Abstract
Ninety ejaculates from a total of 76 AI boars were extended in Beltsville Thawing Solution (BTS). Boar identity, breed, weight of the ejaculate and sperm concentration were registered. Motility and acrosome integrity were assessed after storage at 16–18°C for 6, 30, 54, 78, and 102 h. Storage time had a significant influence on both motility (p < 0.01) and acrosome integrity (p < 0.001). The Least Square Means for percentage of motility showed a small decline from 79.8% after 6 h of storage to 78.4% at 102 h. Motility at 78 and 102 h was significantly different from motility at 6 h (p < 0.05). The percentage of sperm cells with normal acrosomes declined throughout the experiment. The Least Square Means for 6, 30, 54, 78, and 102 h of storage were 93.9%, 90.6%, 88.0%, 84.8%, and 78.2%, respectively. The decrease in acrosome integrity from one storage time to the next was highly significant throughout the trial (p < 0.001). There was a significant influence of boar (p < 0.001) and sperm concentration (p < 0.01) on motility, while acrosome integrity was affected only by boar (p < 0.001). Breed of the boars and weight of the ejaculate did not influence the dependent variables.
Keywords: longtime storage, sperm concentration
Introduction
In Norway, artificial insemination (AI) in pigs is performed with liquid semen extended in Beltsville Thawing Solution (BTS) [1]. Extended semen is distributed to the whole country from one single AI-centre. Due to large geographical distances more than 70% of AI is performed with semen stored for 24 to 60 h [2]. It is, however, a fact that there is considerable variation among boars concerning the fertilizing capacity of semen during storage [20].
There are, on the other hand, several other factors which might influence fertility of stored semen. Individual variation concerning the chemical composition of the ejaculate as well as the amount of seminal plasma might be of importance. Seminal plasma is important for progressive motility of sperm cells. Spermatozoa gain motility during ejaculation as pH and bicarbonate concentration increase during mixing of sperm and seminal plasma [17]. Further, transfer of sperm cells from seminal plasma to artificial media has shown to decrease motility and increase sperm agglutination [8], which indicates that seminal plasma might be of importance to protect membranes and maintain fertilizing capacity during storage. It has also become evident that seminal plasma is of importance during the process of fertilization. In the female, seminal plasma has a regulatory function on the time of ovulation [23]. It has been demonstrated that intrauterine infusion of seminal plasma prior to AI increases fertilization rate [16,22], probably by enhancing passive sperm transport [16,24].
Sperm concentration affects the amount of seminal plasma surrounding each spermatozoa, both in raw and extended semen. As sperm concentration increases, the amount of seminal plasma per sperm cell decreases.
The aim of the present investigation was to study the influence of boar, breed of the boar, sperm concentration and weight of the ejaculate on motility and acrosome integrity of liquid boar semen stored in BTS for 5 days.
Materials and methods
Animals and semen preparation
Ninety ejaculates from a total of 76 AI boars, aged between 12 and 24 months, were allocated to the trial. The ejaculates were obtained by including all AI boars scheduled for semen production 2 consecutive Mondays, including boars of Norwegian Landrace (40), Duroc (12), Yorkshire (10) and Duroc/Landrace (14) breeds. From each boar the sperm-rich fraction was collected using the gloved hand method. Shortly after collection, the semen was filtered through gauze. Sperm concentration, determined by use of a Coulter counter [13], and weight of the ejaculate were registered. Initial extension with BTS (30°C) to approximately one third of the final volume was performed within 15 min after semen collection. The final extension, using BTS (28°C) was performed within one h after the initial extension. The volume of one insemination dose was 80 ml, and the total number of spermatozoa was estimated to be 2.7 × 109. One AI dose was divided into 5 aliquots, and stored at 16–18°C in closed plastic flasks until examination.
Semen quality control
Semen samples were reactivated in a water bath at 35°C for 30 min before examination, which were performed 6, 30, 54, 78, and 102 h after semen collection. The examination after 6 h storage determined the initial point of the trial. Motility was assessed simultaneously but independently by 2 different examiners throughout the trial, using a phase contrast microscope at 100 × magnification and a heating stage (35°C). Three different fields in 2 droplets of semen from each sample were examined, giving 6 motility estimates per examiner for each ejaculate. The average of all estimates per ejaculate was used for the data analysis. The motility was expressed as percentage of progressively motile spermatozoa.
Simultaneously with the motility assessment, semen smears from each sample were prepared for determination of acrosomal status using the dichromatic Spermac® stain [11,14]. After staining, each sample was examined under oil immersion and 1000 × magnification using a bright field microscope. From each smear a total of 100 spermatozoa were evaluated for acrosome integrity. The sperm cells were assessed as having normal or altered acrosomes [11,12]. The acrosomal status was expressed as percentage of sperm cells with normal acrosomal morphology.
Statistical analysis
Analysis of variance was applied to determine possible effects of storage time, breed, boar within breed, weight of the ejaculate and sperm concentration on semen quality assessments. Data on motility and acrosome integrity assessments were analyzed by the general linear-models procedure (SAS). The full statistical model was as follows:
yijk = β0 + Ti + Bj + bjk + β1Wjk + β2Cjk + eijk
where:
yijk = observation of motility and acrosome integrity on boar jk at storage time i
β0 = a constant (intercept)
Ti = fixed effect of storage time i, i = 6, 30, 54, 78 and 102 h
Bj = fixed effect of breed j, j = D, L, L×D and Y
bjk = random effect of boar k within breed j, ~N(0, σb2)
Wjk = weight of ejaculate of boar jk
Cjk = sperm concentration, ejaculate of boar jk
β1, β2 = regression coefficients
eijk = random error associated with observation ijk, ~N(0, σe2)
A backward stepwise elimination procedure was applied until all remaining effects in the model were significant at 0.05 level.
Results
Average sperm concentration and weight of the ejaculates for each breed are shown in Table 1. The results of motility and acrosome integrity assessments are shown as Least Square Means (LS Mean) with Standard Error (SE) in Fig. 1. After 6 h of storage LS Mean for percentage of motile spermatozoa was 79.8%. There was no significant decline when semen was stored for 30 and 54 h, the LS Mean values being 79.3% and 80.1%, respectively. When semen was stored for 78 and 102 h the corresponding values were 78.3% and 78.4%, respectively, representing a statistically significant drop in motility compared to that of semen stored for 6 h (p < 0.05).
Table 1.
Weight of the ejaculate, sperm concentration and number of boars and ejaculates for each of the breeds included in the investigation.
| Breed | No of boars | No of ejaculates | Weight of the ejaculates (g) Mean ± SD | Sperm concentration (106/ml) Mean ± SD |
|---|---|---|---|---|
| Duroc | 12 | 16 | 147 ± 47 | 133 ± 49 |
| Landrace | 40 | 47 | 265 ± 77 | 86 ± 32 |
| Duroc/Landrace | 14 | 16 | 253 ± 72 | 100 ± 21 |
| Yorkshire | 10 | 11 | 245 ± 99 | 117 ± 35 |
Figure 1.
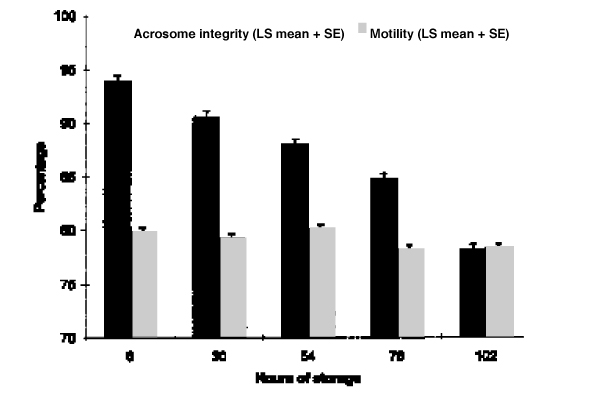
Percentage of motile sperm cells and acrosome integrity expressed as percentage of sperm cells with normal acrosomal morphology after 6, 30, 54, 78 and 102 hours storage of 90 boar ejaculates. Least Square Means (LS Mean) with Standard Error (SE) are shown.
The acrosome integrity expressed as percentage of sperm cells with normal acrosome morphology showed a clear decline throughout the experiment. LS Means for 6, 30, 54, 78, and 102 h storage were 93.9%, 90.6%, 88.0%, 84.8%, and 78.2%, respectively. The decrease in acrosome integrity from one storage time to the next was highly significant throughout the trial (p < 0.001).
The storage time had a highly significant influence on both motility (p < 0.004) and acrosome integrity (p < 0.001), as was the case for the effect of boar within breed (p < 0.0001). Sperm concentration affected motility significantly (p < 0.004), the coefficient of regression being -0.04 giving 0.04% reduction in motility per 4 × 106 cells/ml increase in sperm concentration. There was on the other hand no effect of either breed or weight of the ejaculate on motility and acrosome integrity, and no effect of sperm concentration on acrosome integrity. The level of significance for the influence of storage time, breed, boar, sperm concentration and weight of the ejaculate on motility and acrosome integrity is given in Table 2.
Table 2.
Summary of analysis of variance for motility and acrosome integrity of sperm cells diluted and stored for 5 days in BTS.
| Source of variation | Degree of freedom | Level of significance Motility | Level of significance Acrosome integrity |
|---|---|---|---|
| Storage time (days) | 4 | 0.0039 | 0.0001 |
| Breed | 3 | 0.8 | 0.38 |
| Boar within breed | 72 | 0.0001 | 0.0001 |
| Sperm concentration | 1 | 0.0038 | 0.13 |
| Weight of ejaculate | 1 | 0.43 | 0.29 |
Discussion
This investigation demonstrates that semen quality was gradually reduced during 102 h of storage, a result which is in compliance with other investigations [15,20]. Even though there was a small but significant reduction in motility at the end of the experimental period, the percentage of motile spermatozoa was maintained at a surprisingly high level even after five days storage. There was, however, a subjective impression that the sperm movement changed character during storage, but there was no registration of such parameters in this experiment. Motility is important for semen quality. However, motility alone does not secure fertilizing capacity. Spermatozoa also need intact acrosomes to penetrate the barriers around the ovum. The results from the trial indicate that the acrosome is more susceptible to damage during storage than the organelles being the structural basis of motility. This presumption is in accordance with experiments performed by [3] stating that the decrease of membrane fluidity during storage is greater for head plasma membranes than for sperm body membranes. This is not surprising as storage of diluted semen to some extent may cause sperm capacitation possibly followed by acrosome-reaction [19]. The decrease in acrosome integrity might thus be due to acrosome reaction in addition to membrane damage. When looking at semen quality during storage one should not put too much emphasis on motility estimates alone, but also give attention to other quality parameters to get as close to fertilizing capacity as possible [10].
Looking at the different factors that might have an influence on motility and acrosomal integrity during storage, this study reveals a significant influence of boar. The influence was evident for both motility and acrosome integrity. On the other hand, the dependent variables were not affected by breed of the boars. These results reveal that there is individual variation among boars concerning preservation of semen quality during storage, and that there seems to be no such variation between the breeds investigated in this trial. There might be variation in intrinsic properties of the membranes, possibly being of significance to sperm membrane functionality [6] which might explain the differences between individuals.
There is no influence of weight of the ejaculate on the semen quality parameters investigated during storage in this trial. The sperm concentration seems, however, to play an important role for motility but not for acrosome integrity during storage. The fact that the regression coefficient for sperm concentration in the statistical analysis is negative, demonstrates that the motility is maintained at a higher level during storage when sperm concentration in undiluted semen is low compared to higher sperm concentration. This suggests that there is a positive effect of increasing amount of seminal plasma and furthermore that there might be components in seminal plasma which are beneficial for maintenance of motility, and that the concentration of these components after extension might be important. This presumption is in accordance with results from a study comparing 2 extenders for long-term storage of boar semen, showing one extender to give fecundity of sperm cells superior to the other [9]. The positive effect of additional seminal plasma on viability of bull spermatozoa during extreme extention has been demonstrated by [7]. These investigations indicate that the composition of the surrounding environment is important in order to preserve fertilizing capacity of the sperm cells during storage.
Further, the positive effect of seminal plasma in this trial seems to be limited to motility alone, and does not seem to give any protection to the acrosome membrane during storage. In humans, cholesterol in seminal plasma is claimed to inhibit spermatozoa from undergoing acrosome reaction and to improve survival [4]. The membranes of boar spermatozoa consists of low relative amounts of cholesterol, particularly in comparison to humans [21,5], and one might expect corresponding conditions in seminal plasma. The possible low cholesterol content of seminal plasma, which is even reduced during semen extension, could explain why there seems to be no influence of sperm concentration on preservation of acrosome integrity during storage.
The results of this investigation reveal that the effect of boar is of great importance concerning semen quality during longtime storage. Further, there seems to be a beneficial effect of increasing amount of seminal plasma on motility. Further investigation should be done to compare sperm concentration with field fertility data in order, to some extent, to predict individual differences concerning preservation of semen quality during storage of liquid semen.
References
- Aalbers JG, Rademaker JHM, Grooten HJG, Johnson LA. Fecundity of boar semen stored in BTS, Kiev, Zorlesco, and Modena extenders under field conditions. J Anim Sci. 1983;57(Suppl 1):314–315. [Google Scholar]
- AnonymousAnnual fertility statistics in Norway. Norwegian Pigbreeders' Association, Hamar. 1999.
- Buhr MM. Preservation of boar sperm alters membrane molecular dynamics. Proc 2nd Int Conf Boar Semen Preserv, Beltsville. 1990. pp. 81–93.
- Cross NL. Human seminal plasma prevents sperm from becoming acrosomally responsive to the agonist, progesterone: cholesterol is the major inhibitor. Biol Reprod. 1996;54:138–145. doi: 10.1095/biolreprod54.1.138. [DOI] [PubMed] [Google Scholar]
- De Leeuw FE, Colenbrander B, Verkleij AJ. The role membrane damage plays in cold shock and freezing injury. Proc 2nd Int Conf Boar Semen Preserv, Beltsville. 1990. pp. 95–104.
- Gadella BM, Flesch FM, van Golde LM, Colenbrander B. Dynamics in the membrane organization of the mammalian sperm cell and functionality in fertilization. Vet Q. 1999;21(4):142–146. doi: 10.1080/01652176.1999.9695009. [DOI] [PubMed] [Google Scholar]
- Garner DL, Thomas CA, Gravance CG, Marshall CE, DeJarnette JM, Allen CH. Seminal plasma addition attenuates the dilution effect in bovine sperm. Theriogenology. 2001;56(1):31–40. doi: 10.1016/S0093-691X(01)00540-4. [DOI] [PubMed] [Google Scholar]
- Harrison RAP, Dott HM, Foster GC. Effect of ionic strength, serum albumin and other macromolecules on the maintenance of motility and the surface of mammalian spermatozoa in a simple medium. J Reprod Fert. 1978;52:65–73. doi: 10.1530/jrf.0.0520065. [DOI] [PubMed] [Google Scholar]
- Kuster CE, Althouse GC. The fecundity of porcine semen stored for 2 to 6 days in Androhep and X-CELL extenders. Theriogenology. 1999;52(3):365–376. doi: 10.1016/S0093-691X(99)00135-1. [DOI] [PubMed] [Google Scholar]
- Larsson K. Boar sperm viability after freezing and thawing. Proc 1st Int Conf Deep Freez Boar Semen, Uppsala. 1985. pp. 177–187.
- Oettlé EE. Using a new acrosome stain to evaluate sperm morphology. Vet Med. 1986;81:103–106. [Google Scholar]
- Oettlé EE. Changes in acrosome morphology during cooling and freezing of dog semen. Anim Reprod Sci. 1986;12:145–150. doi: 10.1016/0378-4320(86)90054-0. [DOI] [Google Scholar]
- Paulenz H, Grevle IS, Tverdal Aa, Hofmo PO, Andersen Berg K. Precision of Coulter counter for routine assessment of boar sperm concentration in comparison with haemocytometer and spectrophotometer. Reprod Dom Anim. 1995;30:107–111. doi: 10.1111/j.1439-0531.1995.tb00614.x. [DOI] [Google Scholar]
- Paulenz H, Grevle IS, Andersen Berg K, Thomassen R. The use of a dichromatic stain method (Spermac®) for determining changes in the acrosomal integrity of boar semen during cryopreservation. Reprod Dom Anim. 1995;30:113–116. doi: 10.1111/j.1439-0531.1995.tb00615.x. [DOI] [Google Scholar]
- Perez Marcos C, Sanchez R, Palacio M, Pursel VG, Perez Garcia T, Martin Rillo S. Effects of dilution rate on the motility and acrosome morphology of boar spermatozoa stored at 15°C. Reprod Dom Anim. 1991;26:112–116. doi: 10.1111/j.1439-0531.1991.tb01527.x. [DOI] [Google Scholar]
- Rath D, Weitze KF, Pena Alfaro CE, Andrade Moura JC. Effects of seminal plasma on the number of accessory sperm cells and fertilization in gilts. Zuchthyg. 1989;24:123–127. [Google Scholar]
- Rodriguez-Martinez H, Ekstedt E, Einarsson S. Acidification of the epididymal fluid in the boar. Int J Androl. 1990;13:238–243. doi: 10.1111/j.1365-2605.1990.tb00982.x. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. User's Guide, Statistics. Version 5 Cary, NC. 1985.
- Vishwanath R, Shannon P. Do sperm cells age? A review of the physiological changes in sperm during storage at ambient temperature. Reprod Fertil Dev. 1997;9:321–331. doi: 10.1071/R96088. [DOI] [PubMed] [Google Scholar]
- Waberski D, Meding S, Dirksen G, Weitze KF, Leiding C, Hahn R. Fertility of long-term-stored boar semen: Influence of extender (Androhep and Kiev), storage time and plasma droplets in the semen. Anim Reprod Sci. 1994;36:145–151. doi: 10.1016/0378-4320(94)90061-2. [DOI] [Google Scholar]
- Watson PF, Plummer JM. The response of boar sperm membranes to cold shock and cooling. Proc 1st Int Conf Deep Freez Boar Semen, Uppsala. 1985. pp. 113–127.
- Weitze KF, Rabeler J, Willmen T, Waberski D. Interaction between inseminate, uterine and ovarial function in the sow. I. Influence of seminal plasma and oestrogens in the inseminate on intragenital sperm transport, time of ovulation and fertility results in gilts. Reprod. Dom Anim. 1990;25:191–196. doi: 10.1111/j.1439-0531.1990.tb00459.x. [DOI] [Google Scholar]
- Weitze KF, Lotz JH, Everwand A, Willmen T, Waberski D. Interaction between inseminate, uterine and ovarial function in the sow. II. Investigations into the influencing of ovulation by the use of sperm-free media. Reprod Dom Anim. 1990;25:197–204. doi: 10.1111/j.1439-0531.1990.tb00460.x. [DOI] [Google Scholar]
- Willmen T, Weitze KF, Habeck O, Waberski D. Effect of seminal plasma and different sperm number in the inseminate on fertilization, accessory sperm number and ovulation time. Proc 3rd Int Congr Pig Reprod, Nottingham, UK. 1989. Abstract no 28.


