparison of the PBPs of a penicillinase-nonproducing derivative of strain DU4916 (DU4916-K7) with those of strain DU4916S in intact cells and isolated membranes revealed that PBPs 1 to 4 had similar high beta-lactam antibiotic affinities in both strains and identified an additional PBP, PBP2(1), with low beta-lactam affinity in the methicillin-resistant strain DU4916-K7. The low degree of cross-linking of PG in strain DU4916 cells grown with methicillin was probably due mainly to inhibition of the secondary cross-linking function of PBP 4.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A. O-acetyl groups in the cell wall of Streptococcus faecalis. J Biol Chem. 1958 Feb;230(2):949–959. [PubMed] [Google Scholar]
- Best G. K., Best N. H., Koval A. V. Evidence for participation of autolysins in bactericidal action of oxacillin on Staphylococcus aureus. Antimicrob Agents Chemother. 1974 Dec;6(6):825–830. doi: 10.1128/aac.6.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J. K., Perkins H. R. Effects of beta-lactam antibiotics on peptidoglycan synthesis in growing Neisseria gonorrhoeae, including changes in the degree of O-acetylation. J Bacteriol. 1981 Aug;147(2):633–641. doi: 10.1128/jb.147.2.633-641.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. F., Reynolds P. E. Intrinsic resistance to beta-lactam antibiotics in Staphylococcus aureus. FEBS Lett. 1980 Dec 29;122(2):275–278. doi: 10.1016/0014-5793(80)80455-8. [DOI] [PubMed] [Google Scholar]
- Bruns W., Keppeler H., Baucks R. Suppression of intrinsic resistance to penicillins in Staphylococcus aureus by polidocanol, a dodecyl polyethyleneoxid ether. Antimicrob Agents Chemother. 1985 Apr;27(4):632–639. doi: 10.1128/aac.27.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbusch K., Hallander H. O. Transduction of penicillinase production and methicillin resistance-enterotoxin B production in strains of Staphylococcus aureus. J Gen Microbiol. 1973 May;76(1):1–11. doi: 10.1099/00221287-76-1-1. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Peptidoglycan biosynthesis in Neisseria gonorrhoeae strains sensitive and intrinsically resistant to beta-lactam antibiotics. J Bacteriol. 1983 Jan;153(1):429–435. doi: 10.1128/jb.153.1.429-435.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer D. W., Iandolo J. J. Plasmid-chromosomal transition of genes important in staphylococcal enterotoxin B expression. Infect Immun. 1981 Aug;33(2):450–458. doi: 10.1128/iai.33.2.450-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B., Tomasz A. Altered penicillin-binding proteins in methicillin-resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1981 May;19(5):726–735. doi: 10.1128/aac.19.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff E., Silverman C. S., Adams N. J., Awkard W. S. Extracellular cell wall lytic enzyme from Staphylococcus aureus: purification and partial characterization. J Bacteriol. 1970 Sep;103(3):761–769. doi: 10.1128/jb.103.3.761-769.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin H. H., Gmeiner J. Modification of peptidoglycan structure by penicillin action in cell walls of Proteus mirabilis. Eur J Biochem. 1979 Apr;95(3):487–495. doi: 10.1111/j.1432-1033.1979.tb12988.x. [DOI] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Douglas S. D., Quie P. G., Verhoef J. The key role of peptidoglycan in the opsonization of Staphylococcus aureus. J Clin Invest. 1978 Mar;61(3):597–609. doi: 10.1172/JCI108971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinicke B., Blümel P., Giesbrecht P. Reduced degradability by lysozyme of staphylococcal cell walls after chloramphenicol treatment. Arch Microbiol. 1983 Aug;135(2):120–124. doi: 10.1007/BF00408020. [DOI] [PubMed] [Google Scholar]
- Reynolds P. E., Brown D. F. Penicillin-binding proteins of beta-lactam-resistant strains of Staphylococcus aureus. Effect of growth conditions. FEBS Lett. 1985 Nov 11;192(1):28–32. doi: 10.1016/0014-5793(85)80036-3. [DOI] [PubMed] [Google Scholar]
- Reynolds P. E. Resistance of the antibiotic target site. Br Med Bull. 1984 Jan;40(1):3–10. doi: 10.1093/oxfordjournals.bmb.a071944. [DOI] [PubMed] [Google Scholar]
- Rossi L., Tonin E., Cheng Y. R., Fontana R. Regulation of penicillin-binding protein activity: description of a methicillin-inducible penicillin-binding protein in Staphylococcus aureus. Antimicrob Agents Chemother. 1985 May;27(5):828–831. doi: 10.1128/aac.27.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd S. T., Chase H. A., Reynolds P. E. The separation and properties of two penicillin-binding proteins from Salmonella typhimurium. Eur J Biochem. 1977 Sep;78(2):521–523. doi: 10.1111/j.1432-1033.1977.tb11765.x. [DOI] [PubMed] [Google Scholar]
- Singer H. J., Wise E. M., Jr, Park J. T. Properties and purification of N-acetylmuramyl-L-alanine amidase from Staphylococcus aureus H. J Bacteriol. 1972 Nov;112(2):932–939. doi: 10.1128/jb.112.2.932-939.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley D. W., Wilkinson B. J. Survey of taurine uptake and metabolism in Staphylococcus aureus. J Gen Microbiol. 1983 Aug;129(8):2421–2428. doi: 10.1099/00221287-129-8-2421. [DOI] [PubMed] [Google Scholar]
- Smith P. F., Wilkinson B. J. Differential methicillin susceptibilities of peptidoglycan syntheses in methicillin-resistant Staphylococcus aureus. J Bacteriol. 1981 Nov;148(2):610–617. doi: 10.1128/jb.148.2.610-617.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swim S. C., Gfell M. A., Wilde C. E., 3rd, Rosenthal R. S. Strain distribution in extents of lysozyme resistance and O-acetylation of gonococcal peptidoglycan determined by high-performance liquid chromatography. Infect Immun. 1983 Nov;42(2):446–452. doi: 10.1128/iai.42.2.446-452.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe I., Singer H. J., Wise E. M., Jr, Park J. T. Staphylococcus aureus H autolytic activity: general properties. J Bacteriol. 1970 Apr;102(1):14–19. doi: 10.1128/jb.102.1.14-19.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J. Mechanism of autolysis of isolated cell walls of Staphylococcus aureus. J Bacteriol. 1969 Feb;97(2):837–847. doi: 10.1128/jb.97.2.837-847.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Yamashita N., Konno M. Occurrence of a beta-lactam-inducible penicillin-binding protein in methicillin-resistant staphylococci. Antimicrob Agents Chemother. 1985 May;27(5):851–857. doi: 10.1128/aac.27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Dorian K. J., Sabath L. D. Cell wall composition and associated properties of methicillin-resistant Staphylococcus aureus strains. J Bacteriol. 1978 Dec;136(3):976–982. doi: 10.1128/jb.136.3.976-982.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Kim Y., Peterson P. K. Factors affecting complement activation by Staphylococcus aureus cell walls, their components, and mutants altered in teichoic acid. Infect Immun. 1981 Apr;32(1):216–224. doi: 10.1128/iai.32.1.216-224.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Nadakavukaren M. J. Methicillin-resistant septal peptidoglycan synthesis in a methicillin-resistant Staphylococcus aureus strain. Antimicrob Agents Chemother. 1983 Apr;23(4):610–613. doi: 10.1128/aac.23.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., White P. J. The effect of antibiotics on synthesis of mucopeptide and teichoic acid by Pediococcus cerevisiae and by a substrain that requires methicillin for growth. J Gen Microbiol. 1973 Dec;79(2):195–204. doi: 10.1099/00221287-79-2-195. [DOI] [PubMed] [Google Scholar]
- Wyke A. W., Ward J. B., Hayes M. V., Curtis N. A. A role in vivo for penicillin-binding protein-4 of Staphylococcus aureus. Eur J Biochem. 1981 Oct;119(2):389–393. doi: 10.1111/j.1432-1033.1981.tb05620.x. [DOI] [PubMed] [Google Scholar]



