Abstract
The plasmid determinant of resistance to fosfomycin (For) was cloned into pBR322 and located in a 0.7-kilobase segment of DNA by transposon mutagenesis and in vitro deletion analysis. It encodes an 18-kilodalton protein located in the cytoplasm of resistant cells. Its synthesis is constitutive. The For genetic determinant is common to all plasmids isolated since 1975 in an hospital environment as determined by DNA-DNA hybridization. However, plasmids which carry For can be divided into two groups on the basis of size, pattern of antibiotic resistances, incompatibility specificity, and restriction and hybridization properties.
Full text
PDF
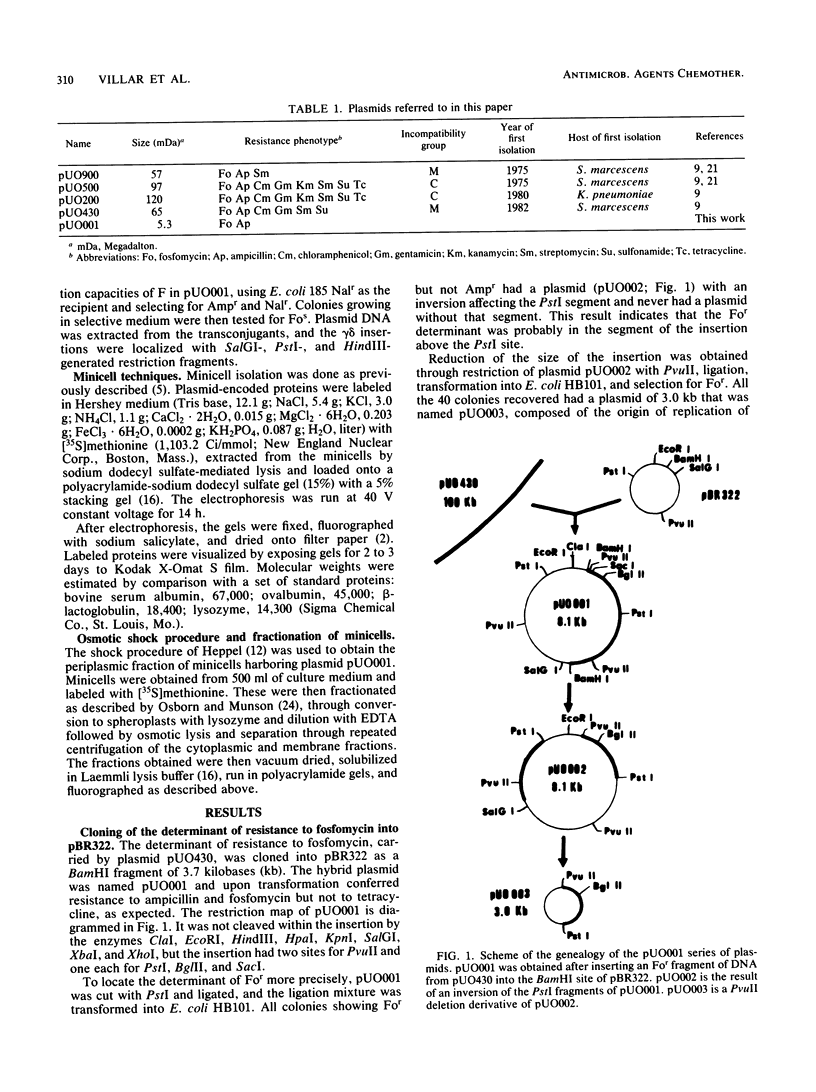



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtieu A. L., Drugeon H., Billaudel S. Susceptibility to fosfomycin of hospital strains isolated in Nantes (France). Frequency of mutation to resistance. Chemotherapy. 1977;23 (Suppl 1):25–36. doi: 10.1159/000222023. [DOI] [PubMed] [Google Scholar]
- Crooks J. H., Ullman M., Zoller M., Levy S. B. Transcription of plasmid DNA in Escherichia coli minicells. Plasmid. 1983 Jul;10(1):66–72. doi: 10.1016/0147-619x(83)90058-6. [DOI] [PubMed] [Google Scholar]
- García-Lobo J. M., León J., Navas J., Ortiz J. M. Cloning and expression in minicells of the determinant of resistance to fosfomycin from the transposon Tn2921. Plasmid. 1984 May;11(3):243–247. doi: 10.1016/0147-619x(84)90030-1. [DOI] [PubMed] [Google Scholar]
- García-Lobo J. M., Ortiz J. M. Tn292l, a transposon encoding fosfomycin resistance. J Bacteriol. 1982 Jul;151(1):477–479. doi: 10.1128/jb.151.1.477-479.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer M. S. The gamma delta sequence of F is an insertion sequence. J Mol Biol. 1978 Dec 15;126(3):347–365. doi: 10.1016/0022-2836(78)90045-1. [DOI] [PubMed] [Google Scholar]
- Hardisson C., Villar C. J., Llaneza J., Mendoza M. C. Prédominance et dispersion des plasmides conférant la résistance á la fosfomycine chez des entérobactéries. Pathol Biol (Paris) 1984 Sep;32(7):755–758. [PubMed] [Google Scholar]
- Havekes L. M., Lugtenberg B. J., Hoekstra W. P. Conjugation deficient E. coli K12 F- mutants with heptose-less lipopolysaccharide. Mol Gen Genet. 1976 Jul 5;146(1):43–50. doi: 10.1007/BF00267981. [DOI] [PubMed] [Google Scholar]
- Hendlin D., Stapley E. O., Jackson M., Wallick H., Miller A. K., Wolf F. J., Miller T. W., Chaiet L., Kahan F. M., Foltz E. L. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science. 1969 Oct 3;166(3901):122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- Kadner R. J., Winkler H. H. Isolation and characterization of mutations affecting the transport of hexose phosphates in Escherichia coli. J Bacteriol. 1973 Feb;113(2):895–900. doi: 10.1128/jb.113.2.895-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan F. M., Kahan J. S., Cassidy P. J., Kropp H. The mechanism of action of fosfomycin (phosphonomycin). Ann N Y Acad Sci. 1974 May 10;235(0):364–386. doi: 10.1111/j.1749-6632.1974.tb43277.x. [DOI] [PubMed] [Google Scholar]
- Kestle D. G., Kirby W. M. Clinical pharmacology and in vitro activity of phosphonomycin. Antimicrob Agents Chemother (Bethesda) 1969;9:332–337. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- León J., García-Lobo J. M., Ortiz J. M. Fosfomycin resistance plasmids do not affect fosfomycin transport into Escherichia coli. Antimicrob Agents Chemother. 1982 Apr;21(4):608–612. doi: 10.1128/aac.21.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llaneza J., Villar C. J., Salas J. A., Suarez J. E., Mendoza M. C., Hardisson C. Plasmid-mediated fosfomycin resistance is due to enzymatic modification of the antibiotic. Antimicrob Agents Chemother. 1985 Jul;28(1):163–164. doi: 10.1128/aac.28.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Mendoza C., Garcia J. M., Llaneza J., Mendez F. J., Hardisson C., Ortiz J. M. Plasmid-determined resistance to fosfomycin in Serratia marcescens. Antimicrob Agents Chemother. 1980 Aug;18(2):215–219. doi: 10.1128/aac.18.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rodríguez A., Gallego A., Olay T., Mata J. M. Bacteriological evaluation of fosfomycin in clinical studies. Chemotherapy. 1977;23 (Suppl 1):247–258. doi: 10.1159/000222055. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suarez J. E., Chater K. F. DNA cloning in Streptomyces: a bifunctional replicon comprising pBR322 inserted into a Streptomyces phage. Nature. 1980 Jul 31;286(5772):527–529. doi: 10.1038/286527a0. [DOI] [PubMed] [Google Scholar]
- Tsuruoka T., Yamada Y. Charactertization of spontaneous fosfomycin (phosphonomycin)-resistant cells of Escherichia coli B in vitro. J Antibiot (Tokyo) 1975 Nov;28(11):906–911. doi: 10.7164/antibiotics.28.906. [DOI] [PubMed] [Google Scholar]