Abstract
Objective To evaluate the effectiveness of self management as a first line intervention for men with lower urinary tract symptoms.
Design Randomised controlled trial.
Setting A teaching hospital and a district general hospital in London.
Participants 140 men (mean age 63 (SD 10.7) years), recruited between January 2003 and April 2004, referred by general practitioners to urological outpatient departments with uncomplicated lower urinary tract symptoms.
Interventions Self management and standard care (n=73) or standard care alone (n=67). The self management group took part in three small group sessions comprising education, lifestyle advice, and training in problem solving and goal setting skills.
Main outcome measures The primary outcome measure was treatment failure measured at 3, 6, and 12 months. Symptom severity (international prostate symptom score; higher scores represent a poorer outcome) was used as a secondary outcome.
Results At three months, treatment failure had occurred in 7 (10%) of the self management group and in 27 (42%) of the standard care group (difference=32%, 95% confidence interval 18% to 46%). Corresponding differences in the frequency of treatment failure were 42% (27% to 57%) at six months and 48% (32% to 64%) at 12 months. At three months, the mean international prostate symptom score was 10.7 in the self management group and 16.4 in the standard care group (difference=5.7, 3.7 to 7.7). Corresponding differences in score were 6.5 (4.3 to 8.7) at six months and 5.1 (2.7 to 7.6) at 12 months.
Conclusions Self management significantly reduced the frequency of treatment failure and reduced urinary symptoms. Because of the large observed benefit of self management, the results of this study support the case for a large multicentre trial to confirm whether self management could be considered as first line treatment for men with lower urinary tract symptoms.
Trial registration National Research Register N0263115137; Clinical trials NCT00270309.
Introduction
Approximately 10 years ago, Wasson and colleagues showed that watchful waiting was a safe alternative to transurethral resection of the prostate for men with moderately severe lower urinary tract symptoms.1 Since then, standard care for men with lower urinary tract symptoms has developed into a “cascade” that escalates from “watchful waiting” or “active monitoring” through a variety of drugs to either minimally invasive interventions or more traditional forms of surgery.2 3
A recent survey of British healthcare professionals involved in the care of men with lower urinary tract symptoms showed that many routinely advise lifestyle modifications (such as fluid management, avoidance of caffeine, and bladder retraining). However, the type of advice given varied considerably.4 In response to this observed variation, a formal consensus development exercise was carried out to define the lifestyle modifications that are likely to be effective in improving lower urinary tract symptoms.5
Simply informing and advising patients about lifestyle modifications is rarely sufficient to bring about an improvement in health status.6 This has led to the development of interventions that incorporate not only provision of information and advice but also techniques that can help to promote behavioural change. These interventions vary, but most aim to involve patients in the day to day management of their disease by enhancing their problem solving and goal setting skills. Self management interventions that contain these attributes have been shown to be effective for several chronic conditions, such as type 2 diabetes, arthritis, and asthma.6
We developed a self management intervention for men with lower urinary tract symptoms that incorporated the recommendations of the consensus panel.5 The purpose of the programme was to reduce urinary symptoms and to delay or avoid an escalation in treatment. To determine the effectiveness of this intervention, we did a randomised controlled trial in two centres to compare men with lower urinary tract symptoms who participated in a self management programme in addition to standard care with those who received standard care alone.
Methods
Patient population
We recruited men with uncomplicated lower urinary tract symptoms from the outpatient departments of two urological centres in London, a teaching hospital and a district general hospital. We randomised the men either to attend a self management programme in addition to standard care or to standard care alone.
During the recruitment period from January 2003 to April 2004, all patients aged over 40 with lower urinary tract symptoms who were referred for the first time by their general practitioner to one of the two participating urological outpatient departments were eligible for inclusion. We excluded men who had received any form of medical treatment (α blocker, 5 α reductase inhibitor, or anticholinergic drug) in the previous three months or had had previous prostatic surgery or pelvic radiotherapy; men who had severe symptoms necessitating immediate medical or surgical treatment; men with complications potentially related to their symptoms (prostate specific antigen >4 ng/ml, residual volumes >200 ml, creatinine >130 µmol/l, bladder stones, haematuria, urinary retention, or recurrent urinary tract infections); men who were unable to speak or understand English; and men with uncontrolled diabetes, dementia, or end stage cardiac or respiratory failure.
Randomisation
We did the randomisation by telephoning a third party (the secretariat of the Clinical Effectiveness Unit of the Royal College of Surgeons of England) who held a randomisation list. We used computer randomisation without restriction to generate this list, with an equal chance of allocating each patient to the self management group or the standard care group. Owing to difficulties in contacting the third party out of hours, randomisation from the 41st patient included onwards was done by telephoning the secretariats of the urological outpatient departments of the two participating hospitals. All participants were included in the study before the results of the randomisation were available.
Standard care
Standard care in the two participating centres began with watchful waiting. Escalation to medical treatment and surgery was left entirely to the discretion of the clinician and patient. All patients, irrespective of treatment allocation, received standard written information about lower urinary tract symptoms.
Intervention
In addition to standard care, the intervention group took part in small group sessions (five to eight men), each lasting between 1.5 and 2 hours, which were scheduled one, two, and six weeks after randomisation. Two urology nurse specialists facilitated the standardised sessions; they were trained in group facilitation skills and in techniques to enhance self management skills. The aim of these sessions was to bring about modification of lifestyle (for example, fluid management, avoidance of caffeine, and use of alcohol) and specific changes in behaviour (such as bladder retraining, double voiding, and urethral milking). (See appendix on bmj.com as well as reference 5 for a description of the information component of the self management programme.) We designed the sessions to enable the participants to learn techniques of problem solving and goal setting and to receive support from each other by using techniques of brainstorming in the context of group discussions. On request, we can provide the full facilitators' manual as well as information on the essential training of the facilitators of the small group sessions.
At 3, 6, and 12 months, clinicians who were not involved in the conduct of the trial saw participants in the urology outpatient departments. We asked participants not to reveal to the clinicians which treatment group they had been randomised to.
Outcomes
The primary outcome was treatment failure (rise of 3 points or more on the international prostate symptom score, use of drugs to control lower urinary tract symptoms, acute urinary retention, or surgical intervention) during follow-up.7 8 Secondary outcomes included severity of symptoms (international prostate symptom score), troublesomeness of symptoms (benign prostatic hypertrophy impact index), and disease specific quality of life (American Urological Association quality of life score).7 9 We collected information on symptom severity, troublesomeness, and quality of life through self administered questionnaires. We asked patients to complete these questionnaires on each of their clinic visits and to send them by post to a third party for data entry.
Sample size
We estimated that a minimum of 84 men should be included in each group to have a 90% chance of detecting a 3 point reduction in mean international prostate symptom score at a 5% level of significance with a standard deviation of 6. We chose this 3 point reduction in the international prostate symptom score because this is considered to represent an improvement in symptoms that is meaningful to patients.8
Statistical analysis
We analysed outcomes at 3, 6, and 12 months separately on an intention to treat basis. We used means and standard deviations to summarise quantitative variables and proportions to summarise qualitative variables. We used two sided t tests to compare means and χ2 tests to compare proportions seen at 3, 6, and 12 months after randomisation. We did multivariate linear and logistic regression to adjust these comparisons for potential imbalances in the baseline characteristics of the two groups (age, severity of symptoms, duration of symptoms, level of education, and number of comorbidities). We considered results to be statistically significant if the P values were less than 0.05.
Results
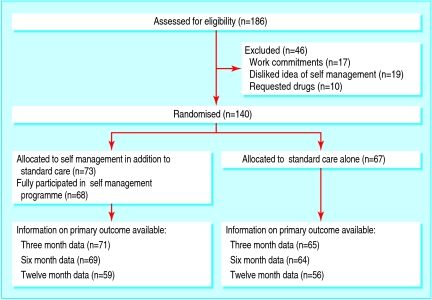
Of the 186 patients who were eligible for randomisation during the recruitment period, 46 were excluded because of work commitments, requests to receive immediate medical treatment, or refusal of self management as a treatment option (figure). Of the 140 men who were included, we randomised 73 to participate in the self management programme and 67 to standard care alone. Compliance with the self management programme was high; 68 (93%) patients attended all three sessions. The five patients who did not attend were included in the self management group for analysis.
Flow diagram of recruitment, randomisation, and follow-up of participants
Baseline characteristics
The distributions of the patient demographics in the self management group and the standard care group were broadly similar (table 1). However, the self management group included more men with a university degree and fewer men with no qualification or only a school or professional qualification. Comorbidity was slightly more frequent in the self management group. The patients in this group also reported slightly more severe prostate symptoms, higher levels of troublesomeness of symptoms, and a poorer quality of life. However, although most patients in the two groups fell in the moderate category of symptom severity, more patients in the standard care group had either mild or severe symptoms.
Table 1.
Patients' characteristics and symptoms at baseline. Values are numbers (percentages) unless stated otherwise
| Self management (n=73) | Standard care (n=67) | |
|---|---|---|
| Mean (SD) age at recruitment (years) | 63.3 (11.1) | 63.4 (10.4) |
| Age range (years) | 42-86 | 40-83 |
| Ethnicity: | ||
| White | 57 (78) | 51 (76) |
| Non-white (black, Asian, other) | 16 (22) | 16 (24) |
| Level of education: | ||
| None | 16 (22) | 22 (33) |
| School or professional qualification | 19 (26) | 26 (39) |
| University degree | 33 (45) | 16 (24) |
| Missing | 5 (7) | 3 (4) |
| No of comorbidities: | ||
| None | 19 (26) | 23 (34) |
| One | 21 (29) | 18 (27) |
| Two | 17 (23) | 15 (22) |
| Three or more | 16 (22) | 11 (16) |
| Mean (SD) duration of symptoms (years) | 3.9 (4.0) | 4.3 (6.7) |
| Mean (SD) IPSS | 16.9 (5.1) | 15.9 (6.5) |
| Symptom severity: | ||
| Mild symptoms (IPSS 1-7) | 0 | 6 (9) |
| Moderate symptoms (IPSS 8-19) | 58 (79) | 43 (64) |
| Severe symptoms (IPSS 20-35) | 15 (21) | 18 (27) |
| Mean (SD) BPH impact index | 5.4 (2.5) | 4.6 (2.6) |
| Mean (SD) AUA-QoL score | 4.0 (1.0) | 3.3 (1.1) |
AUA-QoL=American Urological Association quality of life; BPH=benign prostatic hypertrophy; IPSS=international prostate symptom score.
Primary outcome
At 3, 6, and 12 months, treatment failure was considerably more frequent in patients who were randomised to standard care alone than in those randomised to self management (table 2). The principal reasons for failure were prescription of α blockers or a rise in international prostate symptom score (table 3). Adjustment for baseline characteristics did not change these results. For example, the unadjusted odds ratio for treatment failure at six months was 6.7 (95% confidence interval 3.1 to 14.7), which increased to 11.5 (4.4 to 30) after adjustment for differences in baseline characteristics.
Table 2.
Primary and secondary outcomes at 3, 6, and 12 months. Values are mean (SD) unless stated otherwise
| Self management (n=73) | Standard care (n=67) | Difference (95% CI) | P value | ||||
|---|---|---|---|---|---|---|---|
| Value | No missing | Value | No missing | ||||
| Three month outcomes | |||||||
| Treatment failures (% (No)) | 10 (7) | 2 | 42 (27) | 2 | 32 (18 to 46) | <0.001 | |
| IPSS | 10.7 (5.9) | 2 | 16.4 (5.8) | 3 | 5.7 (3.7 to 7.7) | <0.001 | |
| BPH impact index | 3.3 (2.8) | 2 | 4.7 (2.6) | 3 | 1.4 (0.5 to 2.3) | 0.003 | |
| AUA-QoL score | 2.8 (1.2) | 2 | 3.4 (1.1) | 3 | 0.7 (0.3 to 1.1) | <0.001 | |
| Six month outcomes | |||||||
| Treatment failures (% (No)) | 19 (13) | 4 | 61 (39) | 3 | 42 (27 to 57) | <0.001 | |
| IPSS | 10.4 (6.1) | 6 | 16.9 (6.4) | 6 | 6.5 (4.3 to 8.7) | <0.001 | |
| BPH impact index | 3.5 (2.9) | 7 | 4.8 (2.8) | 6 | 1.4 (0.4 to 2.4) | 0.008 | |
| AUA-QoL score | 2.6 (1.3) | 6 | 3.3 (1.4) | 6 | 0.6 (0.2 to 1.1) | 0.008 | |
| Twelve month outcomes | |||||||
| Treatment failures (% (No)) | 31 (18) | 14 | 79 (44) | 11 | 48 (32 to 64) | <0.001 | |
| IPSS | 10.2 (6.1) | 20 | 15.4 (6.6) | 16 | 5.1 (2.7 to 7.6) | <0.001 | |
| BPH impact index | 3.0 (3.3) | 18 | 4.3 (2.9) | 16 | 1.2 (0 to 2.4) | 0.04 | |
| AUA-QoL score | 2.6 (1.3) | 19 | 3.1 (1.2) | 15 | 0.5 (0 to 1.0) | 0.03 | |
AUA-QoL=American Urological Association quality of life; BPH=benign prostatic hypertrophy; IPSS=international prostate symptom score.
Table 3.
Reasons for treatment failure at 3, 6, and 12 months. Values are numbers of patients
| Self management | Standard care | |
|---|---|---|
| Treatment failures at three months | 7 | 27 |
| Reasons for treatment failures at three months: | ||
| α blocker | 3 | 12 |
| Finasteride | 0 | 0 |
| Anticholinergic | 1 | 1 |
| Surgery | 1 | 1 |
| Catheterised for acute urinary retention | 0 | 2 |
| Other drug treatment | 0 | 0 |
| Rise in IPSS of ≥3 points | 4 | 20 |
| Treatment failures at six months | 13 | 39 |
| Reasons for failures between three and six months: | ||
| α blocker | 0 | 4 |
| Finasteride | 0 | 1 |
| Anticholinergic | 3 | 0 |
| Surgery | 0 | 0 |
| Catheterised for acute urinary retention | 0 | 0 |
| Other drug treatment | 0 | 1 |
| Rise in IPSS of ≥3 points | 3 | 6 |
| Treatment failures at 12 months | 18 | 44 |
| Reasons for failures between six and 12 months: | ||
| α blocker | 3 | 2* |
| Finasteride | 1 | 0 |
| Anticholinergic | 0 | 0 |
| Surgery | 0 | 0 |
| Catheterised for acute urinary retention | 0 | 0 |
| Other drug treatment | 0 | 0 |
| Rise in IPSS of ≥3 points | 1 | 4* |
IPSS=international prostate symptom score.
*One patient was prescribed an α blocker and had a rise in IPSS of ≥3 points.
For a few patients, information on treatment failure was not available at three and six months. However, considerably more patients did not provide information on treatment failure at 12 months. The results in table 2 represent patients with complete information. When we imputed missing values by using results of the previous measurements (three month results imputed with baseline values, six month results with three month values, and 12 months results with six month values), only small changes occurred in the differences between the two groups and these differences remained statistically significant.
Secondary outcomes
At 3, 6, and 12 months, patients who were randomised to self management had less severe symptoms than patients randomised to standard care alone (table 2). The differences in international prostate symptom score increased only slightly when we adjusted them for baseline characteristics. For example, the difference at six months increased from 6.5 (4.3 to 8.7) without adjustment to 7.5 (5.7 to 9.4) with adjustment. Patients who were randomised to self management were also less troubled by their symptoms and had a better quality of life than patients who were randomised to standard care alone (table 2). When we imputed missing values by using results of the previous measurements, the differences in symptom severity, troublesomeness, and quality of life remained statistically significant at 3, 6, and 12 months.
Discussion
Self management in addition to standard care significantly reduced the rate of treatment failure and improved urinary symptoms, compared with standard care alone. The large differences in the rates of treatment failure show the potential effectiveness of this intervention. The difference in symptoms (six international prostate symptom score points) between the treatment groups is twice as large as that seen when medical treatment is compared with placebo.2 The benefits of self management were seen early and were sustained at six and 12 months, which shows that our results do not depend on the timing of the follow-up measurements.
Methodological considerations
Two practical problems confronted us during the trial. Firstly, patient recruitment was slower than anticipated, and at the end of the recruitment period only 140 patients were included, 28 fewer than the minimum number indicated by the power calculation. Fortunately, the evidence that we found for an effect of self management is so strong that this lower sample size does not affect our conclusions. Secondly, contacting the central randomisation office out of hours was sometimes difficult. As a consequence, we changed the randomisation procedure halfway through the recruitment period, and randomisation was then done within the participating hospitals. However, we made sure that the randomisation lists were never available to the investigators involved in the recruitment of patients (CTB and TY) and that patients were formally included before the outcome of the randomisation was made available to the investigators recruiting the patients.
A further methodological problem is that the treatment of men in the standard care group may have been contaminated or influenced in a systematic way because of the conduct of the trial. This contamination, which would have reduced the difference between the self management and standard care groups, may have occurred either through changes in the advice the clinicians gave to their patients or through direct communication between patients allocated to different groups. However, the crucial difference between the two treatment groups is not so much the educational content of the programme but the problem solving and goal setting skills that patients acquired as a result of attending the programme. These complex skills are unlikely to have been conferred during the clinical consultation or transferred from one patient to another. Furthermore, we kept the opportunities for the two treatment groups to interact to a minimum by running the self-management sessions in places other than the clinic and on days when no urology consulting took place.
Lack of blinding of patients to the treatment allocation is another source of bias. However, we asked patients not to reveal their allocation to the clinicians doing the consultations after randomisation. Even if clinicians knew to which group a patient had been randomised, the effect of this lack of blinding would be small because the self management programme was carried out only during the first six weeks and the first clinical visit took place at least six weeks after the last session of the programme. By that time, the severity and troublesomeness of symptoms expressed by the patients will have determined treatment decisions rather than the clinicians' awareness of whether a patient had participated in the self management programme. An additional argument against the influence of clinicians knowing whether patients were randomised to the self management group is that we saw large differences in symptom severity and all other outcomes reported by the patients themselves.
Explanations for observed results
Our study indicates that self management may be a very effective treatment for men with lower urinary tract symptoms. A possible explanation is that the influences of lifestyle modification for lower urinary tract symptoms are immediately apparent to patients, in contrast to the delayed and less apparent effects that are associated with lifestyle modifications for other chronic diseases, such as diabetes, chronic obstructive airway disease, and osteoarthritis. For example, drinking less in the evening, avoiding caffeinated drinks, and voiding twice within a short period before going to bed will have a noticeable effect on voiding patterns that same night. This immediacy of effect provides positive feedback. Furthermore, patients can try out the effects of the different lifestyle modifications and adapt them according to their individual circumstances. In this way, patients quickly become experts on their own condition.
Severity of symptoms in the standard care group remained more or less the same compared with baseline. This apparent lack of effect of standard care in our study contributes to the large difference in symptom severity between the two treatment groups. We would have expected an improvement in symptoms with standard care either through “regression to the mean” or as a result of some of the men being offered medical or surgical treatment. However, the impact of regression to the mean is likely to be small in our study. In contrast to most other studies in this area, symptom severity was not one of our inclusion criteria.10 Moreover, the time interval between referral and recruitment was long (four to six months) and variable, which will have diminished the link between the severity of symptoms at the time of referral and at the time of recruitment. Furthermore, only about 20% of the men who received standard care alone were given medical or surgical treatment, and the rest remained untreated.
A further possible explanation is that the observed results could be the effect of two exceptionally competent and committed facilitators of the small group sessions. However, the facilitators had received specific training and they used a facilitators' manual to standardise the delivery of the intervention, which should strengthen the generalisability of the results. Nevertheless, a multicentre pragmatic randomised controlled trial needs to be done to determine whether our results can be replicated in everyday clinical practice.11
Conclusion
The results from this small two centre study are sufficiently impressive that a policy of self management as described in this paper has the potential to become the ideal first line treatment for men with uncomplicated lower urinary tract symptoms, provided that further studies show their generalisability. An additional argument for this conclusion is that the only imaginable harm that can result from self management is that for some patients medical or surgical treatment is postponed.
What is already known on this topic
Standard treatment for men with lower urinary tract symptoms has developed into a “cascade” that starts with watchful waiting and moves up to drugs and surgery
Self management interventions that aim to enhance patients' problem solving and goal setting skills have been shown to be an effective treatment for arthritis, diabetes, and asthma.
What this study adds
Self management was at least as effective as medical treatment for men with uncomplicated lower urinary tract symptoms
Supplementary Material
We thank all the men who agreed to take part in this trial. We also acknowledge the work of Jane Coe and Daphne Colpman, urology nurse specialists, University College London Hospitals NHS Foundation Trust, for acting as facilitators to the self management groups.
Contributors: ME, CTB, SPN, and JvdM conceived and designed the study. CTB and ME defined the lifestyle modifications, and SPN, LS, and KM developed the format of the small group sessions to enhance self management skills. CTB, TY, and LR coordinated the data collection. DAC analysed the data, supported by CTB, TY, and JvdM. JvdM and ME wrote the manuscript, with contributions from all authors. JvdM and ME are the guarantors.
Funding: BUPA Foundation Project Grant. CTB received a research fellowship from the Royal College of Surgeons of England, funded by Cazenove & Co. JvdM is funded by a national public health career scientist award from the Department of Health and NHS R&D Programme.
Competing interests: ME has received fees from GlaxoSmithKline and Sanofi-Synthelabo for acting as a consultant, giving lectures, and working as an investigator. All other authors: none declared.
Ethical approval: Medical research ethics committees of the participating hospitals.
References
- 1.Wasson JH, Reda DJ, Bruskewitz RC, Elinson J, Keller AM, Henderson WG. Comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. N Engl J Med 1995:332:75-9. [DOI] [PubMed]
- 2.American Urological Association. Guidelines on the management of benign prostatic hyperplasia. 2003. www.auanet.org/guidelines/bph.cfm (accessed 15 Dec 2005).
- 3.Madersbacher S, Alivizatos G, Nordling J, Rioja Sanz C, Emberton M, De la Rosette JJMCH. EAU 2004 guidelines on assessment, therapy and follow-up of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (BPH guidelines). Eur Urol 2004;46:547-54. [DOI] [PubMed] [Google Scholar]
- 4.Brown CT, Van der Meulen J, Mundy AR, Emberton M. Lifestyle and behavioural interventions for men on watchful waiting with uncomplicated lower urinary tract symptoms: a national multidisciplinary survey. BJU Int 2003;92:53-7. [DOI] [PubMed] [Google Scholar]
- 5.Brown CT, Van der Meulen J, Mundy AR, O'Flynn E, Emberton M. Defining the components of a self-management programme for men with uncomplicated lower urinary tract symptoms: a consensus approach. Eur Urol 2004;46:254-63. [DOI] [PubMed] [Google Scholar]
- 6.Newman S, Steed L, Mulligan K. Self-management interventions for chronic illness. Lancet 2004;364:1523-37. [DOI] [PubMed] [Google Scholar]
- 7.Barry MJ, Fowler FJ Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 1992;148:1549-57. [DOI] [PubMed] [Google Scholar]
- 8.Barry MJ, Williford WO, Chang Y, Machi M, Jones KM, Walker-Corkery E, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 1995;154:1770-4. [DOI] [PubMed] [Google Scholar]
- 9.Barry MJ, Fowler FJ Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK. Measuring disease-specific health status in men with benign prostatic hyperplasia. Med Care 1995;33:135-45. [PubMed] [Google Scholar]
- 10.Sech SM, Montoya JD, Bernier PA, Barnboym E, Brown S, Gregory A, et al. The so-called “placebo effect” in benign prostatic hyperplasia treatment trials represents partially a conditional regression to the mean induced by censoring. Urology 1998;51:242-50. [DOI] [PubMed] [Google Scholar]
- 11.Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.