Abstract
Infection with the intracellular microsporidium Encephalitozoon cuniculi can cause serious disease, encephalitozoonosis, in the blue fox (Alopex lagopus). The disease diagnosis is based on clinical signs and pathological findings, and detection of E. cuniculi or circulating antibodies directed against the parasite. Indirect immunofluorescence (IFAT) and carbon immunoassay (CIA) are the most commonly used serological methods for diagnosis in this species. In the present study, an indirect ELISA (enzyme linked immunosorbent assay) was established and evaluated against IFAT by testing of 205 field samples from blue foxes. There was high agreement between the results of the ELISA and CIA (κ = 0.99), and the ELISA and IFAT (κ = 0.958). There was no significant statistical difference between the tests (p > 0.05). It was concluded that the ELISA could be used to identify seropositive farmed blue foxes. The advantage of the ELISA lies in the potential of screening large numbers of animals with the goal of eradicating E. cuniculi infection in the farms.
Keywords: microsporidia, encephalitozoonosis, ELISA, carbon immunoassay, indirect immunofluorescence
Introduction
The intracellular microsporidium Encephalitozoon cuniculi is an obligate parasite that infects a wide range of vertebrate animals [4]. In some hosts, such as rabbit and carnivores, infection can cause disease. The development of clinical disease in different species is highly influenced by natural innate species susceptibility and by the immune status of the individual. For example, severe disease can occur in young dogs or in the blue fox (Alopex lagopus) whereas the farmed silver fox (Vulpes vulpes) is resistant [22]. E. cuniculi infection has also been recorded to cause clinical disease in humans suffering from AIDS or other immune depressive disorders [33].
Outbreaks of encephalitozoonosis lead to heavy losses of pups in blue fox farms. Pups infected in embryonic or foetal life develop encephalitozoonosis 1 to 3 months after birth. The diagnosis of encephalitozoonosis in blue foxes is based on clinical signs and pathological findings [22], and on the detection of E. cuniculi or of circulating antibodies directed against the parasite [20]. At necropsy, parasites may be identified by histological examination, mainly of the brain and kidney. In live animals, a number of methods have been applied to detect E. cuniculi infection. There are methods for detecting spores in urine or faeces. The methods for detection of microsporidia include specific stains, for instance the chromotrope stain [32], and isolation of spores [9]. These methods are specific, but not practically applicable as diagnostic methods in veterinary medicine [24].
Several serological methods have been used for diagnosing E. cuniculi infection in live blue foxes, including indirect immunofluorescence (IFAT, [20]) and carbon immunoassay (CIA, [19]). The CIA uses purified spores of E. cuniculi to which antibodies bind. This reaction is visualised when carbon particles are bound to these immunoglobulins. India ink is thought to bind to the Fc-end of both rabbit and blue fox immunoglobulin G [19,30]. Enzyme linked immunosorbent assays (ELISA) have been developed to test sera of canine, mouse, rabbit, squirrel, monkey and human origin for antibodies to E. cuniculi [2,7]. An initial attempt has been made on an ELISA on blue fox sera for E. cuniculi antibodies [27]. The purpose of the present study was to establish and evaluate a new indirect ELISA with antigen produced from a blue fox isolate for serological diagnosis of E. cuniculi infection in the same animal species.
Materials and methods
Field sera
Serum samples were collected from farmed blue foxes in the Norwegian counties of Hedmark and Oppland during an outbreak of encephalitozoonosis in 1992. The animals were classified as positive or negative for E. cuniculi antibodies using CIA, and the samples were then stored at -40°C. The seropositive animals all came from farms where encephalitozoonosis had been confirmed by pathological examination of clinical cases. The seronegative animals were from farms without outbreak of clinical encephalitozoonosis and with no other indication of the disease.
Reference sera
A positive reference serum pool was obtained by the pooling of sera from three farmed blue foxes. The animals had clinical and pathological signs of chronic encephalitozoonosis, and E. cuniculi had been isolated from 2 of them [17]. The negative reference serum pool was mixed from 5 blue foxes in a farm without any sign of encephalitozoonosis.
Production of parasite spores
Spores of the isolate IPZ: N-F82 and N-F220 were produced in monolayer cell cultures of human fibroblast cells (MRC-5, [17]). The cells were grown in modified Eagle medium (MEM) with Earle's buffered saline solution supplemented with inactivated bovine foetal serum (100 μl/ml), sodium bicarbonate (1.12 mM), L-glutamin (4 mM) and antibiotics (penicillin: 100 I.E./ml; streptomycin: 100 μg/ml and amphotericin B: 250 ng/ml) (cells and medium delivered by Bio-Whittaker, Walkersville, MD, USA). The pH was adjusted to 7.4 and the cells were incubated at 37°C in normal atmosphere supplemented with 5 per cent carbondioxide. Spores were harvested in the culture medium at weekly intervals, and stored for up to 3 months at 4°C before preparation.
The spores were washed twice in phosphate buffered saline (PBS; 170 mM, pH 7.4) by centrifugation (10 min, 1,750 × g). The pellet was then resuspended in 10 ml sodium dodecyl sulfate (SDS; 1.25 mg/ml) in PBS, and incubated (30 min, 37°C). Thereafter, the spores were washed twice again, and the pellet was resuspended in PBS to the concentration of 108 spores E. cuniculi per ml for the ELISA, or distilled water for the IFAT.
Preparation of soluble antigen
Following 3 cycles of freezing – thawing, the spores were mixed with solid glass beads (1:3, Jencons (Scientific) Limited, UK), and sonicated (30 min, 60 W). The number of spores before and after disruption was counted in a haematocytometer to ensure at least 95 per cent spore disruption in the homogenate. After centrifugation (10 min, 17,500 × g, 7°C), the protein content of the supernatant was measured by UV detection as described by [14]. With the protocol used for antigen preparation approximately 108 E. cuniculi spores per ml gave a protein concentration in the final antigen solution of 0.3 mg/ml. The soluble antigen solution was aliquoted and stored at -40°C until use. From non-infected cell cultures the supernatant was handled equally to produce a control antigen.
Enzyme linked immunosorbent assay
Polystyrene microtitre plates (Immunoplate Maxisorb; Nunc, Roskilde, Denmark) were coated for 3 days at 4°C with 150 μl diluted antigen solution (Fig. 1). The following coating buffers were used: carbonate bicarbonate buffer (100 mM, pH 9.6), phosphate buffered saline (170 mM, pH 7.4), citrate phosphate buffers (70 mM, pH 4.0 and 150 mM, pH 5.0), ethanol (70% v/w), and distilled water. The plates were washed 3 times in PBS, and some of them were incubated for 1 h with one of the following blocking solutions: bovine serum albumen (BSA: 10 mg/ml), horse serum (100 μl/ml), non-fat dry milk (30 mg/ml). All subsequent incubations were done at 37°C followed by washing 5 times using PBS with Tween 20 (1 μl/ml; ELISA buffer). The ELISA buffer was added to the blocking substance when the plates were incubated with blocking solutions. The serum samples were diluted in ELISA buffer, and 100 ml was added to each well and incubated for 2 h. After washing, horseradish peroxidase (HRP) conjugated goat anti-dog IgG (ICN/Cappel, Aurora, OH, USA) was diluted in the ELISA buffer and added to the plates, which were subsequently incubated (2 h) and washed. Thereafter, 50 mg of the substrate, 2,2'-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid; ABTS™) was mixed in 100.0 ml citrate-phosphate buffer (6.93 mM, pH 4.0) plus 6 μl 30 per cent hydrogen peroxide, and added to the miocrotitre plates (100 μl/well). After incubation in darkness (45 min), the enzymatic colour reaction was stopped by adding 25 μl sodium acid solution (100 ng/ml) to each well, and the optical density was read at 405 nm.
Figure 1.
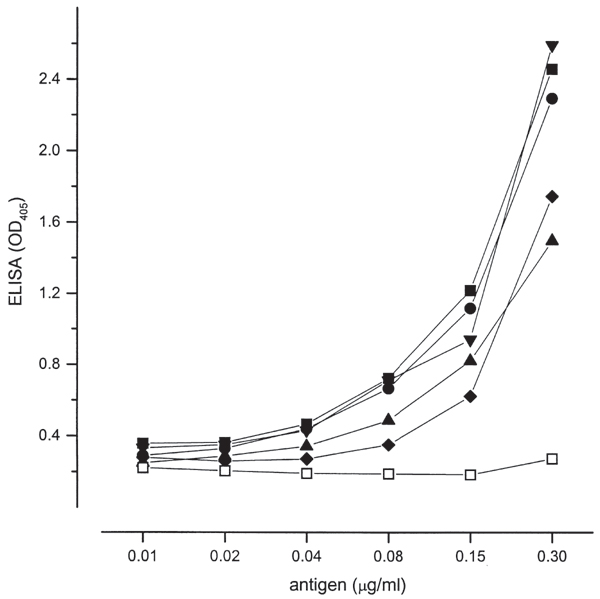
ELISA values for positive reference serum and soluble E. cuniculi antigen diluted 2-fold from 1:1000. Each line represents the following coating buffers: -■- carbonate bicarbonate (100 mM, pH 9.6) -▲- citrate phosphate (70 mM, pH 4.0) -◆- distilled water -●- phosphate buffered saline (PBS; 170 mM, pH 7.4) -▼- citrate phosphate (150 mM, pH 5.0) -□- ethanol (70% v/w).
All sera were tested in 4 wells, 2 wells coated with E. cuniculi antigen and 2 wells coated with control antigen. Thus, each 96-well miocrotitre plate harboured a maximum of 22 test sera and a positive and a negative control. The ELISA values of the sample sera were expressed as sample to positive ratio { S:P-ratio = (OD of sample – OD of negative control)/(OD of positive control – OD of negative control) } [28]. The median value for the 2 pairs of S:P values of each sample was used when selecting cut-off values of the assay. To select the ELISA cut-off values, a 2-graph receiver operating characteristic (TG-ROC) plot was used [13,36]. Thereby, pairs of sensitivity and specificity could be read directly from the pre-assigned threshold values of the TG-ROC plot. For the calculation, a user-defined template was obtained as public domain software [12]. Two cut-off values were selected where the sensitivity and specificity, respectively, were equal to the pre-selected accuracy level of 0.95. Values between the cut-off values were considered intermediate. The estimation of the cut-off values was performed with a non-parametric method. ELISA results were interpreted as follows: when both S:P-ratios were above the upper cut-off value, the serum sample was classified as positive for E. cuniculi antibodies. A sample with both values below the lower cut-off value was regarded as negative. Serum samples, which had both values between the cut-off values, were in the intermediate range (IR) of the test. Samples, which had values on both sides of a cut-off value, were classified as not interpretable (NI).
Carbon immunoassay
On arrival of samples to the laboratory, the CIA was performed according to the manufacturer's instructions (Testman, Uppsala, Sweden, [31]). In brief, each serum sample was diluted 1:50 with PBS. Each diluted sample was mixed with 300000 E. cuniculi spores and incubated at room temperature for 2 min. Then the suspension was mixed with India ink on a glass slide and covered with glass. The result was read immediately at 1000 magnifications with oil in a light microscope. For positive sera, the E. cuniculi spores were stained greyish-black against the background of carbon particles, while in negative sera, the parasite appeared white against the dark background. Sera that agglutinated spontaneously in the test were diluted 1:100 and retested.
Immunofluorescence antibody test
IFAT was performed with spores from isolate IPZ: N-F82 [20]. Each well of 12-welled glass slides was coated with 80000 E. cuniculi spores diluted in ten μl distilled water. The slides were air-dried, fixed in acetone and either used immediately or stored in sealed plastic bags with silica gel at ÷ 40°C. For the assay, each well was blocked with 25 μl rabbit serum (100 μl/ml) in PBS and the slides were incubated in a humidity chamber at room temperature for 30 min. The slides were washed twice for 5 min in PBS under agitation. Serum samples were diluted 1:40 in PBS with rabbit serum (10 ml/ml), and 25 μl of the diluted sample were added per well. Both positive and negative control sera were added to each slide, which was incubated at 4°C. The next day, the slides were washed, and 25 μl of fluoresceinisothiocyante (FITC) conjugated rabbit antidog IgG (Sigma®, St. Louis, MO, USA) diluted 1:25 in PBS with rabbit serum (10 μl/ml) and Evans blue (1:10000) was added to each well. The slides were incubated at room temperature in darkness for 60 min, washed, dried and mounted. Examination of the slides was carried out at a magnification of 400 times in an epifluorescence microscope (Leitz, Wetzlar, Germany) supplied with a 50 W mercury lamp. Wells with strong peripheral fluorescence in at least 50 per cent of the spores were considered positive.
Statistical analysis
The analytical error (s) could be calculated from the ELISA results of the test sera values with the formula: s = square root (Σ d2/2n) where n was the number of duplicate determinations and d was the difference within a certain pair [15]. The agreement (κ) between tests was calculated for the samples classified as positive and negative in the ELISA compared to the results of the IFAT [16]. The agreement was considered low, moderate and high, i.e., 0.4 ≤ κ < 0.6, 0.6 ≤ κ<0.8 and 0.8 ≤ κ ≤ 1, respectively. The Fisher exact test was used to evaluate if differences between tests were significant or not [1].
Results
Establishment
Among the coating buffers used, PBS, carbonate bicarbonate and citrate phosphate buffer (pH 5.0) gave the highest optical density values for the reaction between E. cuniculi and positive reference serum (Fig. 1). In addition, the reaction between positive serum and control antigen was the highest using PBS and citrate phosphate buffers, and below 0.25 OD405 with the other buffers. The absorbency for the reaction between negative serum and E. cuniculi antigen was low for all buffers used (data not shown).
Non-fat dry milk and BSA used to block unspecific antigen-antibody interactions gave high absorbencies when the reaction between E. cuniculi antigen and positive serum was assayed. The ratio between positive and negative serum was slightly greater for non-fat milk than for BSA. Therefore not-fat dry milk was selected as the blocking reagent.
Among the possible alternatives of coating buffer, blocking agent and dilutions of antigen, serum and conjugate, the optimal combination was selected to give the most correct measurement of the amount of specific antibody present in the samples. The best conditions were obtained for the ELISA when the antigen concentration was 0.15 μg/ml carbonate bicarbonate buffer, with non-fat dry milk used for blocking and in the sample buffer, with a serum dilution of 1:100 and the conjugate diluted 1:5000. This resulted in minimal unspecific reaction and strong reaction between positive reference serum and E. cuniculi antigen. Under these conditions, the positive reference serum gave a good response when diluted 1:200. The ELISA OD405 value for the positive reference serum diluted 1:100 was 1.61. The values for CIA test and IFAT were 1:3200 and 1:5120, respectively. The negative reference serum diluted 1:100 had ELISA OD405 value 0.262, and was negative in CIA and IFAT.
Examination of field samples
Of 205 blue fox blood sera from the field, 99 were positive and 106 negative in the CIA.
a) Results of the ELISA measurements, where the OD values from the wells coated with control antigen were subtracted, gave a difference in average S:P value between negative and positive samples of 0.478, and an analytical error of 0.119. The cut-off values were 0.101 and 0.128, respectively. Ninety-one samples were positive, 93 negative, 1 intermediate and 20 not interpretable. All ELISA positive samples were also positive in the CIA test. In addition, 2 ELISA negative samples had been positive with CIA. The agreement (κ) between these tests was 0.978.
b) When the ELISA S:P-ratio was calculated for the E. cuniculi antigen coated wells only, the difference in average S:P value between positive and negative samples was 0.465, and the analytical error was 0.085. The cutoff values could be read from the TG-ROC plot (Fig. 2) giving an intermediate range between 0.116 and 0.151. Thus, 93 samples were positive, 100 negative and 12 not interpretable. One negative sample had been positive with CIA (Table 1).
Figure 2.
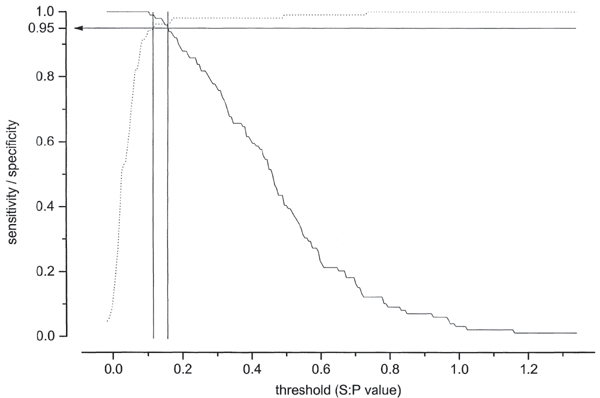
Two-graph receiver operating curve (TG-ROC) analysis for 205 sera from blue foxes tested in the E. cuniculi ELISA. Based on CIA results, the 2 graphs represent sensitivity (solid line) and specificity (dotted line) for thresholds of the ELISA S:P values, where the intersection is the point were sensitivity and specificity are equal. An intermediate range lies between 2 cut-off values were sensitivity and specificity are 0.95, respectively. ELISA results below the lower cut-off value are considered negative, and S:P values above the upper cut-off value are positive. The S:P values represent only wells coated with E. cuniculi.
Table 1.
Blue fox sera classified as positive or negative for antibodies to E. cuniculi with the ELISA compared to IFAT and CIA results. (Results for wells coated with E. cuniculi antigen only).
| CIA | IFAT | ||||
| + | - | + | - | ||
| ELISA | + | 93 | 0 | 86 | 4 |
| - | 1 | 99 | 0 | 100 | |
| Total | 193 | 190 | |||
| Agreement | κ | 0.99 | 0.958 | ||
The agreement (κ) between ELISA and CIA was 0.99.
There was full compliance for 179 sera that were either positive (n = 89) or negative (n = 90) in both ELISA applications.
The results of the application without control antigen were used for the comparison with IFAT. Ninety of 202 field sera were found positive in the IFAT, and of these, 86 were positive and 4 could not be classified with the ELISA. Of 112 negative samples in the IFAT, 100 were negative also with ELISA, 8 were not classified and 4 were positive (Table 1). The agreement between the 2 tests was high with κ = 0.958. The statistical differences between all tests were not significant (p > 0.05).
Discussion
In the present study an indirect ELISA for detection of circulating antibodies against the protozoan parasite E. cuniculi is described and compared with IFAT and CIA. The discrimination between 93 positive and 100 negative samples in the ELISA was good.
The soluble antigen used for the ELISA differs from the antigen used for IFAT and CIA, where the serological response to outer proteins of whole spores is measured. In E. cuniculi infected animals, antibodies are produced against the spore wall, the polar tube and the sporoplasm of the parasite [29]. With silver staining after SDS PAGE about 35 protein bands can be visualised from E. cuniculi, and the blue fox mounts a humoral immune response to at least 25 of these [17]. The main obstacle in the present study was to clean parasite spores from cell debris and then to disrupt them without loosing too much antigen. For the evaluation of the ELISA, sera were also added to wells coated with control antigen only. When the control wells were subtracted, the ELISA gave almost twice as many not interpretable results (20 samples) as when the control wells were neglected (12). Also, the analytical error was higher. The reason for this could be the difficulty in preparing an antigen with the same amounts of cell debris as the E. cuniculi antigen. Thus, the control wells were excluded from the assay.
The cut-off value for serological tests has to be defined so that infected animals are positive in the test, but also to avoid unnecessary cross-reactions. Humoral immune response to E. cuniculi is strong and serological titres in naturally diseased animals with clinical signs of encephalitozoonosis are usually high. Typical IFAT titres in blue foxes are from 1:800 and higher [20], and typical CIA titres are also in the same range (Åkerstedt et al. unpublished). A low cut off has been used for most reported diagnostic assays. Usually, an animal has been regarded as seropositive when the IFAT was positive at a serodilution of 1 to 10. In the CIA spontaneous agglutination readily occurs at the dilution of 1:10 and below. From experience with this method in our laboratory, even dilutions of 1:25 of sub-optimally sampled sera may cause agglutination at a rather high rate. For routine testing the dilution of 1:50 is used. In this study IFAT titres equal to or above 1:40 were considered positive, and titres of 1:50 or higher were considered positive in the CIA. To be able to use the ELISA as a diagnostic test for infection, cut-off levels had to be chosen. Since the true serological status of the collected serum samples was not known, the cut-off values were calculated by comparing the results of the ELISA with the CIA results. IFAT was used for evaluation and the ELISA produced results that strongly agreed with IFAT.
The immunofluorescence test is very sensitive when compared to histological detection of E. cuniculi, intradermal test or complement fixation test [23]. Both IFAT and CIA have proven successful in monitoring and establishing rabbit colonies free of encephalitozoonosis [3,6]. The ELISA may prove to be an alternative test to reach this goal in blue fox farms.
Compared with IFAT the ELISA produced a few "false" positive results, but from a practical point of view these are usually not a problem in blue fox farming. All animals tested positive can be isolated and removed when foxes are pelted. However, for epidemiological studies where the prevalence is investigated serologically, false positive results may be crucial. This is especially the case when the true prevalence is low and most positive reactions will be false. Antigenic similarities of the spore wall of microsporidia make antibody cross-reactions possible between different species of microsporidia [21,34]. Since microsporidia are parasites of virtually all vertebral species, relatively high amounts of microsporidia antigens may therefore be consumed by individuals supplied with feed of animal origin [4]. The possibility of seroconversion due to other microsporidia can thus not be neglected, and some authors even conclude that serology is not useful for the diagnosis of human microsporidian infection [10]. When applying serological methods for epidemiological studies in areas where the true prevalence is low, positive reactions should be verified by alternative techniques.
Serology is used to confirm clinical cases of encephalitozoonosis, but also to screen farms for possibly infected animals. The IFAT has the advantage in detecting antibodies at an early stage of the infection [19]. The CIA test, on the other hand, profits by being technically simple, requiring only uncomplicated reagents and a minimum of laboratory equipment, and has so far been the most commonly used serological method in blue fox farming. Both these methods have the disadvantage of being time consuming and require an experienced technician for the evaluation of the result, whereas the optical densities of the ELISA can be measured photometrically. The ELISA technique furthermore has the potential of being applied in rapid test kits that can be used under field conditions. The results of the positive, intermediate or doubtful sera can, when necessary, be confirmed with a repeated ELISA or with IFAT or CIA technique. The latter techniques will therefore in any case remain valuable in the future for confirmation of the ELISA results and testing small number of samples.
In conclusion, the results of this study show that the described indirect ELISA can be used in order to detect circulating antibodies against the protozoan parasite E. cuniculi in blue foxes and thereby infection. It is a supplement to the existing serological methods for the same purpose. ELISA can increase the diagnostic capacity of the laboratory and make it practically possible and economically advantageous to have farms screened for E. cuniculi infection. This makes it possible to take appropriate measures with the goal of eradicating encephalitozoonosis from populations of blue foxes.
Acknowledgments
Acknowledgements
The study was financed by grant 103.107 from the Norwegian Research Council.
References
- Altman DG. Practical statistics for medical research. 1. Chapman & Hall, London, England; 1993. p. 611. [Google Scholar]
- Beckwith C, Peterson N, Liu JJ, Shadduck JA. Dot enzyme-linked immunosorbent assay (dot ELISA) for antibodies to Encephalitozoon cuniculi. Lab Anim Sci. 1988;38:573–576. [PubMed] [Google Scholar]
- Bywater JEC, Kellett BS. The eradication of Encephalitozoon cuniculi from a specific pathogenfree rabbit colony. Lab Anim Sci. 1978;28:402–404. [PubMed] [Google Scholar]
- Canning EU, Lom J. The microsporidia of vertebrates. Academic Press, London; 1986. p. 289. [Google Scholar]
- Cox JC, Gallichio HA. An evaluation of indirect immunofluorescence in the serological diagnosis of Nosema cuniculi infection. Res Vet Sci. 1977;22:50–52. [PubMed] [Google Scholar]
- Cox JC, Gallichio HA, Pye D, Walden NB. Application of immunofluorescence to the establishment of an Encephalitozoon cuniculi-free rabbit colony. Lab Anim Sci. 1977;27:204–209. [PubMed] [Google Scholar]
- Cox JC, Horsburgh R, Pye D. Simple diagnostic test for antibodies to Encephalitozoon cuniculi based on enzyme immunoassay. Lab Anim. 1981;15:41–43. doi: 10.1258/002367781780958513. [DOI] [PubMed] [Google Scholar]
- Cox JC, Pye D. Serodiagnosis of nosematosis by immunofluorescence using cell-culture-grown organisms. Lab Anim. 1975;9:297–304. doi: 10.1258/002367775780957124. [DOI] [PubMed] [Google Scholar]
- Cox JC, Walden NB, Nairn RC. Presumptive diagnosis of Nosema cuniculi in rabbits by immunofluorescence. Res Vet Sci. 1972;13:595–597. [PubMed] [Google Scholar]
- Didier ES, Kotler DP, Dieterich DT, Orenstein JM, Aldras AM, Davis R, et al. Serological studies in human microsporidiosis. AIDS. 1993;7:S8–S11. [Google Scholar]
- Gannon JV. The immunoperoxidase test diagnosis of Encephalitozoon cuniculi in rabbits. Lab Anim. 1978;12:125–127. doi: 10.1258/002367778780936322. [DOI] [PubMed] [Google Scholar]
- Greiner M. Two-graph receiver operating characteristic (TG-ROC): a Microsoft-EXCEL template for the selection of cut-off values in diagnostic tests. J Immunol Methods. 1995;185:145–146. doi: 10.1016/0022-1759(95)00078-O. [DOI] [PubMed] [Google Scholar]
- Greiner M, Sohr D, Göbel P. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J Immunol Methods. 1995;185:123–132. doi: 10.1016/0022-1759(95)00121-P. [DOI] [PubMed] [Google Scholar]
- Harlov E, Lane D. Antibodies – a laboratory manual. Cold Spring Harbor Laboratory, New York; 1988. p. 726. [Google Scholar]
- Larsson B. Mucosal disease and persistent infection with bovine virus diarhoea virus: studies on mononuclear cells in relation to their activities. Swedish university of agricultural sciences. 1987. p. 118.
- Martin SW, Meek AH, Willeberg P. Veterinary Epidemiology – principles and methods. 1. Iowa State University Press, Ames; 1987. p. 343. [Google Scholar]
- Mathis A, Åkerstedt J, Tharaldsen J, Ødegaard Ø, Deplazes P. Isolates of Encephalitozoon cuniculi isolated from farmed blue foxes (Alopex lagopus) from Norway differ from isolates from Swiss domestic rabbits (Oryctolagus cuniculus) Parasitol Res. 1996;82:727–730. doi: 10.1007/s004360050192. [DOI] [PubMed] [Google Scholar]
- McInnes EF, Stewart CG. The pathology of subclinical infection of Encephalitozoon cuniculi in canine dams producing pups with overt encephalitozoonosis. J S Afr Vet Med Assoc. 1991;62:51–54. [PubMed] [Google Scholar]
- Mohn SF. Encephalitozoonosis in the blue fox: comparison between the India-ink immuno-reaction and the indirect fluorescent antbody test in detecting Encephalitozoon cuniculi antibodies. Acta Vet Scand. 1982;23:99–106. doi: 10.1186/BF03546826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn SF, Ødegaard ØA. The indirect fluorescent antibody test (IFAT) for the detection of Nosema cuniculi antibodies in the blue fox (Alopex lagopus) Acta Vet Scand. 1977;18:290–292. doi: 10.1186/BF03548458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkorn JY, Shadduck JA, Weidner E. Antigenic cross-reactivity among different microsporidian spores as determined by immunofluorescence. J Parasitol. 1980;66:675–677. doi: 10.2307/3280530. [DOI] [PubMed] [Google Scholar]
- Nordstoga K, Mohn SF, Loftsgaard G. Nosematose hos blårev. Proc 12th Nordic Congress. 1974. pp. 183–186.
- Pakes SP, Shadduck JA, Feldman DB, Moore JA. Comparison of tests for the diagnosis of spontaneous encephalitozoonosis in rabbits. Lab Anim Sci. 1984;34:356–359. [PubMed] [Google Scholar]
- Pye D, Cox JC. Isolation of Encephalitozoon cuniculi from urine samples. Lab Anim. 1977;11:234. doi: 10.1258/002367777780936549. [DOI] [PubMed] [Google Scholar]
- Shadduck JA, Bendele R, Robinson GT. Isolation of the causative organism of canine encephalitozoonosis. Vet Pathol. 1978;15:449–460. doi: 10.1177/030098587801500402. [DOI] [PubMed] [Google Scholar]
- Shadduck JA, Geroulo MJ. A simple method for the detection of antibodies to Encephalitozoon cuniculi in rabbits. Lab Anim Sci. 1979;29:330–334. [PubMed] [Google Scholar]
- Tharaldsen J. Detection of antibodies against Encephalitozoon cuniculi in the blue fox (Alopex lagopus) by ELISA. The joint Baltic Scandinavian symposium on parasitic zoonoses and ecology of parasites. Vilnius, Lithuania Bull Soc Scand Parasit. 1995;5:53. [Google Scholar]
- Tijssen P. Practice and theory of enzyme immunoassays. 1. Elsevier Science Publishers B.V., Amsterdam; 1985. p. 549. [Google Scholar]
- Visvesvara GS, Leitch GJ, Moura H, Wallace S, Weber R, Bryan RT. Culture, electron microscopy, and immunoblot studies on a microsporidian parasite isolated from the urine of a patient with AIDS. J Protozool. 1991;38:105S–111S. [PubMed] [Google Scholar]
- Waller T. The india-ink immunoreaction: a method for the rapid diagnosis of encephalitozoonosis. Lab Anim. 1977;11:93–97. doi: 10.1258/002367777781005604. [DOI] [PubMed] [Google Scholar]
- Waller T, Bergquist NR. Rapid simultaneous diagnosis of toxoplasmosis and encephalitozoonosis in rabbits by carbon immunoassay. Lab Anim Sci. 1982;32:515–517. [PubMed] [Google Scholar]
- Weber R, Bryan RT, Owen RL, Wilcox CM, Gorelkin L, Visvesvara GS, et al. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. N Engl J Med. 1992;326:161–166. doi: 10.1056/NEJM199201163260304. [DOI] [PubMed] [Google Scholar]
- Weber R, Bryan RT, Schwartz DA, Owen RL. Human microsporidial infections. Clin Microbiol Rev. 1994;7:426–461. doi: 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LM, Cali A, Levee E, Laplace D, Tanowitz HB, Simon D, et al. Diagnosis of Encephalitozoon cuniculi infection by western blot and the use of cross-reactive antigens for the possible detection of microsporidiosis in humans. Am J Trop Med Hyg. 1992;47:456–462. doi: 10.4269/ajtmh.1992.47.456. [DOI] [PubMed] [Google Scholar]
- Wosu NJ, Shadduck JA, Pakes SP, Frenkel JK. Diagnosis of encephalitozoonosis in experimentally infected rabbits by intradermal and immunofluorescence tests. Lab Anim Sci. 1977;27:210–216. [PubMed] [Google Scholar]
- Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]


