Abstract
Chemical reaction systems operating in nonequilibrium open-system states arise in a great number of contexts, including the study of living organisms, in which chemical reactions, in general, are far from equilibrium. Here we introduce a theorem that relates forward and reverse fluxes and free energy for any chemical process operating in a steady state. This relationship, which is a generalization of equilibrium conditions to the case of a chemical process occurring in a nonequilibrium steady state in dilute solution, provides a novel equivalent definition for chemical reaction free energy. In addition, it is shown that previously unrelated theories introduced by Ussing and Hodgkin and Huxley for transport of ions across membranes, Hill for catalytic cycle fluxes, and Crooks for entropy production in microscopically reversible systems, are united in a common framework based on this relationship.
Introduction
For a reaction occurring in an isothermal and isobaric system the chemical driving force ΔG—the Gibbs free energy difference—characterizes how far a chemical reaction is away from equilibrium. If we take a simple bimolecular reaction in a dilute solution
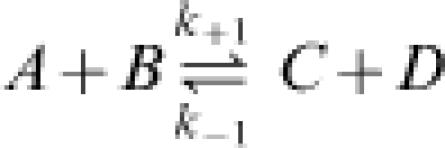 |
as an example, then ΔG is related to the concentrations of the reactants and products, as well as the equilibrium constant Keq, through the well-known thermodynamic equation
If we further assume that the law of mass action governs the reaction's kinetics, then the forward and reverse reaction fluxes and equilibrium constant are
where k +1 and k −1 are constants that do not depend on the concentrations. Combining Equations (2) and (3) yields
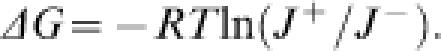 |
Expressing ΔG in terms of Equation (4) has many advantages: It is apparent that if ΔG = 0, then J
+ = J
−. This equilibrium relationship is required by the principle of detailed balance, which states that at equilibrium the forward and reverse fluxes are equal for all existing independent mechanisms for the reaction A B [1]. Furthermore, Equation (4) can be generalized to many other situations. For example, for reversible enzyme reactions governed by Michaelis-Menten kinetics, although both J
+ and J
− are complex, nonlinear functions of reactant and substrate concentrations, Equation (4) still holds true.
B [1]. Furthermore, Equation (4) can be generalized to many other situations. For example, for reversible enzyme reactions governed by Michaelis-Menten kinetics, although both J
+ and J
− are complex, nonlinear functions of reactant and substrate concentrations, Equation (4) still holds true.
Another nontrivial example of Equation (4) that arises in cycle kinetics in unimolecular systems is due to T.L. Hill [2]–[5]. As in the example above, the law of mass action is assumed in all of Hill's work. The novelty of this note is to show a wide range of validity of Equation (4) based solely on conservation of mass, without invoking any assumptions of rate laws such as Equation (3). Hence, Equation (4) is in fact a fundamental relation for any chemical process operating in an open-system steady state.
The relation is also intimately related to the fluctuation theorem [6], [7], [8], [9]. However, the most significant insight from the present work is that the relation between one-way-fluxes and ΔG can be established without any supposition on the dynamics of a system.
Analysis
Flux and Free Energy in a Nonequilibrium Open System
For the reaction A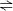 B, the Gibbs free energy change per mole of molecules that transform from state A to state B is expressed
B, the Gibbs free energy change per mole of molecules that transform from state A to state B is expressed
where NA and NB are the number of molecules in states A and B, respectively. In equilibrium, the ratio NB/NA is equal to 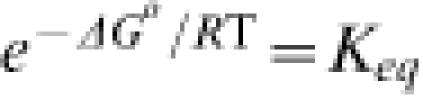 and the net reaction flux is J = J
+−J
− = 0, where J
+ and J
− are the forward and reverse reaction fluxes, respectively. When ΔG<0, the net flux J from A to B is positive.
and the net reaction flux is J = J
+−J
− = 0, where J
+ and J
− are the forward and reverse reaction fluxes, respectively. When ΔG<0, the net flux J from A to B is positive.
To determine how flux and free energy are related for systems not in equilibrium we consider, without loss of generality, the case where NB/NA<Keq and J>0. In a nonequilibrium steady state NA and NB are held constant by pumping A molecules into the system, and pumping B molecules out of the system, at the steady-state flux J.
Next imagine that we are able to place a label on each molecule that converts from state B to state A. These particles we denote by A*. Apart from the label, A* molecules are identical in every way to unlabeled A molecules in this thought experiment. In addition, imagine that A* molecules lose their label when they convert to B molecules. Thus if we continue to pump A and B molecules into and out of the system at the constant flux J, then a steady state will be reached for which NA *, the number of labeled molecules in state A*, is less than or equal to NA, the total number of labeled plus unlabeled molecules in state A.
The steady state is reached when the rate of conversion of labeled A* molecules into state B is equal to the rate of conversion from B to A*. Since there is no transport of A* into or out of the system, then in the steady state the NA
* molecules in state A* will be in equilibrium with the NB molecules in state B: NB/NA
* = Keq. Mass conservation requires that the forward flux of A*→B equal the reverse flux of B→A*, or 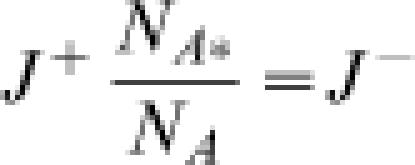 . Combining these equations, we have:
. Combining these equations, we have:
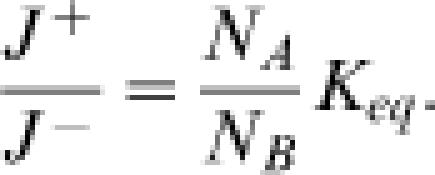 |
This relationships hold for a reaction operating in any steady state, including thermodynamic equilibrium. In equilibrium, J + = J −, and
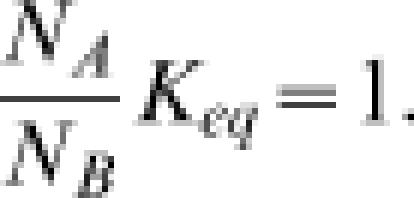 |
Thus it is trivial that Equation (6) holds in equilibrium. The more interesting case is a nonequilibrium steady state for which Equations (5) and (6) yield Equation (4). Therefore Equation (4) is a condition that does not depend on the details of the kinetic reaction mechanism that is operating in a particular system. In addition, the above proof is easily generalized to apply to multimolecular (non-uni-unimolecular) chemical reactions or any spontaneous process transforming or transporting mass from one state to another. Therefore Equation (4) represents a fundamental property of any chemical process in dilute solution.
Relationship to other Theories
Hill Equation for Catalytic Cycles
For the case of a catalytic cycle with J +/J − equal to the ratio of the forward-to-reverse cycle flux and ΔG equal to the thermodynamic driving force for the cycle, Equation (4) is identical to the relationship introduced by Hill [2], [5], [10] and proved by Kohler and Vollmerhaus [11] and by Qian et al. [12] for cycles in Markov systems. (See Equations (3.7) and (7.8) in [5].) Therefore the relationship between J +/J − and ΔG introduced by Hill for linear cycle kinetics is a special case of Equation (4).
As specific example, consider the well known Michaelis-Menten enzyme mechanism:
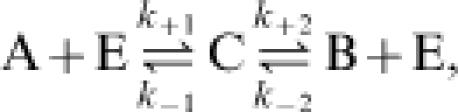 |
in which E is an enzyme involved in converting substrate A into product B. The steady-state flux through this mechanism is
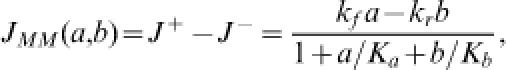 |
where Eo is the total enzyme concentration, a = [A], b = [B], kf = Eok +1 k +2/(k −1+k +2), kr = Eok −1 k −2/(k −1+k +2), Ka = (k −1+k+2)/k +1, and Kb = (k −1+k +2)/k −2. Identifying J + as the positive term and J − as the negative term in Equation (9), it is straightforward to verify that J + and J − satisfy Equation (4), where ΔGo = −RTln(k +1 k +2/k −1 k −2). In application in biochemical enzyme kinetics, it is sometimes assumed that k −2[B] = 0, resulting in the well known Michaelis-Menten equation for an irreversible reaction (see below).
Crooks Fluctuation Theorem
Again, we consider a system made up of molecules that can transition between two states: A B. The system is sustained in a steady-state with constant numbers of A and B; therefore, each transition brings the system back to its starting state in a cyclic fashion. If Γ+ is the mean forward transition rate (number of transitions per unit time) for which the system is driven in the forward direction, then the probability of n forward transitions in a finite time period τ is given by the Poisson distribution:
B. The system is sustained in a steady-state with constant numbers of A and B; therefore, each transition brings the system back to its starting state in a cyclic fashion. If Γ+ is the mean forward transition rate (number of transitions per unit time) for which the system is driven in the forward direction, then the probability of n forward transitions in a finite time period τ is given by the Poisson distribution: 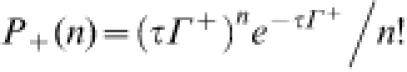 . Likewise, the probability of m reverse transitions is
. Likewise, the probability of m reverse transitions is 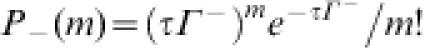 , where Γ– is the reverse transition rate. Hence, the probability of net forward turnover l = m − n is given by
, where Γ– is the reverse transition rate. Hence, the probability of net forward turnover l = m − n is given by
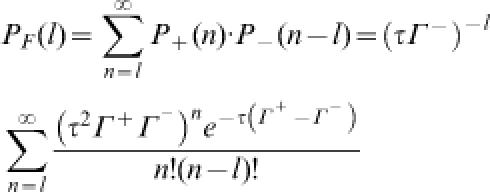 |
and the probability of l net reverse turnovers is
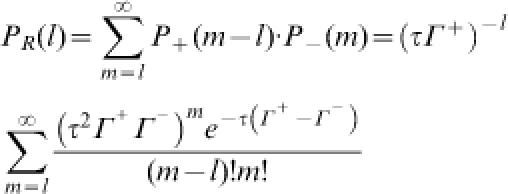 |
Associated with net l forward turnover cycles is the isothermal heat dissipation which is equal to the entropy production (e.p.) of −(lΔG); the net l reverse turnovers have an e.p. of +(lΔG). Therefore, the ratio of the probability of e.p. = σ to the probability of e.p. = −σ, within a finite time interval, is
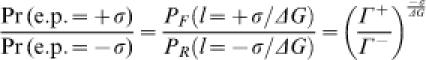 |
Since the chemical flux is proportional to the number of transitions per unit time, Γ+/Γ− = J +/J −. Connecting this result with Equation (4), we have
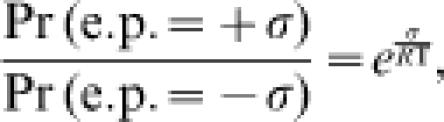 |
which is known as the Crooks fluctuation theorem [6].
Ussing Flux Ratio
When a charged species is transported across a biological membrane, the ionic flux is influenced by any electrostatic potential difference that exists across the membrane. Using the convention that the electrostatic potential across a cell is measured as the outside potential subtracted from inside potential, Equation (4) is expressed
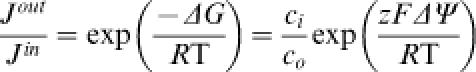 |
for passive transport of a single ion across a cell membrane. Here, ci and co in Equation (14) denote the concentrations of the ion on the inside and outside of the cell, respectively; z is the valence number of the ion; F is Faraday's constant; ΔΨ is the electrostatic potential (defined as inside potential minus outside potential); and Jin and Jout are in inward and outward one-way fluxes. The flux ratio in form of Equation (14) was introduced in 1949 by Ussing [13] for the case of passive transport of single ions and is known as the Ussing flux ratio. Based on the assumption that Jout is independent of co and that Jin is independent of ci, Hodgkin and Huxley derived the same expression in 1952 [14].
The current work shows that the theory of Ussing and Hodgkin and Huxley is a special case of Equation (4). In addition to single-ion channel fluxes for which the Ussing flux ratio has been developed and applied, the flux ratio of Equation (4) applies to all active and passive transport processes as well as multiple-ion transporters.
Additional Consequences
Net Flux for Nearly Irreversible Reactions is Proportional to Reverse Flux
We can study nearly irreversible systems based on Equation (4). The net flux through a chemical process is J = J +−J −; thus, e −ΔG/RT = J/J −+1, which leads to the approximation
 |
for nearly irreversible reactions (J≫J −1). Thus the net flux through an enzyme in a reaction operating far from equilibrium is proportional to the reverse flux.
For the quasi-steady approximation of Equation (9) the reverse flux is J − = krb/(1+a/Ka+b/Kb); thus for a nearly irreversible reaction
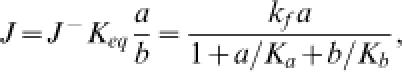 |
which is the expression that we would arrive at by setting kr = 0 in Equation (9). Note that the usual irreversible Michaelis-Menten equation derives from the assumption that k −2 = 0, which results in
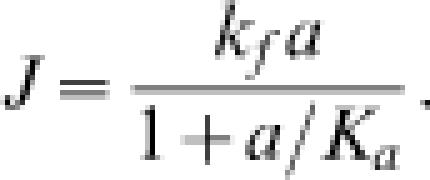 |
This analysis illustrates that the assumption k −2 = 0 is a special case of the irreversible single-substrate enzyme. Equation (16) is the general approximation for the case of |ΔG/RT|≫1, where k −2 may be finite.
Net Flux for Highly Reversible Reactions is Proportional to Reverse Flux
Near equilibrium (for |ΔG|≪RT) the flux can be approximated as linearly proportional to the thermodynamic driving force: J = −XΔG, where X is the Onsager coefficient [15], [16]. When the near-equilibrium approximation |ΔG|≪RT holds, the flux ratio J +/J − is approximately equal to 1. In this case Equation (4) is approximated
From this expression, we have
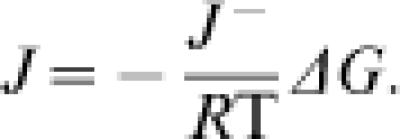 |
Therefore for highly reversible systems, the net flux is proportional to the reverse flux times the thermodynamic driving force; the Onsager coefficient is equal to J −/RT.
Application to Transport Processes
In addition to application to chemical reactions, Equation (4) is directly applied to transport processes. For example, one-dimensional transport of particles in a complex medium is governed by a Fokker-Planck equation with spatially dependent diffusion coefficient D(x) and potential function u(x) [17]:
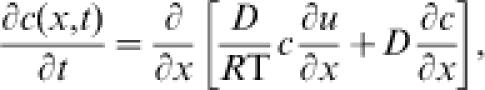 |
over the domain 0≤x≤1. The steady-state transport flux predicted by this equation is [17]
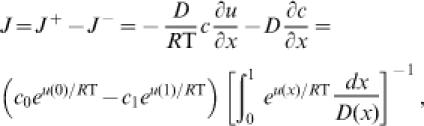 |
where c 0 and c1 are the concentrations of the two reservoirs at x = 0 and x = 1. Recognizing that ΔG = −{u(1)−u(0)+RTln(c 1/c 0)} for this system, we have Equation (4).
Exchange of Isotope Labels
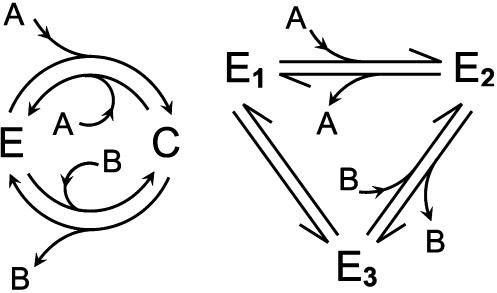
A variety of isotope labeling methods are used to determine in vivo metabolic fluxes. In some cases, it is possible to estimate not only the net flux of a given reaction, but also the forward and reverse rate at which an isotope label exchanges between species involved in a chemical reaction [18]. Consider as examples the enzyme-mediated catalysis schemes for the reaction A B illustrated in Figure 1.
B illustrated in Figure 1.
Figure 1. Example enzyme mechanisms for the reaction A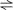 B.
B.
The left panel illustrates the Michaelis-Menten scheme of Equation (8), in which enzyme binds to substrate A, forming a complex C. The product B reversibly dissociates from the complex C, forming unbound enzyme E. The right panel illustrates a more complex mechanism involving three enzyme states E1, E2, and E3. Enzyme kinetic theory assumes that the state transitions follow mass-action kinetics, as described by Equation (8) for the left panel and Equation (23) for the right panel.
For the reversible Michaelis-Menten example of the left panel, which is described by Equations (8) and (9), the exchange flux ratio—the rate at which a label on A molecules is transferred to B molecules divided by the rate at which a label on B molecules is transferred to A molecules is given by Equation (4),
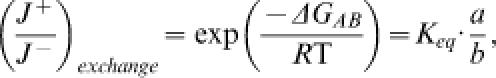 |
where ΔGAB and Keq are the Gibbs free energy and equilibrium constant for the reaction.
For the mechanism illustrated in the right panel of the figure, the exchange flux ratio takes a slightly different form. This enzyme mechanism has the following elementary steps:
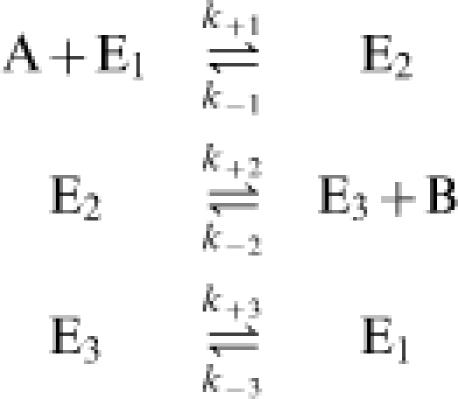 |
where Keq = (k
+1·k
+2·k
+3)/ (k
−1·k
−2·k
−3). The overall flux ratio for the enzyme cycle E1+A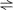 E1+B, has the same form as Equation (22)
E1+B, has the same form as Equation (22)
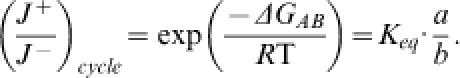 |
The exchange flux ratio follows from applying Equation (4) or (6) to the reaction E1+A E3+B:
E3+B:
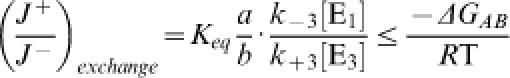 |
where the apparent equilibrium constant for the reaction E1+A E3+B is K = Keq
k
−3/k
+3. The inequality in Equation (25) is for the case that the net flux is positive and therefore k
−3[E1]≤k
+3[E3].
E3+B is K = Keq
k
−3/k
+3. The inequality in Equation (25) is for the case that the net flux is positive and therefore k
−3[E1]≤k
+3[E3].
Discussion
In summary, we have demonstrated that Equation (4) is a fundamental condition that is satisfied by any chemical process operating in a steady state in dilute solution. This equation is a generalization of the well known equilibrium conditions ΔG = 0 and J − = J + to the case of a chemical process occurring in a nonequilibrium steady state, such as a chemical reaction in an open system [19], [20]. It provides a novel equivalent definition for the reaction free energy, or thermodynamic driving force. Based on this relationship, related theories of Ussing [13] and Hodgkin and Huxley [14] for ionic transport across membranes, Hill [2]–[5], [10] for enzyme cycle kinetics, and Crooks [6] for entropy production and work done by microscopically reversible systems, are united in a common framework.
Acknowledgments
We thank J.B. Bassingthwaighte, A. Cornish-Bowden, and M. E. Fisher for valuable comments on the manuscript. Discussions regarding the Ussing flux ratio with James Sneyd were of great value, as were discussions with Wolfgang Wierchert on labeled isotope exchange.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by NIH grant GM0680610.
References
- 1.Lewis GN. A new principle of equilibrium. Proc. Natl. Acad. Sci. USA. 1925;11:179–183. doi: 10.1073/pnas.11.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill TL. Studies in irreversible thermodynamics IV. Diagrammatic representation of steady state fluxes for unimolecular systems. J. Theoret. Biol. 1966;10:442–459. doi: 10.1016/0022-5193(66)90137-8. [DOI] [PubMed] [Google Scholar]
- 3.Hill TL. Free Energy Transduction in Biology. New York, , NY: Academic Press; 1977. [Google Scholar]
- 4.Hill TL. Discrete-time random walks on diagrams (graphs) with cycles. Proc. Natl. Acad. Sci. USA. 1988;85:5345–5349. doi: 10.1073/pnas.85.15.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill TL. Free Energy Transduction and Biochemical Cycle Kinetics. New York, , NY: Dover Publications, Inc; 2004. [Google Scholar]
- 6.Crooks GE. Entropy production fluctuation theorem and the nonequilibrium work relation for free energy differences. Phys. Rev. E. 1999;60:2721–2726. doi: 10.1103/physreve.60.2721. [DOI] [PubMed] [Google Scholar]
- 7.Gallavotti G, Cohen EGD. Dynamic ensembles in nonequilibrium statistical chanics. Phys. Rev. Lett. 1995;74:2694–2697. doi: 10.1103/PhysRevLett.74.2694. [DOI] [PubMed] [Google Scholar]
- 8.Qian H. Nonequilibrium steady-state circulation and heat dissipation functional. Phys. Rev. E. 2001;64:022101. doi: 10.1103/PhysRevE.64.022101. [DOI] [PubMed] [Google Scholar]
- 9.Qian H, Xie, XS Generalized Haldane equation and fluctuation theorem in the steady state cycle kinetics of single enzymes. Phys. Rev. E. 2006;74:010902(R). doi: 10.1103/PhysRevE.74.010902. [DOI] [PubMed] [Google Scholar]
- 10.Hill TL, Chen YD. Stochastics of cycle completions (fluxes) in biochemical kinetic diagrams. Proc. Natl. Acad. Sci. USA. 1975;72:1291–1295. doi: 10.1073/pnas.72.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohler HH, Vollmerhaus E. The frequency of cyclic processes in biological multistate systems. J Math Biol. 1980;9:275–290. doi: 10.1007/BF00276029. [DOI] [PubMed] [Google Scholar]
- 12.Qian C, Qian M, Qian MP. Markov chain as a model of Hill's theory on circulation. Scientia Sinica. 1981;24:1431–1447. [PubMed] [Google Scholar]
- 13.Ussing HH. The distinction by means of tracers between active transport and diffusion. The transfer of iodide across the isolated frog skin. Acta Physiologica Scandinavica. 1949;19:43–56. [Google Scholar]
- 14.Hodgkin AL, Huxley AF. Currents carried by sodium and potassium ions through the membrane of the giant axon of loligo. J. Physiol. 1952;116:449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onsager L. Reciprocal relations in irreversible processes. I. Phys. Rev. 1931;37:405–426. [Google Scholar]
- 16.Onsager L. Reciprocal relations in irreversible processes. II. Phys. Rev. 1931;38:2265–2279. [Google Scholar]
- 17.Risken H. The Fokker-Planck Equation. Methods of Solution and Applications, second edition. New York: Springer; 1996. p. 474 p. [Google Scholar]
- 18.Wiechert W, de Graaf AA. Bidirectional reaction steps in metabolic networks: I. Modeling and simulation of carbon isotope labeling experiments. Biotechnol. Bioeng. 1997;55:101–117. doi: 10.1002/(SICI)1097-0290(19970705)55:1<101::AID-BIT12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Qian H, Beard DA. Thermodynamics of stoichiometric biochemical networks in living systems far from equilibrium. Biophys. Chem. 2005;114:213–220. doi: 10.1016/j.bpc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Qian H, Beard DA, Liang SD. Stoichiometric network theory for nonequilibrium biochemical systems. Eur. J. Biochem. 2003;270:415–421. doi: 10.1046/j.1432-1033.2003.03357.x. [DOI] [PubMed] [Google Scholar]